Abstract
Illicit psychostimulant addiction remains a significant problem worldwide, despite decades of research into the neural underpinnings and various treatment approaches. The purpose of this review is to provide a succinct overview of the neurocircuitry involved in drug addiction, as well as the acute and chronic effects of cocaine and amphetamines within this circuitry in humans. Investigational pharmacological treatments for illicit psychostimulant addiction are also reviewed. Our current knowledge base clearly demonstrates that illicit psychostimulants produce lasting adaptive neural and behavioral changes that contribute to the progression and maintenance of addiction. However, attempts at generating pharmacological treatments for psychostimulant addiction have historically focused on intervening at the level of the acute effects of these drugs. The lack of approved pharmacological treatments for psychostimulant addiction highlights the need for new treatment strategies, especially those that prevent or ameliorate the adaptive neural, cognitive, and behavioral changes caused by chronic use of this class of illicit drugs.
Keywords: substance abuse, pharmacotherapy, cocaine, amphetamine, methamphetamine, addiction, human
Introduction
Drug addiction, also referred to as substance dependence, is a serious and chronically relapsing disease wherein the afflicted individual has difficulty limiting drug intake, exhibits high motivation to take the drug, continues using the drug despite negative consequences, and experiences negative emotional and physiological states when the drug is withheld.1 In the United States, the 2010 prevalence rates (current and past-month use, in persons aged 12 years or older) for illicit drug use (including marijuana, cocaine, and heroin) reached 22.6 million (8.9%).2 The estimated number of persons aged 12 years or older classified with substance dependence (including illicit drugs and alcohol) in 2010 was 22.1 million, representing 8.7% of the US population.2 Furthermore, 20.5 million Americans were classified as needing treatment for an illicit drug or alcohol use problem.2 Of the 1 million persons that felt that they needed treatment for illicit drug or alcohol use problems, only 33% made an effort to seek treatment.2 These surprisingly high numbers and lack of effort to seek treatment clearly indicate that illicit drug addiction remains a significant problem in the US.
The most frequently abused illicit psychostimulants include cocaine and amphetamines, the latter of which represents a class of structurally related molecules, including D-amphetamine (AMPH), methamphetamine (METH), and 3,4-methylenedioxymethamphetamine (MDMA, commonly referred to as Ecstasy). Recent epidemiological data showed that in 2010 there were 1.5 million current cocaine users aged 12 or older, which comprised 0.6% of the population.2 The number of METH users represented 0.1% of the population, and the number of MDMA users was approximately 0.5%.2 In addition to the use of these classical illicit psychostimulants, the years 2010 and 2011 were characterized by a dramatic rise in the number of users of a newer class of amphetamine-like psychostimulants called synthetic cathinones, more frequently referred to as “bath salts”.3 However, due to the very recent emergence of the use of synthetic cathinones, national epidemiological data on the prevalence of their use as well as long-term effects on the brain are not yet available.
Using both animal models of addiction as well as advanced neuroimaging techniques, researchers have identified various neural substrates of addiction to psychostimulants, with primary emphasis on the ability of addictive drugs to “hijack” the brain’s natural reward circuitry.4 In recent years, it has become apparent that while acute psychostimulant use activates this reward circuitry, chronic drug use progressively “rewires” the brain and produces many lasting neuroadaptations that have been characterized as a pathology of “staged neuroplasticity.”5 The present review will provide a general overview of the reward and addiction neurocircuitries, the initial subjective effects of illicit psychostimulants and their mechanisms of action, the neuropsychological, psychiatric, and neurological sequelae of chronic psychostimulant use in humans, and functional and structural changes in the neurocircuitry of addiction. Since MDMA and synthetic cathinones possess unique pharmacological and hallucinogenic properties, and their addictive potential is less well established, our review will focus on the traditional psychostimulants cocaine, AMPH, and METH. In light of the fact that no pharmacological treatment has yet been approved by the US Food and Drug Administration (FDA) specifically for psychostimulant dependence, we will also summarize the newer and more promising investigational treatments and approaches.
Theories of addiction
The transition from drug use to drug dependence is impacted by numerous factors, including genetics, environmental influences (such as stress and early life experiences), and neurochemical and neuroanatomical modifications in the brain that result from repeated drug use.6,7 Initial drug use can be attributed to the ability of the drug to act as a reward (ie, a pleasurable emotional state or positive reinforcer), which can lead to repeated drug use and dependence.8,9 A great deal of research has focused on the molecular and neuroanatomical mechanisms of the initial rewarding or reinforcing effect of drugs of abuse. However, more recent research on the long-term neuroanatomical and molecular changes in the brain that result from chronic drug use has revealed drug addiction to be highly complex and involve brain systems beyond the canonical reward circuitry.
Several parallel and intersecting theories of drug addiction include changes in behavior that are supported by various alterations in the underlying neurocircuitry of addiction. Robinson and Berridge hypothesized that repeated drug use produces alterations in the brain reward and associative learning systems, such that the drug user becomes increasingly sensitive to both the drug and drug-associated cues, resulting in pathological drug seeking or “wanting.”10,11 Koob and Le Moal have postulated that repeated drug use and dependence are a result of decreased functioning of the brain’s reward system coupled with an increase in the engagement of the brain’s antireward or stress circuitries.12,13 These two opposing neurocircuitries are hypothesized to interact in a cyclical manner that ultimately manifests as addiction.12 Everitt and Robbins have theorized that drug addiction is the result of transitions from initial, voluntary drug use to habitual and eventually compulsive drug use, which results from a switch in the relative engagement of the neurocircuitries underlying these behaviors.14 Finally, Hyman and colleagues have also suggested has also suggested that addiction is a disorder characterized by the “hijacking” of normal learning and memory processes involved in reward seeking and drug–cue associations.4
These theories all agree that drug addiction is characterized by a progression or shift from initial stages, where the drug user is in control, to the end stages, where the drug user has lost control over drug use. Furthermore, this continuum of behavioral adaptation is proposed to be mediated by drug-induced alterations in the neurocircuitries underlying reward, executive function, and learning and memory. Despite these recent advances in our understanding of the addicted brain, the majority of FDA-approved treatments for drug addiction aim to intervene at the level of the acute rewarding effects of drugs of abuse.
The neurocircuitry of addiction
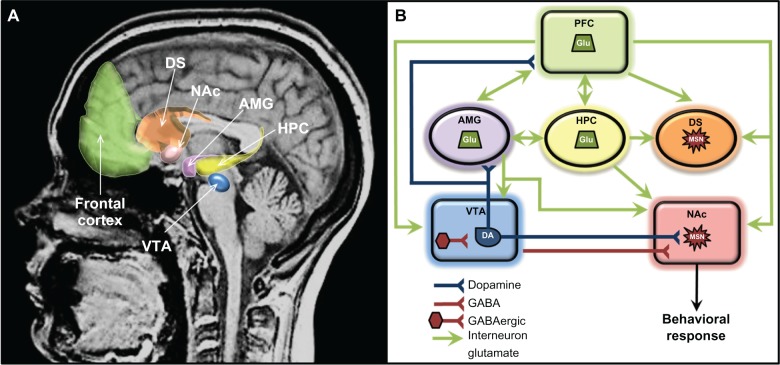
The brain reward circuitry underlies all motivated behavior for natural rewards (eg, food, water, and sex) as well as drug rewards. Some of the key brain regions of the reward circuitry include the ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex (PFC), basal ganglia, dorsal striatum (DS), amygdala (AMG), and hippocampus (HPC) (Figure 1). The rewarding effects of drugs and the progression from initial drug use to dependence are highly dependent on complex interactions between and adaptations within these regions. The key neurotransmitter that has been historically thought to mediate reward is dopamine (DA). However, as discussed below, psychostimulants also increase extracellular levels of glutamate (Glu), norepinephrine (NE), serotonin (5-HT), and other neurotransmitters within this neurocircuitry, which contribute to many of the long-term molecular and behavioral changes underlying addiction.15,16
Figure 1.
(A and B) Components of the principal reward neurocircuitries in the brain. (A) Neuroanatomical location of reward circuitry; (B) schematic of reward circuitry with primary neurotransmitter projections.
Note: Frontal cortex includes prefrontal, orbitofrontal, and anterior cingulate cortex.
Abbreviations: AMG, amygdala; DA, dopaminergic neurons; DS, dorsal striatum; GABA, γ-aminobutyric acid; Glu, glutamatergic neurons; HPC, hippocampus; MSN, medium spiny neurons; NAc, nucleus accumbens; PFC, prefrontal cortex; VTA, ventral tegmental area.
Reward systems
Ventral tegmental area
The brain reward circuit functionally begins with the VTA, which contains a cluster of dopaminergic cells with efferent projections to the NAc and to other brain regions, such as the PFC and AMG. Many natural rewards and drugs of abuse activate the dopaminergic neurons of the VTA to release DA in these brain regions.10,17–20 The VTA is also modulated by glutamatergic inputs from the PFC and AMG, in addition to a subpopulation of inhibitory γ-aminobutyric acid (GABA), interneurons within the VTA.21 Recent evidence also suggests that inputs to the VTA containing the stress-related peptide corticotropin releasing factor (CRF) may be responsible for modulating stress-induced influences on drug-seeking behavior, while orexin-containing inputs to the VTA from hypothalamic regions may also be involved in relapse-like behaviors.22,23 These neurotransmitter and neuropeptide interactions result in numerous cellular and molecular changes that contribute to reward-associated learning and the development of addiction.24
Nucleus accumbens
Most drugs of abuse, particularly psychostimulants, induce DA release at the level of the terminal fields in the NAc, PFC, and AMG.25 The release of DA in the NAc appears to be crucial for the rewarding effects of drugs of abuse. The NAc, which is contained within the ventral striatum, is composed primarily of GABAergic medium spiny neurons (MSNs). The NAc can be functionally subdivided into two distinct subregions, the core and shell, which respond to rewarding stimuli differently and are thought to mediate distinct aspects of addictive processes. The release of DA in the NAc shell contributes to the initial rewarding and reinforcing effects of drugs and the assignment of salience to rewarding stimuli, whereas DA release in the NAc core is thought to be more associated with the expression of learned behaviors in response to rewarding stimuli, such as drug seeking.26–30 Drug-induced elevations in extracellular DA in the NAc and other brain regions of the reward circuitry are modulated by numerous other neurotransmitter systems, including GABA, Glu, endogenous opioids and cannabinoids, 5-HT, NE, and various neuropeptides such as CRF.15,16,25,31–33 Furthermore, these neurotransmitter systems can modulate psychostimulant intake and drug-seeking behavior. For example, various ionotropic and metabotropic glutamate receptors in both the NAc core and shell mediate context-induced reinstatement of cocaine seeking.34
Executive function and habit systems
Prefrontal cortex
The PFC is a key regulator of cognitive and emotional processes, and contains many functionally distinct subregions. The dorsolateral PFC, orbitofrontal cortex (OFC), and anterior cingulate cortex are involved in salience attribution, decision-making, inhibition of inappropriate behaviors, behavioral and cognitive flexibility, and the regulation of emotion.35–37 These regions of the PFC have dense populations of glutamatergic cell bodies with efferent pathways that synapse onto the same cell populations (ie, MSNs) in the NAc that receive dopaminergic afferents from the VTA.38 In addition, glutamatergic efferents from the PFC also project to the AMG, VTA, DS, and indirectly to the HPC.39–41 Thus, the PFC is situated to modulate the rewarding effects of drugs of abuse and drug-seeking behavior, as well as relapse to drug taking.42 The PFC coordinates the flexibility with which an organism adapts its goal-directed behavior at many stages in the addiction process, and is modulated by inputs from other cortical and subcortical regions.
Dorsal striatum and cortical-basal ganglia loops
Regions of the basal ganglia, which include the dorsal and ventral striatum, internal and external segments of the globus pallidus, subthalamic nucleus, and dopaminergic cell bodies in the substantia nigra, are highly implicated not only in fine motor control but also in PFC function.43 Of these regions, the NAc (described above) and the DS (described below) are most frequently examined with respect to addiction. Thus, only a brief description of the modulatory role of the basal ganglia in addiction-relevant circuits will be mentioned here. The overall output of the basal ganglia is predominantly via the thalamus, which then projects back to the PFC to form cortico-striatal-thalamo-cortical (CSTC) loops. Three CSTC loops are proposed to modulate executive function, action selection, and behavioral inhibition. In the dorsolateral prefrontal circuit, the basal ganglia primarily modulate the identification and selection of goals, including rewards.44 The OFC circuit modulates decision-making and impulsivity, and the anterior cingulate circuit modulates the assessment of consequences.44 These circuits are modulated by dopaminergic inputs from the VTA to ultimately guide behaviors relevant to addiction, including the persistence and narrowing of the behavioral repertoire toward drug seeking, and continued drug use despite negative consequences.43–45
The DS (also referred to as the caudate-putamen in primates) is associated with transitions from goal-directed to habitual drug use, due in part to its role in stimulus–response learning.28,46 As described above, the initial rewarding and reinforcing effects of drugs of abuse are mediated by increases in extracellular DA in the NAc shell, and after continued drug use in the NAc core.47,48 After prolonged drug use, drug-associated cues produce increases in extracellular DA levels in the DS and not in the NAc.49 This lends to the notion that a shift in the relative engagement from the ventral to the dorsal striatum underlies the progression from initial, voluntary drug use to habitual and compulsive drug use.28 In addition to DA, recent evidence indicates that glutamatergic transmission in the DS is important for drug-induced adaptations and plasticity within the DS.50
Learning and memory systems
Hippocampus
The HPC is highly involved in normal spatial memory function, in part due to its critical involvement in the integration of contextual and environmental cues or information.51 Interestingly, the ventral subiculum, the primary output of the HPC, projects to several limbic regions, including the NAc and DS.52 The ventral subiculum has been found to play a key role in cocaine-seeking behavior, suggesting the importance of contextual information in psychostimulant addiction.53 In addition, the ventral subiculum-to-NAc pathway has been implicated in psychostimulant sensitization in a DA-dependent manner.54,55 Recently, it was found that a trans-synaptic link between the CA3 region of the HPC and the VTA may also mediate the ability of contextual information to modulate motivational behaviors.56 Microinjection studies by Fuchs and colleagues have also demonstrated a role for Glu transmission in the dorsal HPC in context-induced cocaine-seeking.57
Amygdala
The AMG plays a prominent role in conditioned learning, wherein strong associations between environmental stimuli and the rewarding effects of drug use are made. Glutamatergic efferents from the AMG to the PFC, NAc, and HPC mediate behavioral responses to these stimuli.58–60 The AMG is composed of multiple nuclei that play distinct roles in addiction related associative learning. The stress neuropeptide CRF within the central nucleus of the AMG mediates various aspects of stress-motivated behaviors pertinent to addiction, and drug-induced adaptations in CRF signaling have been found to occur within the AMG.61,62 In addition, the basolateral AMG is critical for cue-induced relapse, while the central nucleus of the AMG is a key neural substrate underlying stress-induced relapse.63–66 The reciprocal connections between the basolateral AMG and the anterior cingulate cortex may be particularly important for cocaine cue-induced relapse.67,68 Interestingly, the AMG and ventral subiculum themselves have reciprocal connections and have been found to project to the same population of NAc neurons.69,70 Thus, the outputs of the AMG and HPC interact and converge to modulate the reward circuitry and potentially solidify reward–context and reward–cue associations that contribute to the chronic relapsing characteristics of addiction.
Acute effects of psychostimulants
Mechanisms of action
As discussed above, a common neurobiological effect of many drugs of abuse is the direct or indirect modulation of DA-containing cell bodies in the VTA that target the NAc, resulting in the selective enhancement of extracellular DA levels in the NAc.20,72–74 However, not all drugs act directly on VTA dopaminergic neurons at the level of the cell bodies. Psychostimulants exert their effects through interactions with vesicular and plasma membrane monoamine reuptake transporters on presynaptic terminals in the NAc.75 Cocaine binds transporters for DA, NE, and 5-HT and competitively inhibits their function, resulting in increased extracellular levels of DA, NE, and 5-HT in the NAc.76 In transgenic mice lacking DA transporters (DATs), administration of cocaine did not result in enhanced locomotor activity or increased DA levels in the striatum, though these mice could learn to self-administer cocaine, potentially due to action at the NE and 5-HT transporters.77–79
Amphetamines (AMPH and METH) exert their effects in a more complex manner. Rather than simply blocking DATs (or NE and 5-HT transporters) to increase extracellular monoamine levels in the NAc, amphetamines function to reverse the transport function of DAT, resulting in increased extracellular levels of DA. The exact mechanism of this reversal remains unclear.80,81 As part of this effect on DAT, amphetamines are transported into the presynaptic terminal via DATs and accumulate within the cytosol.82 An additional mechanism of action for amphetamines is to cause the redistribution of monoamines from synaptic vesicles to the cytosol.83,84 Several mechanisms for this redistribution have been suggested. Two primary hypotheses are that amphetamines act as a substrate at the vesicular monoamine transporters (VMAT2 in neurons) or that they act as a weak base to collapse the vesicular pH gradient (see Rudnick and Clark75 for review). Furthermore, amphetamines prolong monoaminergic transmission by inhibiting monoamine oxidase (MAO) activity and enhancing the activity of the rate-limiting DA synthetic enzyme tyrosine hydroxylase (TH).85–87 While NE and 5-HT levels also increase in the NAc as a result of stimulant use, increased extracellular DA plays a more prominent role in the initial rewarding and reinforcing properties of stimulants.78 Interestingly, AMPH and METH are reported to be more potent releasers of NE than DA, and much less potent at releasing 5-HT.89
Subjective effects and pharmacokinetics
Psychostimulants are associated with euphoria, increased self-confidence as well as increased energy, wakefulness and activity, with associated decreased fatigue and appetite.90–93 Adrenergic (sympathetic nervous system) effects of these drugs include elevated heart and respiration rate, blood pressure, body temperature, and sweating, in addition to increased tremor and exaggeration of reflexes.90,92,94,95 While different classes of illicit psychostimulants exert largely similar subjective effects, they differ in their pharmacokinetic properties. Cocaine has a short half-life of 30 minutes to 2 hours, and the time to peak subjective effects differs by route of administration.96 When acidic cocaine (cocaine hydrochloride) is snorted, peak effects are felt within 10–15 minutes, while injection of acidic cocaine leads to peak effects within 3–4 minutes.97–99 Basic cocaine (“crack”) can be smoked and is considered more addictive, primarily due to the rapid peak effects (1–2 minutes).97,98 METH can also be administered via several routes, including oral, intravenous, and smoked.99 As with cocaine, smoking METH leads to a more rapid “high,” potentially contributing to increased addiction liability of this route of administration.100 METH has a much longer half-life (10–12 hours) compared to cocaine.101 AMPH is primarily used via oral or intravenous routes and also has a longer half-life of 10–14 hours.99,102 Various AMPH salt mixtures, such as D-AMPH, L-AMPH, are also used in medications to treat attention deficit/hyperactivity disorder, and are often described and alternatively categorized as prescription drugs. It is commonly thought that METH is more addictive than AMPH, which is supported by the finding that METH may have a stronger effect on DAT-mediated cell physiology and DA clearance in the NAc, though METH and AMPH do not differ in other pharmacokinetic properties or in the magnitude of DA release in the DS.103,104
Chronic effects of psychostimulants
As previously described, drug addiction is considered a transitional process from casual drug use to habitual and uncontrolled drug use to dependence. Thus, different neurobiological adaptations likely underlie different stages of addiction. Several types of long-term changes can occur following prolonged drug use, including impairments in cognition and behavior, dysregulated functional connectivity within the brain, altered monoamine (primarily DA) receptor and transporter expression, and neurotransmitter synthesis and release. The focus of this section will be to provide a general picture of the long-term effects of chronic illicit psychostimulant use in humans. The majority of clinical research has focused on cocaine and METH users, with the frequent assumption that chronic AMPH use leads to similar outcomes as METH. Due to the variability in duration of drug abstinence in human subjects, the present review focuses on studies that utilized current psychostimulant users, or users in early stages of abstinence. For more detailed information on psychostimulant relapse, the reader is referred to several recently published reviews.105–107 In addition, a couple of caveats relevant to research on the chronic effects of psychostimulants in humans deserve to be mentioned. First, it is important to note that not every individual that experiences the acute effects of psychostimulants will become a chronic user. Individual predisposing factors, such as genetic vulnerabilities, trait impulsivity, socioeconomic status, early life experiences, and stress exposure, all significantly impact the addiction process. Secondly, only longitudinal studies in humans can truly determine whether the neuropsychological, psychiatric, or neuroanatomical differences in chronic psychostimulant users are a result of drug use or predisposing factors.
Neuropsychological effects
Chronic use of psychostimulants results in widespread impairments of neuropsychological functioning, including measures of memory, executive function, and mental flexibility. The primary brain regions mediating these cognitive impairments are the same as those implicated in the neurocircuitry of addiction, namely the PFC, AMG, HPC, and CSTC loops. Chronic cocaine users demonstrated impaired executive functions, including measures of attention, working memory, set-shifting, mental flexibility, and response inhibition.108 In one measure of PFC-mediated executive function, the Wisconsin Card Sorting Task, both METH-dependent subjects and chronic cocaine users demonstrated significant impairments (ie, increased perseverative responding).109–111 In addition, cocaine-dependent subjects exhibit poor performance on a delayed-discounting and reversal-learning task.112 In another study, chronic cocaine users, but not AMPH users, demonstrated increased perseverative responding in a probabilistic reversal-learning task.113
METH-dependent subjects also exhibit impaired global neuropsychological function, specifically in the domains of executive function, learning, motor ability, and speed of information processing.114 These impairments improved significantly following a period of abstinence.114 The same study found no effects of chronic METH use on measures of memory, working memory, or verbal abilities.114 However, other studies found that METH-dependent subjects exhibited impaired visual memory and executive control of verbal learning.115,116 Differences between these studies on the effects of METH on verbal processes and memory may be in part due to differing histories of METH use in METH-dependent subjects across studies.2,3 METH-dependent subjects were also impaired in measures of real-life skills, such as comprehension, finance, transportation, communication, and medication management.109 In addition, METH abusers demonstrated impaired social cognition, as measured by facial emotion-recognition tasks, especially with respect to fear recognition.111 Interestingly, impairments in executive function and social cognition have been found to be positively correlated.4,111,117 In another study, METH-dependent subjects demonstrated slower reaction times and increased error and response-inhibition rates on a “go/no go” task.118 These performance deficits were more severe in the presence of cue-elicited craving.118
Neurological and psychiatric effects
Psychosis is a common effect of chronic psychostimulant abuse. Psychotic symptoms include both hallucinations and delusions. Lifetime use of cocaine, but not AMPH, is associated with the experience of psychotic symptoms after adjusting for demographic covariates, comorbidity, and childhood adversity.119 Severity of psychosis as a result of long-term psychostimulant abuse is increased in those that begin using at younger ages and use for longer periods of time.120 Interestingly, cocaine- and METH-dependent women are more likely than men to experience a variety of psychotic symptoms, including delusions of grandeur and tactile hallucinations.121 Chronic use of psychostimulants is also associated with various other psychiatric disorders, including mood and anxiety disorders. METH users are more likely than cocaine users to have a comorbid psychiatric diagnosis, such as major depression, bipolar disorder, and anxiety disorders.122 A separate study found that METH-dependent subjects reported increased depressive symptoms and acute affective distress compared to control subjects, and that these symptoms improved following a period of abstinence.114
A more unusual effect of chronic psychostimulant use is formication, the sensation of organisms crawling under one’s skin, which has been reported by both chronic METH and cocaine users.94 These “meth mites” or “crank bugs” frequently lead to excessive skin picking, sores, and infections.123,124 Other neurological effects of chronic psychostimulant use include punding (engagement in purposeless and repetitive activities, such as assembling and disassembling electronics), grooming behaviors, and dyskinesias.125–127 With regard to the latter, choreoathetoid movements (involuntary and aimless movements; also known as “crack dancing”) are associated with both AMPH and cocaine abuse.128 Interestingly, many of the neurological and psychiatric effects of chronic psychostimulant use, with the exception of altered mood and psychosis, are symptoms related to altered functioning in the striatal region of the basal ganglia and are likely a result of damage to dopaminergic terminals.
Neurocircuitry: brain imaging
Functional effects
Chronic psychostimulant use is associated with alterations in functional connectivity, white- and gray-matter densities, and activation of distinct brain regions underlying reward and cognitive function. Connectivity and activation studies are conducted with various iterations of magnetic resonance imaging (MRI), while density studies utilize either MRI or diffusion tensor imaging. A greater number of studies have been conducted in cocaine-dependent individuals than AMPH or METH users. In a detailed study using resting-state functional MRI connectivity analysis with seed voxels, cocaine users exhibited decreased connectivity between (1) the VTA and the region encompassing the thalamus/lentiform nucleus/NAc, (2) the AMG and mPFC, and (3) between the HPC and dorsal mPFC.129 These changes in the neurocircuitry of the cocaine-addicted brain are consistent with maladaptive or impaired reward processing, learning, memory, and emotional regulation.129 Interestingly, connectivity between the NAc and cortical regions was not reduced, potentially lending credence to the role of habitual drug use in addiction, which is more tightly regulated by the DS.129 Several studies suggest that cocaine addiction results in altered engagement of frontoparietal networks during attentional tasks.130,131 Recently it was found that cocaine abusers exhibited reduced functional connectivity between the midbrain, where DA neurons are situated, and cortical and subcortical brain regions during an attentional Stroop task that tested the processing of drug and neutral words.132 Reduced activation in the right parietal cortex, a region involved in attention, during the two-back verbal working memory task was also found in cocaine-dependent males.133 In a study investigating the neural underpinnings of drug craving, chronic cocaine use was associated with enhanced reactivity to drug-associated cues, paralleled by enhanced connectivity between the left dorsolateral PFC and occipital cortex compared to healthy control subjects.134 In addition, the OFC was activated significantly more in cocaine users in response to cocaine cues, but not appetitive stimuli.134 It has also been found that chronic cocaine users demonstrate impaired sensorimotor abilities that are accompanied by abnormal activity in the cortical and subcortical brain areas involved in motor control.135
Structural effects
Cocaine users have reduced subcortical gray-matter density which negatively correlates with impaired performance in psychomotor performance, recognition and working memory, and planning.136 Interestingly, in a study that included cocaine- and AMPH-dependent subjects, reduced gray-matter volume in the medial OFC was found, which correlated with increased high-risk decisions in a modified gambling task.137 Likewise, cocaine-dependent subjects demonstrated impaired decision-making on the Iowa gambling task, which correlated with compromised frontal, parietal, and corpus callosum white-matter integrity.138 In addition, abnormalities in white-matter microstructure underlying the PFC and HPC are associated with chronic METH use, during both late and early abstinence.139,140 Chronic METH use is also associated with gray-matter deficits in the cingulate and limbic cortices, reduced hippocampal volume, and white-matter hypertrophy in the temporal regions around the HPC.141 These cortical and hippocampal abnormalities likely contribute to impaired memory performance found in chronic METH users, while white-matter hypertrophy may be related to altered glial changes.141 Additionally, decreased temporal lobe, but not frontal lobe, volume was found in both AMPH- and cocaine-dependent subjects.142
Effects on molecular substrates: DA receptors, DAT, and VMAT
Postmortem studies
Repeated psychostimulant use can alter DA receptor and transporter expression in several brain regions. Most human postmortem studies have focused on DAT expression in cocaine-dependent subjects. The majority of these studies, which used either saturation-binding or autoradiography techniques, demonstrated increased DAT binding in the striatum of cocaine-dependent subjects compared to control subjects (see Table 1 in Rajesh and Diana).143 However, others have found decreased DAT-binding sites in the postmortem PFC from chronic cocaine users compared to non-cocaine-using controls.144 In the postmortem striatum of METH users, decreased TH immunoreactivity and DAT levels were found.145 In a subset of individuals, VMAT2 levels were also significantly reduced, though this were not common.145 Another postmortem study in chronic METH users found decreased levels of DA, TH, and DAT in the striatum, but normal levels of L-3,4-dihydroxyphenylalanine (DOPA) decarboxylase (the enzyme that converts L-DOPA into DA) and VMAT2.146 The authors concluded that chronic METH use does not cause permanent degeneration of striatal DA nerve terminals. Postmortem studies of VMAT2 expression in the striatum of cocaine abusers have had mixed results, with two studies finding decreased VMAT2 and one study finding no change.147–149
Table 1.
Summary of acute and chronic effects of psychostimulants in humans
| Acute effects | Cocaine | METH/AMPH |
|---|---|---|
| Primary site of action | Presynaptic monoaminergic terminals in NAc. | Presynaptic monoaminergic terminals in NAc. |
| Mechanism of action | Inhibition of DA, NE and 5-HT transporters. | Reverse DA, NE, 5-HT transporter function. Redistribution monoamines to cytosol via vMAT2. |
| Half-life | 30 minutes–2 hours. | 10–14 hours. |
| Subjective effects | Euphoria, increased libido and self-confidence. Increased energy, wakefulness and activity. Decreased appetite and fatigue. | |
| Adrenergic effects | Elevated heart rate, blood pressure and rapid breathing. Elevated body temperature and sweating. Tremor and exaggeration of reflexes. | |
|
| ||
| Chronic effects | ||
|
| ||
| Neuropsychological | Impaired executive function, learning, mental flexibility. Impaired motor abilities and reaction time. Impaired information processing and social cognition. | |
| Psychiatric and Neurological | Psychosis, comorbid psychiatric disorders such as depression and anxiety. Formication (skin picking at “METH mites” or “crank bugs”), punding (purposeless and stereotyped behavior) and choreoathetoid (twisting and aimless) movements. | |
| Neurocircuitry | Decreased functional connectivity and activation within the reward circuitry and cortical and subcortical regions mediating executive function, learning and memory. Reduced subcortical gray matter. Reduced integrity and abnormalities in white matter in the HPC, PFC and other cortical regions. | |
| Molecular substrates | Majority find increased striatal DAT. | Decreased striatal DA, DAT and TH levels. |
| Post-mortem | No consensus on striatal VMAT2. | Normal striatal vMAT2 levels. Increased number of microglia in the striatum. |
| Molecular substrates | Decreased striatal VMAT2 availability. | Increased striatal VMAT2, abstinence-dependent. |
| Imaging | Blunting of striatal DA system function. Decreased striatal D2 receptor availability. |
Decreased DAT in the striatum, PFC, and AMG. |
Note: See text for citations, more detailed descriptions, and discrepancies.
Abbreviations: AMG, amygdala; AMPH, D-amphetamine; DA, dopamine; DAT, dopamine transporter; HPC, hippocampus; METH, methamphetamine; NAc, nucleus accumbens; NE, norepinephrine; PFC, prefrontal cortex; VMAT2, vesicular monoamine transporter 2; 5-HT, 5-hydroxytryptamine (serotonin).
Imaging studies
Imaging studies of receptor and transporter expression are typically conducted with positron-emission tomography (PET). Imaging studies, while sacrificing spatial resolution, avoid some of the limitations of postmortem studies, such as antemortem tissue events, varied time since death, and undocumented life-history events. The majority of these types of studies have again focused on the availability of DA receptors and transporters and VMAT2, as well as DA uptake and synthesis. In chronic cocaine abusers, VMAT2 availability was decreased in the NAc (referred to as the ventral striatum) and DS (referred to as the associative and sensorimotor striatum) compared to control subjects.150 This may reflect potential compensatory downregulation in response to loss of DA terminals.150 Stimulant-induced DA release, as measured by displacement of DA receptor-antagonist radiotracers, was decreased in the striatum of cocaine-dependent subjects with 2–6 weeks of abstinence, indicating a blunting of striatal DA system function as a result of chronic cocaine use.151,152 PET studies have also found a significant decrease in striatal DA synthesis, as measured by [18F]6-FDOPA uptake, in cocaine-dependent subjects. However, this was only evident in subjects that had been abstinent for greater than 10 days.153 Decreased striatal D2 receptor availability was also found in both cocaine- and METH-dependent subjects.152,154–156
In METH users, striatal VMAT2 binding was significantly increased with decreasing duration of abstinence (ie, 3 vs 21 days).157 These findings are in opposition to the slight increase in striatal VMAT2 binding found in METH abusers after long-term abstinence (ie, years).158 The disparity between these findings suggests that the time since last drug use is a critical variable that must be accounted for and controlled for in human studies. Most studies in METH-dependent subjects use currently abstinent subjects, but in those that were abstinent for an average of 6 months, decreased DAT was found in the DS (referred to as the caudate and putamen), NAc, PFC, OFC, and AMG.159–161 Unlike with VMAT2, increased duration of abstinence was associated with increased striatal DAT density.162
Note on inflammatory effects
Repeated use of high doses of METH results in neurotoxicity in preclinical models, particularly in the striatum (for review, see Krasnova and Cadet).163 While the mechanism underlying METH-induced neurotoxicity has not been fully elucidated, it is likely due to repeated, high levels of extracellular DA leading to production of quinones and free radicals such as reactive oxygen species.164,165 Studies in humans have investigated the role of inflammatory mediators in METH-induced neurotoxicity, due to the fact that microglial activation has been associated with METH neurotoxicity in animal models.166 Interestingly, the number of microglia but not activation of glial cells in the postmortem striatum of METH users was significantly increased.145 A follow-up study confirmed and extended these results in non-abstinent METH users, finding that METH use results in significantly increased striatal microglia in the absence of reactive gliosis.167 However, in abstinent METH abusers, a PET study found a significant increase in binding of a radiotracer (PK11195) for activated microglia in all brain regions.168 In adults in remission from METH dependence, immune-system dysregulation was found, in addition to significant cognitive impairments, which encompassed learning, memory, and attentional/informational processing.169 In the same study, impaired global cognition was significantly and positively correlated with plasma levels of the cytokine alpha-interferon.169 In addition, a decrease in the anti-inflammatory marker interleukin 10 and increased proinflammatory tumor necrosis factor alpha in response to stress exposure has been found in cocaine abusers (compared to social drinkers), potentially indicating an elevated inflammatory state.170 Interestingly, postmortem brain studies suggest that cocaine addiction is not associated with increased activation of markers of apoptotic cell-death pathways.47,171 Together, these initial studies indicate dysregulated inflammatory processes following chronic psychostimulant use in humans and provide evidence to support the need for further investigations.
Implications for the treatment of substance abuse
At present, no pharmacological therapy has been approved by the FDA to treat psychostimulant addiction. Many drugs have been tested, but none have shown conclusive efficacy with tolerable side effects in humans.172 These drugs have included DA-receptor ligands, such as DA receptor agonists, partial agonists, and antagonists, as well as DA-reuptake inhibitors.173,174 One newer dopaminergic drug that has shown some promise is the antipsychotic medication aripiprazole, a partial agonist at D2-like receptors, which is currently approved for the treatment of schizophrenia, depression, and bipolar disorder.175 Clinical trials have thus far been mixed, with one study finding reduced cocaine craving and use, and another study showing increased cocaine use.176,177
Recent evidence for dysregulation of glutamatergic signaling in addiction has led to the testing of N-acetylcysteine, a derivative of the amino acid cysteine that normalizes extracellular levels of Glu following cocaine administration.178,179 Clinical trials found that N-acetylcysteine treatment attenuates cocaine craving and use and normalizes brain glutamate levels.180–182 Other recent studies found trends toward significant reductions in METH use and craving produced by the antidepressant buproprion and the anticonvulsant topiramate, which has glutamate release-inhibiting properties.183,184 While these compounds have not demonstrated overwhelming efficacy in reducing psychostimulant use or craving in all subjects tested, trends toward effects were observed and thus merit further investigation.
Other compounds that have been tested, with disappointing results, include β-adrenergic antagonists, opioid-receptor antagonists, 5-HT3-receptor antagonists, antidepressants, and anticonvulsants.172,185–190 Despite the lack of past successes, several newer medications are currently being investigated and have shown some initial success, such as the smoking-cessation aid varenicline, the α2 adrenergic agonist clonidine, and the antidepressant mirtazapine.191–194 In addition, preclinical evidence strongly suggests potential therapeutic effects of compounds targeting receptors for endocannabinoids, the neuropeptide orexin, and CRF for the prevention of relapse to psychostimulant use.195–197
Future approaches for the treatment of psychostimulant-induced cognitive deficits and dependence include the use of cognitive enhancers.198–200 Two promising examples include prescription stimulants, such as modafinil and methylphenidate, which have shown modest but potential treatment effects in METH users and cocaine users with comorbid attention deficit/hyperactivity disorder, respectively.201–205 In addition, galantamine, a cholinergic modulator and cognitive enhancer, has been reported to both decrease cocaine use and improve sustained attention in dependent subjects.206,207 Together, these studies indicate that clinical trials of cognitive enhancers in the treatment of psychostimulant addiction are warranted.
In addition to the pharmacological trials reviewed above, less conventional strategies that are gaining scientific momentum include vaccine therapies to immunoneutralize drug molecules and impede penetrance across the blood–brain barrier, enzyme conjugates that dramatically increase the metabolic breakdown of abused drugs, pharmacogenetic approaches based on individual genetic polymorphisms in addiction-related genes, and epigenetic modulators of drug-induced changes in gene expression.208–211 While still in their relative infancy, these exciting new avenues of research offer a significant expansion of possible biologically based targets for the treatment of psychostimulant addiction.
Conclusion
Even with over half a century of research directed at understanding the mechanisms and treatment of psychostimulant addiction, this disorder remains a serious socioeconomic problem. Psychostimulants exert their acute rewarding and reinforcing actions primarily via direct and indirect release of extracellular DA in the NAc. DA release in other brain regions, such as the PFC and AMG, play a role in behavioral control and learned associations between environmental stimuli and the subjective rewarding effects. In addition to DA release, psychostimulants also alter other neurotransmitter systems, such as 5-HT, NE, Glu, endocannabinoids, and various neuropeptides. With continued drug use, other regions, such as the DS and CSTC, may mediate such behaviors as action control and habitual or compulsive drug seeking. Chronic drug use leads to persistent adaptive changes within the reward circuitry, including altered receptor and transporter expression, neurotransmitter release, and functional connectivity between brain regions. Ultimately, these adaptive changes are associated with an impaired cognitive state and neuropsychiatric symptoms. While some of the effects of chronic psychostimulant use may dissipate with increasing abstinence, many chronic users are unable to achieve or maintain even a short period of abstinence.
The tremendous need for more effective pharmacological treatments for psychostimulant addiction is a mainstay of contemporary addiction research. However, the recent downsizing of many major pharmaceutical companies away from psychiatric indications (including addiction) due to the lack of efficacy of experimental compounds in humans may require a sea change in the translational research approach.212,213 A new emphasis on larger-scale biomarker, genetic, and epigenetic research focused on the molecular targets of mental disorders has been recently advocated.212 In addition, the integration of cognitive and behavioral modification of circuit-wide neuroplasticity (ie, computer-based training to enhance executive function) may prove to be an effective adjunct-treatment approach for addiction, particularly when combined with cognitive enhancers.198,213–216 Furthermore, in order to be effective, all pharmacological or biologically based treatments for addiction need to be integrated into other established forms of addiction rehabilitation, such as cognitive behavioral therapy, individual and group psychotherapy, behavior-modification strategies, twelve-step programs, and residential treatment facilities.
Acknowledgments
The authors wish to acknowledge the support of NIH grants DA024355 and DA025606.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: American Psychiatric Association; 2000. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville (MD): SAMHSA; 2011. [Google Scholar]
- 3.Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): legal status and patterns of abuse. Ann Pharmacother. 2012;46(3):436–441. doi: 10.1345/aph.1Q628. [DOI] [PubMed] [Google Scholar]
- 4.Hyman SE. Addiction: A disease of learning and memory. Am J Psychiatry. 2005;162(8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 5.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2007;33(1):166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 6.Li CY, Zhou WZ, Zhang PW, Johnson C, Wei L, Uhl GR. Meta-analysis and genome-wide interpretation of genetic susceptibility to drug addiction. BMC Genomics. 2011;12:508. doi: 10.1186/1471-2164-12-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav. 1980;13(Suppl 1):213–223. doi: 10.1016/s0091-3057(80)80033-5. [DOI] [PubMed] [Google Scholar]
- 9.Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13(5):170–176. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 11.Berridge KC, Robinson TE. The mind of an addicted brain: neural sensitization of wanting versus liking. Curr Dir Psychol Sci. 1995;4(3):71–76. [Google Scholar]
- 12.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 14.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 15.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 16.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 17.McClure SM, Daw ND, Montague PR. A computational substrate for incentive salience. Trends Neurosci. 2003;26(8):423–428. doi: 10.1016/s0166-2236(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 18.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 19.Taber MT, Fibiger HC. Feeding-evoked dopamine release in the nucleus, accumbens: regulation by glutamatergic mechanisms. Neuroscience. 1997;76(4):1105–1112. doi: 10.1016/s0306-4522(96)00450-2. [DOI] [PubMed] [Google Scholar]
- 20.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahler SV, Smith RJ, Aston-Jones G. Interactions between vta orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2012 Mar 13; doi: 10.1007/s00213-012-2681-5. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25(22):5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69(6):375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 25.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sellings LHL, Clarke PBS. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23(15):6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89(3):637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- 28.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25(3):341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 31.Serrano A, Parsons LH. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol Ther. 2011;132(3):215–241. doi: 10.1016/j.pharmthera.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols DE. Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs. 1986;18(4):305–313. doi: 10.1080/02791072.1986.10472362. [DOI] [PubMed] [Google Scholar]
- 33.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 34.Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17(2):287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breiter HC, Gollub RL, Weisskoff RM, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19(3):591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 37.Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320(2):145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 39.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 40.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290(2):213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 41.Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71(6):601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev. 2008;32(3):408–422. doi: 10.1016/j.neubiorev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35(2):248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 46.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 47.Di Chiara G, Bassareo V, Fenu S, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7(4):389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 49.Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22(14):6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22(3):545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarrard LE. What does the hippocampus really do? Behav Brain Res. 1995;71(1–2):1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 52.Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23(1):103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 53.Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23(32):10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47(2):255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28(31):7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333(6040):353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208(1):1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 59.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38(1–2):247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 60.McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 61.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zorrilla EP, Wee S, Zhao Y, et al. Extended access cocaine self-administration differentially activates dorsal raphe and amygdala corticotropin-releasing factor systems in rats. Addict Biol. 2012;17(2):300–308. doi: 10.1111/j.1369-1600.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42(1):1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- 64.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22(9):3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54(1):1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 66.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 67.Kilts CD, Schweitzer JB, Quinn CK, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 68.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119(1):19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 70.French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981(1–2):160–167. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- 71.Wise RA. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotox Res. 2008;14(2–3):169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 73.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 74.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 75.Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144(3):249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 76.Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46(9):635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- 77.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 78.Rocha BA. Stimulant and reinforcing effects of cocaine in monoamine transporter knockout mice. Eur J Pharmacol. 2003;479(1–3):107–115. doi: 10.1016/j.ejphar.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 79.Rocha BA, Fumagalli F, Gainetdinov RR, et al. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1(2):132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- 80.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69(4):628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 82.Zaczek R, Culp S, De Souza EB. Interactions of [3H]amphetamine with rat brain synaptosomes. II. Active transport. J Pharmacol Exp Ther. 1991;257(2):830–835. [PubMed] [Google Scholar]
- 83.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15(5 Pt 2):4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson JB. Stereoselectivity and isoenzyme selectivity of monoamine oxidase inhibitors. Enantiomers of amphetamine, N-methylamphetamine and deprenyl. Biochem Pharmacol. 1985;34(23):4105–4108. doi: 10.1016/0006-2952(85)90201-1. [DOI] [PubMed] [Google Scholar]
- 86.Fung YK, Uretsky NJ. The importance of calcium in the amphetamine-induced stimulation of dopamine synthesis in mouse striata in vivo. J Pharmacol Exp Ther. 1982;223(2):477–482. [PubMed] [Google Scholar]
- 87.Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22(20):8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Espana RA, Jones SR. Presynaptic dopamine modulation by stimulant self-administration. Front Biosci (Schol Ed) 2013;5:261–276. doi: 10.2741/s371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rothman RB, Baumann MH, Dersch CM, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39(1):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 90.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104(7):1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 91.National Institute on Drug Abuse . Cocaine: Abuse and Addiction. Bethesda (MD): NIDA; 2010. [Google Scholar]
- 92.National Institute on Drug Abuse . Methamphetamine Abuse and Addiction. Bethesda (MD): NIDA; 2006. [Google Scholar]
- 93.Hando J, Topp L, Hall W. Amphetamine-related harms and treatment preferences of regular amphetamine users in Sydney, Australia. Drug Alcohol Depend. 1997;46(1–2):105–113. doi: 10.1016/s0376-8716(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 94.Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Neurol Clin. 2011;29(3):641–655. doi: 10.1016/j.ncl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ciccarone D. Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Prim Care. 2011;38(1):41–58. doi: 10.1016/j.pop.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jufer RA, Wstadik A, Walsh SL, Levine BS, Cone EJ. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol. 2000;24(7):467–477. doi: 10.1093/jat/24.7.467. [DOI] [PubMed] [Google Scholar]
- 97.Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? JAMA. 1996;276(19):1580–1588. [PubMed] [Google Scholar]
- 98.Volkow ND, Wang GJ, Fischman MW, et al. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67(12):1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- 99.National Institute on Drug Abuse Prescription drugs. [Accessed October 5, 2012]. Available from: http://www.drugabuse.gov/drugs-abuse/prescription-drugs.
- 100.Kish SJ. Pharmacologic mechanisms of crystal meth. CMAJ. 2008;178(13):1679–1682. doi: 10.1503/cmaj.071675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris DS, Boxenbaum H, Everhart ET, Sequeira G, Mendelson JE, Jones RT. The bioavailability of intranasal and smoked methamphetamine. Clin Pharmacol Ther. 2003;74(5):475–486. doi: 10.1016/j.clpt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 102.Food and Drug Administration Adderall (CII) 2007. [Accessed December 19, 2012]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/011522s040lbl.pdf.
- 103.Goodwin JS, Larson GA, Swant J, et al. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284(5):2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274(1):90–96. [PubMed] [Google Scholar]
- 105.Ross S, Peselow E. The neurobiology of addictive disorders. Clin Neuropharmacol. 2009;32(5):269–276. doi: 10.1097/wnf.0b013e3181a9163c. [DOI] [PubMed] [Google Scholar]
- 106.Gamberino WC, Gold MS. Neurobiology of tobacco smoking and other addictive disorders. Psychiatr Clin North Am. 1999;22(2):301–312. doi: 10.1016/s0193-953x(05)70078-2. [DOI] [PubMed] [Google Scholar]
- 107.Van den Oever MC, Spijker S, Smit AB. The synaptic pathology of drug addiction. Adv Exp Med Biol. 2012;970:469–491. doi: 10.1007/978-3-7091-0932-8_21. [DOI] [PubMed] [Google Scholar]
- 108.Madoz-Gurpide A, Blasco-Fontecilla H, Baca-Garcia E, Ochoa-Mangado E. Executive dysfunction in chronic cocaine users: an exploratory study. Drug Alcohol Depend. 2011;117(1):55–58. doi: 10.1016/j.drugalcdep.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 109.Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hosak L, Preiss M, Bazant J, Tibenska A, Cermakova R, Cermakova E. Comparison of Wisconsin card sorting test results between Czech subjects dependent on methamphetamine versus healthy volunteers. Psychiatr Danub. 2012;24(2):188–193. [PubMed] [Google Scholar]
- 111.Kim YT, Kwon DH, Chang Y. Impairments of facial emotion recognition and theory of mind in methamphetamine abusers. Psychiatry Res. 2011;186(1):80–84. doi: 10.1016/j.psychres.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 112.Camchong J, MacDonald AW, 3rd, Nelson B, et al. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69(11):1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197(3):421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iudicello JE, Woods SP, Vigil O, et al. Longer term improvement in neurocognitive functioning and affective distress among methamphetamine users who achieve stable abstinence. J Clin Exp Neuropsychol. 2010;32(7):704–718. doi: 10.1080/13803390903512637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morgan EE, Woods SP, Poquette AJ, Vigil O, Heaton RK, Grant I. Visual memory in methamphetamine-dependent individuals: deficient strategic control of encoding and retrieval. Aust N Z J Psychiatry. 2012;46(2):141–152. doi: 10.1177/0004867411433212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Woods SP, Rippeth JD, Conover E, et al. Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology. 2005;19(1):35–43. doi: 10.1037/0894-4105.19.1.35. [DOI] [PubMed] [Google Scholar]
- 117.Rolls ET. The representation of information about faces in the temporal and frontal lobes. Neuropsychologia. 2007;45(1):124–143. doi: 10.1016/j.neuropsychologia.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 118.Tolliver BK, Price KL, Baker NL, et al. Impaired cognitive performance in subjects with methamphetamine dependence during exposure to neutral versus methamphetamine-related cues. Am J Drug Alcohol Abuse. 2012;38(3):251–259. doi: 10.3109/00952990.2011.644000. [DOI] [PubMed] [Google Scholar]
- 119.Kuzenko N, Sareen J, Beesdo-Baum K, et al. Associations between use of cocaine, amphetamines, or psychedelics and psychotic symptoms in a community sample. Acta Psychiatr Scand. 2011;123(6):466–474. doi: 10.1111/j.1600-0447.2010.01633.x. [DOI] [PubMed] [Google Scholar]
- 120.Lichlyter B, Purdon S, Tibbo P. Predictors of psychosis severity in individuals with primary stimulant addictions. Addict Behav. 2011;36(1–2):137–139. doi: 10.1016/j.addbeh.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 121.Mahoney JJ, 3rd, Hawkins RY, De La Garza R, 2nd, Kalechstein AD, Newton TF. Relationship between gender and psychotic symptoms in cocaine-dependent and methamphetamine-dependent participants. Gend Med. 2010;7(5):414–421. doi: 10.1016/j.genm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Copeland AL, Sorensen JL. Differences between methamphetamine users and cocaine users in treatment. Drug Alcohol Depend. 2001;62(1):91–95. doi: 10.1016/s0376-8716(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 123.Cohen AL, Shuler C, McAllister S, et al. Methamphetamine use and methicillin-resistant Staphylococcus aureus skin infections. Emerg Infect Dis. 2007;13(11):1707–1713. doi: 10.3201/eid1311.070148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Siegel RK. Cocaine hallucinations. Am J Psychiatry. 1978;135(3):309–314. doi: 10.1176/ajp.135.3.309. [DOI] [PubMed] [Google Scholar]
- 125.Rylander G. Psychoses and the punding and choreiform syndromes in addiction to central stimulant drugs. Psychiatr Neurol Neurochir. 1972;75(3):203–212. [PubMed] [Google Scholar]
- 126.Fasano A, Barra A, Nicosia P, et al. Cocaine addiction: from habits to stereotypical-repetitive behaviors and punding. Drug Alcohol Depend. 2008;96(1–2):178–182. doi: 10.1016/j.drugalcdep.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Rhee KJ, Albertson TE, Douglas JC. Choreoathetoid disorder associated with amphetamine-like drugs. Am J Emerg Med. 1988;6(2):131–133. doi: 10.1016/0735-6757(88)90050-2. [DOI] [PubMed] [Google Scholar]
- 128.Bartzokis G, Beckson M, Wirshing DA, Lu PH, Foster JA, Mintz J. Choreoathetoid movements in cocaine dependence. Biol Psychiatry. 1999;45(12):1630–1635. doi: 10.1016/s0006-3223(98)00238-8. [DOI] [PubMed] [Google Scholar]
- 129.Gu H, Salmeron BJ, Ross TJ, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kübler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21(7):1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- 132.Tomasi D, Volkow ND, Wang R, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5(5):e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bustamante JC, Barrós-Loscertales A, Ventura-Campos N, et al. Right parietal hypoactivation in a cocaine-dependent group during a verbal working memory task. Brain Res. 2011;1375:111–119. doi: 10.1016/j.brainres.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 134.Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115(1–2):137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res. 2010;181(1):15–23. doi: 10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011;218(4):681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tanabe J, Tregellas JR, Dalwani M, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65(2):160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lane SD, Steinberg JL, Ma L, et al. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5(7):e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tobias MC, O’Neill J, Hudkins M, Bartzokis G, Dean AC, London ED. White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology (Berl) 2010;209(1):13–24. doi: 10.1007/s00213-009-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salo R, Nordahl TE, Buonocore MH, et al. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol Psychiatry. 2009;65(2):122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thompson PM, Hayashi KM, Simon SL, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bartzokis G, Beckson M, Lu PH, et al. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98(2):93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 143.Rajesh N, Diana M. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62(11):851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- 144.Hitri A, Casanova MF, Kleinman JE, Wyatt RJ. Fewer dopamine transporter receptors in the prefrontal cortex of cocaine users. Am J Psychiatry. 1994;151(7):1074–1076. doi: 10.1176/ajp.151.7.1074. [DOI] [PubMed] [Google Scholar]
- 145.Kitamura O. Detection of methamphetamine neurotoxicity in forensic autopsy cases. Leg Med (Tokyo) 2009;11(Suppl 1):S63–S65. doi: 10.1016/j.legalmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 146.Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2(6):699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 147.Little KY, Krolewski DM, Zhang L, Cassin BJ. Loss of striatal vesicular monoamine transporter protein (VMAT2) in human cocaine users. Am J Psychiatry. 2003;160(1):47–55. doi: 10.1176/appi.ajp.160.1.47. [DOI] [PubMed] [Google Scholar]
- 148.Wilson JM, Levey AI, Bergeron C, et al. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol. 1996;40(3):428–439. doi: 10.1002/ana.410400312. [DOI] [PubMed] [Google Scholar]
- 149.Staley JK, Talbot JZ, Ciliax BJ, et al. Radioligand binding and immunoautoradiographic evidence for a lack of toxicity to dopaminergic nerve terminals in human cocaine overdose victims. Brain Res. 1997;747(2):219–229. doi: 10.1016/s0006-8993(96)01196-1. [DOI] [PubMed] [Google Scholar]
- 150.Narendran R, Lopresti BJ, Martinez D, et al. In vivo evidence for low striatal vesicular monoamine transporter 2 (VMAT2) availability in cocaine abusers. Am J Psychiatry. 2012;169(1):55–63. doi: 10.1176/appi.ajp.2011.11010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Martinez D, Narendran R, Foltin RW, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 152.Volkow ND, Wang GJ, Fowler JS, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 153.Wu JC, Bell K, Najafi A, et al. Decreasing striatal 6-FDOPA uptake with increasing duration of cocaine withdrawal. Neuropsychopharmacology. 1997;17(6):402–409. doi: 10.1016/S0893-133X(97)00089-4. [DOI] [PubMed] [Google Scholar]
- 154.Martinez D, Broft A, Foltin RW, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29(6):1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- 155.Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine d2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 156.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine d2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 157.Boileau I, Rusjan P, Houle S, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28(39):9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Johanson CE, Frey KA, Lundahl LH, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185(3):327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- 159.Sekine Y, Minabe Y, Ouchi Y, et al. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160(9):1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- 160.Sekine Y, Iyo M, Ouchi Y, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 161.Volkow ND, Chang L, Wang GJ, et al. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001;158(3):383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- 162.Volkow ND, Chang L, Wang GJ, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19(4):1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28(10):1145–1150. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- 166.Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367(3):349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 167.Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, Kubo S. Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg Med (Tokyo) 2010;12(2):57–62. doi: 10.1016/j.legalmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Sekine Y, Ouchi Y, Sugihara G, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28(22):5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20(1):59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fox HC, D’Sa C, Kimmerling A, et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol. 2012;27(2):156–166. doi: 10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Alvaro-Bartolomé M, La Harpe R, Callado LF, Meana JJ, García-Sevilla JA. Molecular adaptations of apoptotic pathways and signaling partners in the cerebral cortex of human cocaine addicts and cocaine-treated rats. Neuroscience. 2011;196:1–15. doi: 10.1016/j.neuroscience.2011.08.074. [DOI] [PubMed] [Google Scholar]
- 172.Kampman KM, Leiderman D, Holmes T, et al. Cocaine Rapid Efficacy Screening Trials (CREST): lessons learned. Addiction. 2005;100(Suppl 1):102–110. doi: 10.1111/j.1360-0443.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 173.Amato L, Minozzi S, Pani PP, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst Rev. 2011;12:CD003352. doi: 10.1002/14651858.CD003352.pub3. [DOI] [PubMed] [Google Scholar]
- 174.Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. Medication discovery for addiction: translating the dopamine d3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882–890. doi: 10.1016/j.bcp.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154(2):261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meini M, Moncini M, Cecconi D, et al. Aripiprazole and ropinirole treatment for cocaine dependence: evidence from a pilot study. Curr Pharm Des. 2011;17(14):1376–1383. doi: 10.2174/138161211796150783. [DOI] [PubMed] [Google Scholar]
- 177.Haney M, Rubin E, Foltin RW. Aripiprazole maintenance increases smoked cocaine self-administration in humans. Psychopharmacology (Berl) 2011;216(3):379–387. doi: 10.1007/s00213-011-2231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Baker DA, Shen H, Kalivas PW. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23(1–3):161–162. doi: 10.1007/s00726-001-0122-6. [DOI] [PubMed] [Google Scholar]
- 179.Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–351. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 180.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of n-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389–394. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 181.LaRowe SD, Myrick H, Hedden S, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164(7):1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 182.Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37(9):2143–2152. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297–1306. doi: 10.1111/j.1360-0443.2011.03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 185.Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1(9):710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- 186.Karila L, Gorelick D, Weinstein A, et al. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11(3):425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 187.Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- 188.Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108(1):94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 189.Pani PP, Trogu E, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst Rev. 2011;12:CD002950. doi: 10.1002/14651858.CD002950.pub3. [DOI] [PubMed] [Google Scholar]
- 190.van den Brink W, van Ree JM. Pharmacological treatments for heroin and cocaine addiction. Eur Neuropsychopharmacol. 2003;13(6):476–487. doi: 10.1016/j.euroneuro.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 191.Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: a pilot study. Am J Addict. 2010;19(5):401–408. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Plebani JG, Lynch KG, Yu Q, Pettinati HM, O’Brien CP, Kampman KM. Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug Alcohol Depend. 2012;121(1–2):163–166. doi: 10.1016/j.drugalcdep.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 2011;218(1):83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168–1175. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gysling K. Relevance of both type-1 and type-2 corticotropin releasing factor receptors in stress-induced relapse to cocaine seeking behaviour. Biochem Pharmacol. 2012;83(1):1–5. doi: 10.1016/j.bcp.2011.07.101. [DOI] [PubMed] [Google Scholar]
- 196.Aston-Jones G, Smith RJ, Sartor GC, et al. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parolaro D, Rubino T. The role of the endogenous cannabinoid system in drug addiction. Drug News Perspect. 2008;21(3):149–157. [PubMed] [Google Scholar]
- 198.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nic Dhonnchadha BA, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: insights from animal models. Pharmacol Biochem Behav. 2011;99(2):229–244. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105(1):38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Castells X, Casas M, Perez-Mana C, Roncero C, Vidal X, Capella D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2010;2:CD007380. doi: 10.1002/14651858.CD007380.pub3. [DOI] [PubMed] [Google Scholar]
- 202.Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am. 2012;35(2):425–439. doi: 10.1016/j.psc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87(1):20–29. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 204.Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1–3):20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shearer J, Darke S, Rodgers C, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104(2):224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 206.Sofuoglu M, Carroll KM. Effects of galantamine on cocaine use in chronic cocaine users. Am J Addict. 2011;20(3):302–303. doi: 10.1111/j.1521-0391.2011.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sofuoglu M, Waters AJ, Poling J, Carroll KM. Galantamine improves sustained attention in chronic cocaine users. Exp Clin Psychopharmacol. 2011;19(1):11–19. doi: 10.1037/a0022213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. Am J Drug Alcohol Abuse. 2009;35(3):161–177. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Narasimhan D, Woods JH, Sunahara RK. Bacterial cocaine esterase: a protein-based therapy for cocaine overdose and addiction. Future Med Chem. 2012;4(2):137–150. doi: 10.4155/fmc.11.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shen X, Kosten TR. Immunotherapy for drug abuse. CNS Neurol Disord Drug Targets. 2011;10(8):876–879. doi: 10.2174/187152711799219352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12(11):623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hyman SE. Revolution stalled. Sci Transl Med. 2012;4(155):155cm111. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- 213.Insel TR. Next-generation treatments for mental disorders. Sci Transl Med. 2012;4(155):155ps19. doi: 10.1126/scitranslmed.3004873. [DOI] [PubMed] [Google Scholar]
- 214.Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73(4):842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Eldar S, Apter A, Lotan D, et al. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am J Psychiatry. 2012;169(2):213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Amir N, Taylor CT. Combining computerized home-based treatments for generalized anxiety disorder: an attention modification program and cognitive behavioral therapy. Behav Ther. 2012;43(3):546–559. doi: 10.1016/j.beth.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]