Abstract
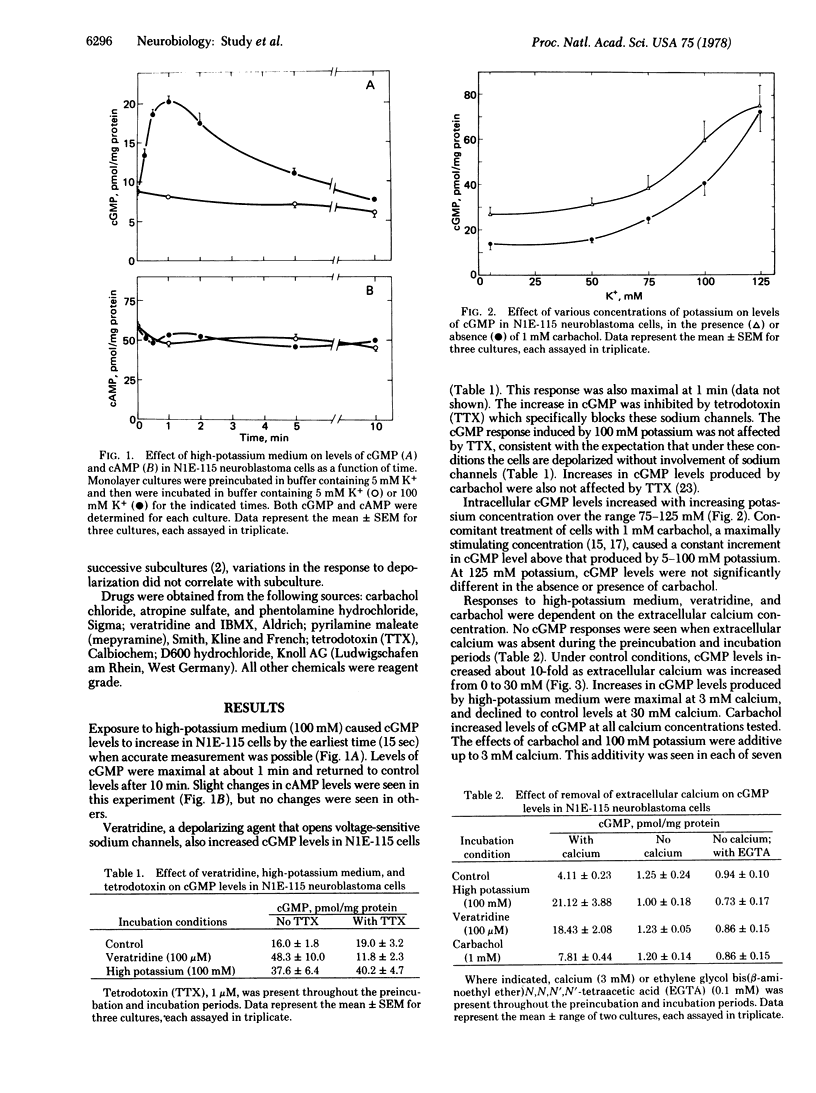
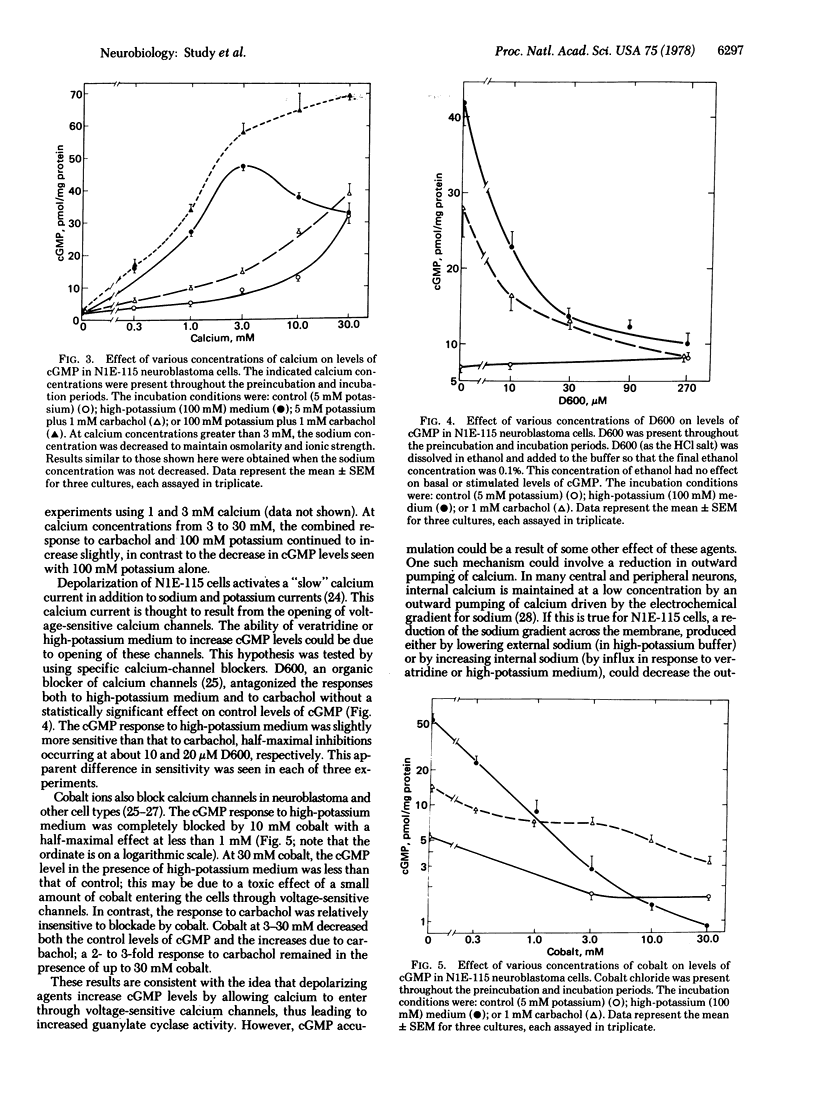
Veratridine or high potassium concentration increased guanosine 3',5'-cyclic monophosphate (cGMP) levels in neuroblastoma cells of clone N1E-115 without affecting levels of adenosine 3',5'-cyclic monophosphate (cAMP). The increases in cGMP appear to be a direct result of the depolarizing action of these agents and not due to the action of substances released from the cells upon depolarization. The increase in cGMP produced by depolarization was dependent upon extracellular calcium and could be prevented by the calcium channel blockers D600 and cobalt. Carbachol, acting on muscarinic acetylcholine receptors, also caused a calcium-dependent increase in cGMP in these cells. The carbachol and potassium effects were additive from 5 to 100 mM potassium and from 1 to 3 mM calcium. The carbachol response was nearly as sensitive as the potassium response to inhibition by D600 but was much less sensitive to inhibition by cobalt. The results suggest that depolarization increases cGMP levels in these cells by opening voltage-sensitive calcium channels and that activation of muscarinic receptors opens separate, voltage-insensitive calcium channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R., Nilsson K., Wikberg J., Johansson S., Mohme-Lundholm E., Lundholm L. Cyclic nucleotides and the contraction of smooth muscle. Adv Cyclic Nucleotide Res. 1975;5:491–518. [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Nestler E. J., Greengard P. Increased cyclic GMP levels associated with contraction in muscle fibres of the giant barnacle. Nature. 1977 Jun 9;267(5611):534–536. doi: 10.1038/267534a0. [DOI] [PubMed] [Google Scholar]
- Biggio G., Guidotti A. Climbing fiver activation and 3', 5'-cyclic guanosine monophosphate (cGMP) content in cortex and deep nuclei of cerebellum. Brain Res. 1976 May 7;107(2):365–373. doi: 10.1016/0006-8993(76)90233-x. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Breakefield X. O. Reserpine sensitivity of catecholamine metabolism in murine neuroblastoma clone N1E-115. J Neurochem. 1975 Dec;25(6):877–882. doi: 10.1111/j.1471-4159.1975.tb04421.x. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey D., Vincent J. P., Làzdunski M. The muscarinic receptor of heart cell membranes. Association with agonists, antagonists and antiarrhythmic agents. FEBS Lett. 1977 Dec 1;84(1):110–114. doi: 10.1016/0014-5793(77)81068-5. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Kinscherf D. A., Chang M. M. Regulation of levels of guanosine cyclic 3',5'-monophosphate in the central nervous system: effects of depolarizing agents. Mol Pharmacol. 1973 Jul;9(4):445–454. [PubMed] [Google Scholar]
- Ferrendelli J. A., Rubin E. H., Kinscherf D. A. Influence of divalent cations on regulation of cyclic GMP and cyclic AMP levels in brain tissue. J Neurochem. 1976 Apr;26(4):741–748. doi: 10.1111/j.1471-4159.1976.tb04447.x. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Goldberg N. D., O'Dea R. F., Haddox M. K. Cyclic GMP. Adv Cyclic Nucleotide Res. 1973;3:155–223. [PubMed] [Google Scholar]
- Kimhi Y., Palfrey C., Spector I., Barak Y., Littauer U. Z. Maturation of neuroblastoma cells in the presence of dimethylsulfoxide. Proc Natl Acad Sci U S A. 1976 Feb;73(2):462–466. doi: 10.1073/pnas.73.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinscherf D. A., Chang M. M., Rubin E. H., Schneider D. R., Ferrendelli J. A. Comparison of the effects of depolarizing agents and neurotransmitters on regional CNS cyclic GMP levels in various animals. J Neurochem. 1976 Mar;26(3):527–530. doi: 10.1111/j.1471-4159.1976.tb01506.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mao C. C., Guidotti A., Costa E. The regulation of cyclic guanosine monophosphate in rat cerebellum: possible involvement of putative amino acid neurotransmitters. Brain Res. 1974 Oct 25;79(3):510–514. doi: 10.1016/0006-8993(74)90449-1. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H., Nirenberg M. Receptor-mediated shifts in cGMP and cAMP levels in neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3472–3476. doi: 10.1073/pnas.72.9.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointer R. H., Butcher F. R., Fain J. N. Studies on the role of cyclic guanosine 3':5'-monophosphate and extracellular Ca2+ in the regulation of glycogenolysis in rat liver cells. J Biol Chem. 1976 May 25;251(10):2987–2992. [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Richelson E. Antipsychotics block muscarinic acetylcholine receptor-mediated cyclic GMP formation in cultured mouse neuroblastoma cells. Nature. 1977 Mar 24;266(5600):371–373. doi: 10.1038/266371a0. [DOI] [PubMed] [Google Scholar]
- Richelson E. Desensitisation of muscarinic receptor-mediated cyclic GMP formation by cultured nerve cells. Nature. 1978 Mar 23;272(5651):366–368. doi: 10.1038/272366a0. [DOI] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Baird C. E., Sutherland E. W. The importance of calcium ions for the regulation of guanosine 3':5'-cyclic monophosphage levels. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3889–3893. doi: 10.1073/pnas.70.12.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I., Kimhi Y., Nelson P. G. Tetrodotoxin and cobalt blockade of neuroblastoma action potentials. Nat New Biol. 1973 Nov 28;246(152):124–126. doi: 10.1038/newbio246124a0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Strange P. G., Birdsall N. J., Burgen A. S. Occupancy of muscarinic acetylcholine receptors stimulates a guanylate cyclase in neuroblastoma cells. Biochem Soc Trans. 1977;5(1):189–191. doi: 10.1042/bst0050189. [DOI] [PubMed] [Google Scholar]



