Abstract
Purpose
The objective of the present study was to conduct a systematic review and meta-analysis of published literature to appraise the prognostic value of lymphovascular invasion (LVI) in radical cystectomy specimens.
Materials and Methods
Following the PRISMA statement, PubMed, Cochrane Library, and SCOPUS database were searched from the respective dates of inception until June 2013.
Results
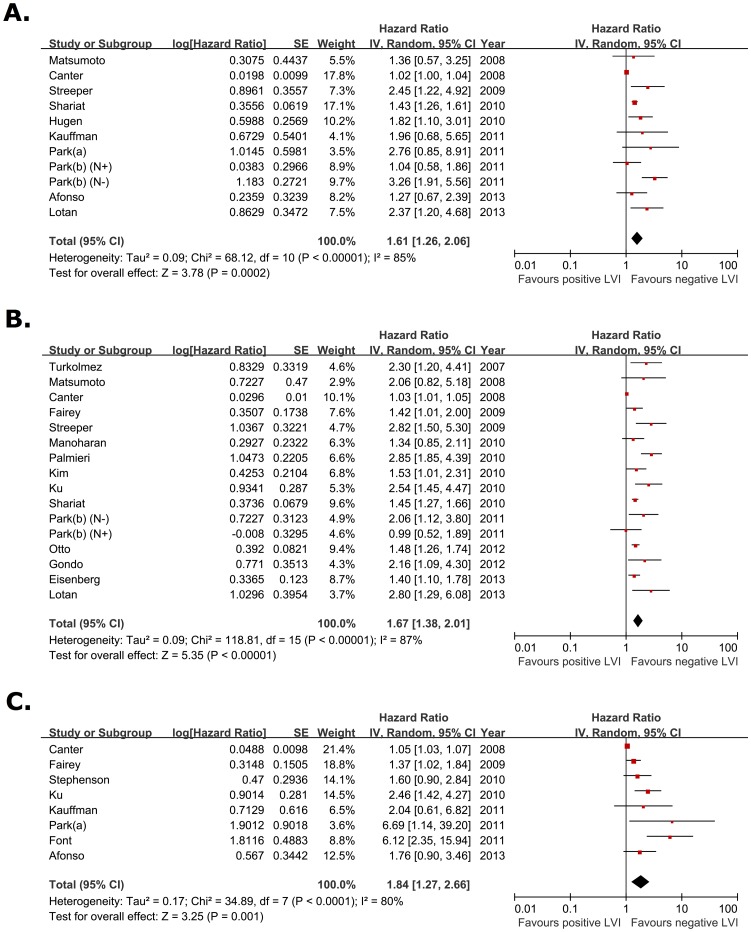
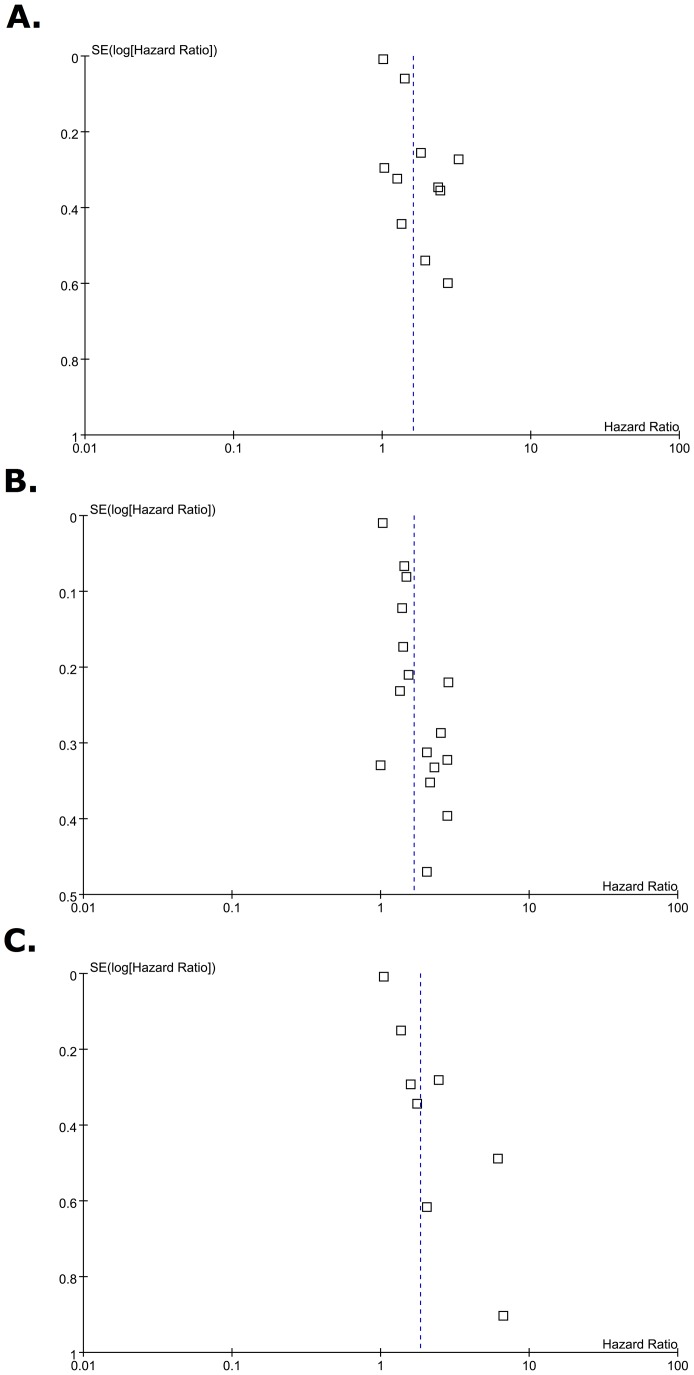
A total of 21 articles met the eligibility criteria for this systematic review, which included a total of 12,527 patients ranging from 57 to 4,257 per study. LVI was detected in 34.6% in radical cystectomy specimens. LVI was associated with higher pathological T stage and tumor grade, as well as lymph node metastasis. The pooled hazard ratio (HR) was statistically significant for recurrence-free survival (pooled HR, 1.61; 95% confidence interval [CI], 1.26–2.06), cancer-specific survival (pooled HR, 1.67; 95% CI, 1.38–2.01), and overall survival (pooled HR, 1.67; 95% CI, 1.38–2.01), despite the heterogeneity among included studies. On sensitivity analysis, the pooled HRs and 95% CIs were not significantly altered when any one study was omitted. The funnel plot for overall survival demonstrated a certain degree of asymmetry, which showed slight publication bias.
Conclusions
This meta-analysis indicates that LVI is significantly associated with poor outcome in patients with bladder cancer who underwent radical cystectomy. Adequately designed prospective studies are required to provide the precise prognostic significance of LVI in bladder cancer.
Introduction
Urothelial carcinoma of the bladder is the fifth most common cancer worldwide, with an estimated incidence of 73,510 cases and 14,880 deaths in the United States for 2012 [1]. While the majority of patients present with non-muscle invasive lesions amenable to local resection, radical cystectomy with pelvic lymphadenectomy continues to represent the gold standard for patients with muscle invasive tumors, as well as for patients with high-risk non-muscle invasive disease. Despite recent multidisciplinary advances in its treatment, bladder cancer continues to carry unacceptably high rates of morbidity and mortality. Thus, the identification of patients at high risk of poor outcome is one of the major concerns for clinicians. New strategies, including the administration of innovative and intensive neoadjuvant/adjuvant therapies, may enable improved survival in these patients.
Lymphovascular invasion (LVI) has been documented as a poor prognostic factor in many solid organ tumors [2], [3]. In a previous study, we have also demonstrated an association between the presence of LVI and poor prognosis in upper urinary tract urothelial carcinoma [4]. The prognostic value of LVI in bladder cancer has been widely investigated, owing to the fact that this feature exhibited an increasing relevance in clinical practice. Although multiple studies have been conducted on bladder cancer patients, the prognostic significance of LVI in radical cystectomy specimens is still controversial. Therefore, we have conducted an up-to-date meta-analysis to appraise the prognostic value of LVI in bladder cancer.
Materials and Methods
Search Strategy
We conducted and reported this systematic review and meta-analysis following the PRISMA statement [5]. A comprehensive literature search of electronic databases PubMed, SCOPUS, and Cochrane Library were performed using the following keywords: [urinary bladder neoplasms] OR [urinary AND bladder AND neoplasms] OR [bladder AND cancer] OR [bladder cancer] AND [lymphovascular] AND [invasion]. The search concluded in June 2013, and no lower date limit was used. Searches were limited to studies published in English. Conference abstracts were not selected for analysis due to the insufficient data reported.
Study Inclusion/Exclusion Criteria
A study was considered eligible if it met all of the following inclusion criteria: (i) the study included proven diagnosis of urothelial carcinoma; (ii) the study evaluated LVI in radical cystectomy specimens; (iii) the study considered radical cystectomy as a treatment modality; (iv) the study assessed the association between LVI and survival of patients with bladder cancer; and, (v) the study provided a hazard ratio (HR) and 95% confidence interval (CI) directly or presented the data that allows for estimation of the HR and 95% CI. Studies were excluded based on any of the following criteria: (i) review articles, letters to the editor, commentaries, or case reports; (ii) laboratory studies, such as studies on bladder cancer cell lines and animal models; and (iii) duplication of previous publications. All studies were carefully examined to avoid inclusion of duplicate data. When more than one of the same patient populations was included in several publications, only the most recent or most complete study was used to avoid duplication of information. Two reviewers (HK and MK) assessed the eligibility of the screened studies independently. Agreements were reached for discrepant opinions through discussion.
Data Extraction
To rule out subjectivity in the data gathering and entry process, data were extracted independently by two reviewers (HK and MK) for each eligible study. The extracted data were recorded by both investigators independently in separate databases. The two completed databases were compared and discussed between the two investigators to reach a consensus. We did not contact authors of eligible studies for additional data. Pre-specified data parameters to be gathered were as follows: (i) publication data including country, first author’s last name, publication year, period of recruitment, study design, inclusion and exclusion criteria, consecutiveness of patient enrollment, definition of LVI, definition of survival, and interpretation of LVI; (ii) demographic data such as sample size, age, gender, treatment, and follow-up period; (iii) tumor data including concomitant carcinoma in situ, variant form, pathological T stage, tumor grade, pathological N stage, and number of lymph nodes retrieved; and (iv) statistical data including the exact data of total and exposed number of subjects in? case and control groups, as well as HRs and their CIs. Multivariate Cox hazard regression analysis data were preferred in our analysis. If this analysis was not available, we extracted univariate Cox hazard regression analysis or Kaplan-Meier survival curves with log-rank p-value of survival outcomes, instead. In studies for which clinical outcomes were estimated using both multivariate and univariate analyses, the results of the multivariate analyses were used to calculate HRs and CIs.
Quality Assessments
Methodological assessment for each of the included studies was performed by three investigators (HK, MK, and JHK), according to three quality scales from the predefined form by De Graeff et al [6], which was adapted from REMARK (Reporting recommendations for tumor MARKer prognostic studies) [7]. The quality scale has seven criteria, and a study with a total score of 8 was considered to have the highest study quality, whereas a score of zero indicated the lowest quality.
Statistical Analysis
We obtained the log-HRs and their 95% CIs from each study and subsequently performed the meta-analysis by a random-effect model. If HRs and the corresponding standard errors were not directly reported, they were estimated according to the available survival data by using the method reported by Palmar et al [8]. An observed HR >1 implied a worse survival for the study group with positive LVI, relative to the reference group. The impact of LVI on outcome was considered statistically significant if the 95% CI did not overlap with 1 and if p<0.05. We also performed subgroup analyses to examine if our pooled estimate of the prognostic value was influenced by publication year, region, number of patients, pathologic N stage, median follow-up, HR estimation, analysis results, and methodological quality scales. To evaluate the robustness of the combined HR and to check the stability of meta-analysis, sensitivity analyses were performed by removing one study at a time. A test of heterogeneity of the combined HRs was carried out using the Chi-square test and Higgins I-squared statistic. P<0.10 was considered to represent substantial heterogeneity between studies. I2>50% indicated large heterogeneity among studies, whereas I2 values between 25% and 50% indicated moderate heterogeneity [9]. Publication bias was evaluated using the funnel plot. The Begg’s rank correlation and Egger’s linear regression were also applied to assess the potential publication bias. The nomnial level of significance was set at 5%. All 95% CIs were two-sided. The meta-analysis was performed using Review Manager (RevMan) software version 5.0 (RevMan 5; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). Publication biases were evaluated by R 2.13.0 (R Development Core Team, Vienna, Austria, http://www.R-project.org).
Results
Study Selection
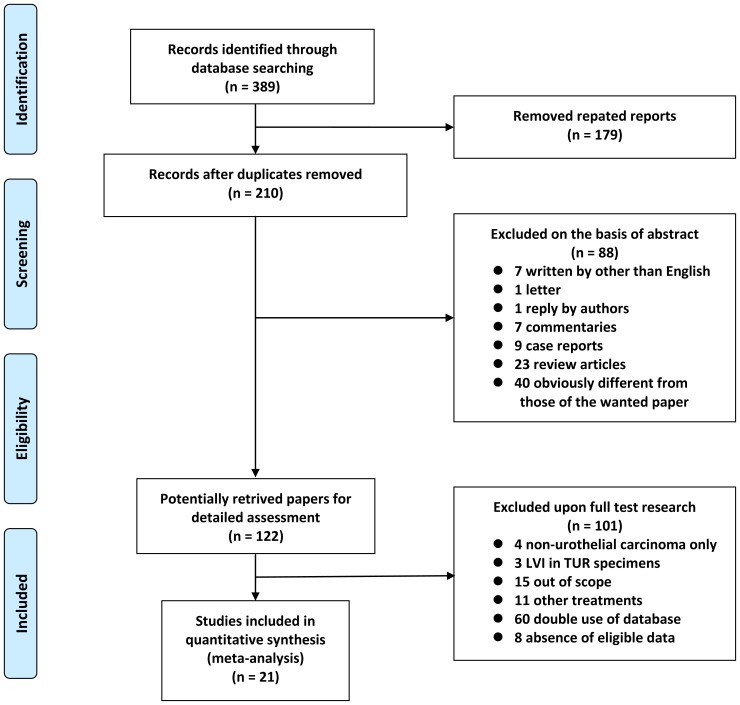
Of the 389 articles initially identified, 179 articles were excluded as duplicate publications. After screening the titles and abstracts, an additional 88 articles were excluded. The remaining 122 articles were reviewed by full text. After the full text review, 15 were excluded because these studies did not perform survival analysis, eight were excluded because these studies did not provide sufficient data for estimation of HRs, three were excluded because LVI was not assessed for radical cystectomy specimens but for transurethral resection specimens, four were excluded because the assessment was conducted on non-urothelial carcinoma, 11 were excluded because study subjects had been treated with modalities other than radical cystectomy, and 60 were excluded for having overlapping data with another study. At the end of this culling process, 21 articles were selected for the systemic review, which included 12,527 patients, ranging from 57 to 4,257 per study [10]–[30]. Figure 1 shows a flow diagram of the selection process for relevant studies.
Figure 1. Flow chart of the literature search used in this meta-analysis.
Methodological Quality of the Studies
The median quality score was 3 for the 21 articles reviewed (mean: 3.6, range: 2–5) (Table 1). Ten of the included studies obtained scores of 4 or more in methodolocal assessment, indicating that they were of high quality. There was no significant correlation between study size and quality scores (Spearman’s r = −0.002, p = 0.992). There were no statistical differences of quality score according to publication year and median follow-up time. However, there was a significant difference in the quality of studies by study origin (3.2 for Asian countries vs. 4.3 for other countries, p = 0.015).
Table 1. Main characteristics of the eligible studies.
| Study | Year | Country | Recruitmentperiod | Study design | Inclusionandexclusioncriteria | Consecutivepatients | Definitionof survival | Definitionof LVI | Interpretationof LVI | Qualityscale |
| Turkolmez [10] | 2007 | Turkey | 1990–2005 | Retrospective | Yes | NA | No | Yes | NA | 3 |
| Canter [11] | 2008 | USA | 1988–2006 | Retrospective | Yes | NA | No | No | NA | 2 |
| Matsumoto [12] | 2008 | Japan | 1990–2004 | Retrospective | Yes | Yes | No | Yes | Blind | 5 |
| Fairey [13] | 2009 | USA | 1994–2007 | Retrospective | Yes | Yes | Yes | No | NA | 3 |
| Streeper [14] | 2009 | USA | 1995–2005 | Retrospective | Yes | Yes | No | Yes | NA | 3 |
| Hugen [15] | 2010 | USA | 1996–2008 | Retrospective | Yes | NA | Yes | No | NA | 2 |
| Kim [16] | 2010 | Korea | 1986–2005 | Retrospective | Yes | NA | No | No | NA | 3 |
| Ku [17] | 2010 | Korea | 1991–2000 | Retrospective | Yes | Yes | Yes | Yes | NA | 4 |
| Manoharan [18] | 2010 | USA | 1992–2008 | Retrospective | Yes | NA | No | Yes | NA | 2 |
| Palmieri [19] | 2010 | Italy | 1995–2007 | Retrospective | Yes | Yes | Yes | Yes | NA | 5 |
| Shariat [20] | 2010 | Multination | 1979–2008 | Retrospective | No | NA | Yes | Yes | Blind | 5 |
| Stephenson [21] | 2010 | USA | 1999–2007 | Retrospective | Yes | Yes | No | No | Blind | 3 |
| Font [22] | 2011 | Spain | 1991–2007 | Retrospective | Yes | NA | No | No | NA | 3 |
| Kauffman [23] | 2011 | USA | 2006–2008 | Prospective | Yes | Yes | No | No | NA | 4 |
| Park(a) [24] | 2011 | Korea | 1999–2009 | Retrospective | Yes | NA | Yes | Yes | NA | 5 |
| Park(b) [25] | 2011 | Korea | 1991–2008 | Retrospective | Yes | Yes | No | No | NA | 3 |
| Gondo [26] | 2012 | Japan | 2000–2009 | Retrospective | Yes | Yes | No | Yes | NA | 4 |
| Otto [27] | 2012 | Germany | 1989–2008 | Retrospective | No | NA | Yes | Yes | NA | 4 |
| Afonso [28] | 2013 | Portugal | 1996–2005 | Retrospective | Yes | NA | Yes | No | NA | 4 |
| Eisenberg [29] | 2013 | USA | 1980–2008 | Retrospective | Yes | NA | Yes | No | Blind | 5 |
| Lotan [30] | 2013 | USA | 2007–2012 | Prospective | No | NA | No | No | NA | 3 |
LVI: lymphovascular invasion, NA: not available.
Study Characteristics
The main features of included studies are listed in Tables 1 and 2. The 21 studies had originated from the United States (9), Europe (5), Asia (6), and multiple countries (1). Two studies were based on a prospective cohort design. Four of these studies included <100 patients, and 11 studies had enrolled >200 patients. The median follow-up durations ranged from 18 months to 10.5 years, while three studies did not provide clear follow-up duration. All of the studies were published between 2007 and 2013. Other characteristics such as tumor characteristics and pathologic results are summarized in Table S1. Of the 12,527 patients included in the meta-alaysis, LVI was detected in 34.6% in radical cystectomy specimens. There were higher frequencies of LVI with higher pathological T stages and tumor grades, as well as lymph node metastasis (Table S2). Of the 35 survival analyses, 33 (94.3%) directly reported HRs or p-values with event number for multivariate analysis. In studies using multivariate analysis, the most common cofactors used to assess the risk of mortality was pathologic T stage (Table S3).
Table 2. Patient characteristics of the eligible studies.
| Study | No. of patients | Median age, range (yr) | Gender (m/f) | Neoadjuvant chemotherapy | Adjuvant chemotherapy | Median FU, range (mon) |
| Turkolmez [10] | 154 | Primary MIBC: 59.8 (mean), NA Secondary MIBC: 60.3 (mean), NA | 134/20 | NA | NA | Primary MIBC: 77.8 (mean), NA Secondary MIBC: 90.3 (mean), NA |
| Canter [11] | 356 | 65.5 (mean), NA | 285/71 | NA | NA | 46.4 (mean), NA |
| Matsumoto [12] | 92 | 63, 40–81 | 75/17 | 0 | 17 | 25.3, 1.1–196.1 |
| Fairey [13] | 468 | 66 (mean), NA | 367/101 | 0 | 82 | NA, NA |
| Streeper [14] | 126 | LVI -: 64.0 (mean), 44–87 | 101/25 | 16 | 41 | LVI -: 1.66 yr, 0.25–10.16 yr |
| LVI+: 64.8 (mean), 35–85 | LVI+: 1.79 yr, 0.04–10.57 yr | |||||
| Hugen [15] | 260 | No recurrence: 64.8 (mean), NA | 193/67 | NA | NA | NA, NA |
| Recurrence: 68.4 (mean), NA | ||||||
| Kim [16] | 406 | 60.8, 27–79 | 360/46 | 0 | 0 | 66.3, 3–232 |
| Ku [17] | 155 | 60.2, 32–84 | 128/27 | 0 | 0 | 34.3, 1.0–162.4 |
| Manoharan [18] | 357 | NA, NA | 285/72 | 0 | NA | NA, NA |
| Palmieri [19] | 265 | 69, 46–93 | 218/47 | 0 | 0 | 108, 1–216 |
| Shariat [20] | 4257 | 67, NA | 3373/864 | 0 | 954 | 43, 0.1–324.0 |
| Stephenson [21] | 134 | 68, 59–75 | 102/32 | 0 | 90 | 23, 10–36 (IQR) |
| Font [22] | 57 | 64, 41–80 | 54/3 | 57 | NA (RT: 5) | 45, 13–190 |
| Kauffman [23] | 85 | 73.5, 41.4–93.8 | 67/18 | 17 | 10* | 18 (mean), NA |
| Park(a) [24] | 155 | 67.8, 38–80 | 127/28 | 0 | 0 | 36.6 (mean), 12–141 |
| Park(b) [25] | 450 | pN-: 63, 38–85 | 408/42 | 0 | 86 | 26.8, 2–204 |
| pN+: 63, 37–80 | ||||||
| Gondo [26] | 194 | 70, 38–85 | 162/32 | 0 | 48 | 26.8, 3.1–131.8 |
| Otto [27] | 2483 | 66.4, 60.1–72.5 (IQR) | 1976/507 | 0 | 245 | 42, 21–79 (IQR) |
| Afonso [28] | 81 | 71, 41–83 | 66/15 | 0 | 0 | 24, 1–132 |
| Eisenberg [29] | 1776 | 68, 62–75 (IQR) | 1464/312 | 0 | 131 (RT: 17) | 10.5 yr, 7.3–15.3 yr (IQR) |
| Lotan [30] | 216 | 70, 62–76 | 171/15 | 48 | 29 | 20, 10–37 (IQR) |
*chemotherapy or radiation therapy.
FU: follow-up, MIBC: muscle-invasive bladder cancer, NA: not available, LVI: lymphovascular invasion, IQR: interquartile range, RT: radiation therapy.
Meta-analysis
According to a priori assumptions about the likelihood for heterogeneity between primary studies, the pooled HR estimate of the each study was calculated by the random effect model. Figure 2 demonstrates a forest plot of the individual HRs and results from the meta-analysis. When 10 eligible studies (11 dataset) were pooled into the meta-analysis for recurrence-free survival (RFS), we found that LVI was significantly associated with worse RFS (pooled HR, 1.61; 95% CI, 1.26–2.06; Z = 3.78). Cochrane Q test (Chi2 = 68.12; p<0.000001) and test of inconsistency (I2 = 85%) could not exclude a significant heterogeneity (Fig. 2a). The meta-analysis was performed on 15 studies (16 dataset) assessing the association of LVI and cancer-specific survival (CSS). The pooled HR was 1.67 (95% CI, 1.38–2.01; Z = 5.35) despite the heterogeneity among studies (p<0.00001 for heterogeneity test; I2 = 87%) (Fig. 2b). Eight studies provided data on overall survival (OS), and meta-analysis of OS suggested that LVI correlated with poor OS (pooled HR, 1.84; 95% CI, 1.27–2.66; Z = 3.25) with a large heterogeneity in the data (p<0.00001 for heterogeneity test; I2 = 80%) (Fig. 2c).
Figure 2. Forest plots of prognosis of lymphovascular invasion.
The horizontal lines correspond to the study-specific hazard ration and 95% confidence interval, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of hazard ratio and 95% confidence interval. (A) Recurrence-free survival. (B) Cancer-specific survival. (C) Overall survival.
Subgroup Analysis
Considering the substantial heterogeneity among the studies, we performed subgroup analyses to investigate if there were differences in results by publication year, the country of origin in which the study was conducted, number of patients, pathologic N stage, median follow-up, HR estimation, analysis results, and methodological quality scales (Table S4 to S6). The pooled HR for almost all subgroup analyses again supported LVI as a prognostic marker. However, the association between LVI and worse RFS did not remain statistically significant in studies of Asian subjects (pooled HR, 1.85; 95% CI, 0.98–3.51; Z = 1.89; p = 0.03 for heterogeneity test; I2 = 67). Notably, many of these subgroup analyses revealed heterogeneities of data.
Sensitivity Analysis
We performed one-way sensitivity analyses by stepwise excluding a single study and calculating again the pooled HR for remaining studies. The pooled HRs and 95% CIs were not significantly altered when any one of the 21 studies was omitted, which indicated that no single study had a significant impact on the combined risk estimates and confirmed the robustness of the result of this meta-analysis. Omitting a certain study did not reduce inter-study heterogeneity significantly in the sensitivity analysis.
Publication Bias
Begg’s funnel plot was used to examine publication bias (Fig. 3). No significant publication bias was found in the meta-analysis of survival outcome except for the association between LVI and OS. The funnel plot for OS demonstrated a certain degree of asymmetry, which suggested a slight publication bias. Begg’s test indicated no publication bias among these studies regarding HR of OS, CSS and OS with p values of 0.103, 0.6915 and 0.1021, respectively, but Egger’s test demonstrated a publication bias (all P<0.05). These results indicated a possibility that publication bias may have played a role in the observed effect.
Figure 3. Begg’s Funnel plots for publication bias test.
Each point represents a separate study for the indicated association. Vertical line represents the mean effects size. (A) Recurrence-free survival. (B) Cancer-specific survival. (C) Overall survival.
Discussion
Despite remarkable advances in treatment, the prognosis of bladder cancer remains unsatisafactory at the present time. Identification of the risk of disease recurrence and mortality in bladder cancer is critical to guide surveillance and select adjuvant therapies. Many studies have investigated potential prognostic factors for patients with bladder cancer, in order to guide therapeutic approaches and improve survival outcomes. LVI has been found in association with lymph node invasion, distant metastasis, and poor prognosis in patients with other sold tumors [31], [32]. Numerous studies have been performed to assess the prognostic value of LVI, but the results are still controversial and ambiguous in the management of bladder cancer.
To our knowledge, this meta-analysis is the first study to systemically assess the association between LVI and prognosis of bladder cancer. This study aggregated the outcomes of 12,527 bladder cancer patients who underwent radical cystectomy, as they were reported in 21 individual studies. We found that LVI was present in 34.6% of patients treated with radical cystectomy for bladder cancer. The significant associations were found between LVI and pathological parameters such as pathologic T stage, tumor grade, and pathologic N stage. Pooled analysis of the included studies found a significant correlation between LVI and poor survival outcome, suggesting that LVI is a significant predictor for poor survival in these patients. Sensitivity analysis demonstrated that omission of any single study did not have a significant impact on the combined risk estimates, further confirming the prognostic value of LVI in bladder cancer. Although subgroup analyses also demonstrated similar results, LVI was not significantly correlated with poor RFS for patients living in Asian countries. The characteristics of bladder cancer in different regions might differ because of diverse environmental and genetic factors, As such, the prognostic value of LVI in bladder cancer might differ across study locations. More studies with larger sample sizes in Asian countries are thus needed to further elucidate the prognostic value of LVI.
In addition to lymphatic metastasis, LVI is most likely associated with hematogenous tumor dissemination. Infiltration of the vascular and/or lymphatic structures by tumor cells is an important step in tumor dissemination [33]–[37]. Malignant cells invade the lymphovascular space, proliferate, and then permeate the local lymphatics or spread more widely [38]. This association is not limited to bladder cancer, and has also been shown in other cancers [39]–[41]. In addition, LVI is an important prognostic factor in various malignancies such as liver, testis, and penile cancer. In other malignancies [42], [43], LVI has been added the TNM staging system, allowing for improved cancer staging and treatment decision-making. Despite the increasing numbers of published studies that have added to the general knowledge about the prognostic role of LVI in bladder cancer, LVI is not a part of the TNM staging system or treatment guidelines for bladder cancer. Upstaging tumors on the basis of LVI might improve the accuracy of prognosis in bladder cancer, and therefore is a worthy consideration.
Several limitations of this meta-analysis should be acknowledged. One weakness of our study was publication bias, which could be seen from the publication bias evaluation of OS outcomes; the reported HR might be an overestimation of the true effect size. Because studies with negative results are less likely to be published than those representing positive data, and even if these results are published, they are more frequently reported in a brief way and not easily available for analysis, meta-analyses of selective reports may often introduce bias. It should be also noted that we could not exclude the bias associated with reviewing articles written in only the English language. Studies with positive results are more frequently published in English language, while studies with negative results tend to be published in the native language of respective authors [44].
The second limitation was heterogeneity. In sensitivity analysis, omission of any individual study did not reduce the heterogeneity. Our meta-analysis relied on publication but not on individual patient data. Studies have differed in baseline characteristics of patients. Though the random effect model takes heterogeneity into account and was used to analyze the studies with heterogeneities, the conclusion drawn in this meta-analysis should be approached with caution.
Moreover, we admit that meta-analysis of prognostic literature is associated with a number of inherent limitations. One of these key limitations is the general prevalence of retrospective study design in this setting. Only two studies included in the current meta-analysis specified a prospective design, with the remaining studies providing a lower level of evidence. There is a clear need for the initiation of a prospective multicenter trial to provide more definite answers.
In addition to these study limitation, significant differences in the assessment of prognostic factors have been observed among pathologists [45]. Because retraction artifacts in the surrounding stromal tissue can mimic vascular invasion, experts have recommended reporting LVI only in unequivocal cases, using immunohistochemistry if necessary [46]. However, the use of immunohistochemical staining to identify lymphatic vessels remains controversial and is not practical for everyday clinical use [47], [48]. It is of utmost importance that strict morphological criteria are established to standardize and render the diagnosis of LVI reproducible, allowing its recommendation in daily clinical settings [33]. In most studies on bladder cancer outcomes, vascular invasion and lymphatic invasion were combined as LVI. One of the reasons for this is that an unequivocal distinction between vascular invasion and lymphatic invasion is often difficult to make without the use of special stains, and that the clinical value of distinguishing vascular invasion from lymphatic invasion to predict bladder cancer outcomes has not been fully investigated. The development of novel markers and further studies are required to examine the significance of the distinction between blood and lymphatic vessels [26].
Conclusions
This meta-analysis indicates that LVI is significantly associated with poorer outcomes in patients with bladder cancer who underwent radical cystectomy. LVI in radical cystectomy specimens not only predicts prognosis, but may also be useful in identifying a subgroup of patients who could benefit from adjuvant therapy. Strict criteria to unify the reproducibility of diagnosis as well as adequately designed prospective studies are required to provide a precise prognostic significance of LVI in bladder cancer.
Supporting Information
Tumor characteristics of the eligible studies.
(DOC)
Lymphovascular invasion according to pathological features.
(DOC)
Estimation of hazard ratio.
(DOC)
Subgroup analysis for recurrence-free survival.
(DOC)
Subgroup analysis for cancer-specific survival.
(DOC)
Subgroup analysis for overall survival.
(DOC)
PRISMA Checklist, page one.
(TIF)
PRISMA Checklist, page two.
(TIF)
Funding Statement
The authors have no support or funding to report.
References
- 1. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Gasparini G, Weidner N, Bevilacqua P, Maluta S, Dalla Palma P, et al. (1994) Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node-negative breast carcinoma. J Clin Oncol 12: 454–466. [DOI] [PubMed] [Google Scholar]
- 3. Herman CM, Wilcox GE, Kattan MW, Scardino PT, Wheeler TM (2000) Lymphovascular invasion as a predictor of disease progression in prostate cancer. Am J Surg Pathol 24: 859–863. [DOI] [PubMed] [Google Scholar]
- 4. Ku JH, Byun SS, Jeong H, Kwak C, Kim HH, et al. (2013) Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: A systematic review and meta-analysis. Eur J Cancer49: 2665–2680. [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J (2009) Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Graeff P, Crijns AP, de Jong S, Boezen M, Post WJ, et al. (2009) Modest effect of p53, EGFR and HER-2/neu on prognosis in epithelial ovarian cancer: a meta-analysis. Br J Cancer 101: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, et al. (2005) Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Türkölmez K, Tokgöz H, Reşorlu B, Köse K, Bedük Y (2007) Muscle-invasive bladder cancer: predictive factors and prognostic difference between primary and progressive tumors. Urology 70: 477–81. [DOI] [PubMed] [Google Scholar]
- 11. Canter D, Guzzo T, Resnick M, Magerfleisch L, Sonnad S, et al. (2008) The presence of lymphovascular invasion in radical cystectomy specimens from patients with urothelial carcinoma portends a poor clinical prognosis. BJU Int 102: 952–957. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto K, Satoh T, Irie A, Ishii J, Kuwao S, et al. (2008) Loss expression of uroplakin III is associated with clinicopathologic features of aggressive bladder cancer. Urology 72: 444–9. [DOI] [PubMed] [Google Scholar]
- 13. Fairey AS, Jacobsen NE, Chetner MP, Mador DR, Metcalfe JB, et al. (2009) Associations between comorbidity, and overall survival and bladder cancer specific survival after radical cystectomy: results from the Alberta Urology Institute Radical Cystectomy database. J Urol 182: 85–92. [DOI] [PubMed] [Google Scholar]
- 14. Streeper NM, Simons CM, Konety BR, Muirhead DM, Williams RD, et al. (2009) The significance of lymphovascular invasion in transurethral resection of bladder tumour and cystectomy specimens on the survival of patients with urothelial bladder cancer. BJU Int 103: 475–479. [DOI] [PubMed] [Google Scholar]
- 15. Hugen CM, Polcari AJ, Fitzgerald MP, Dauw C, Flanigan RC, et al. (2010) Risk factors for recurrence following radical cystectomy for pathologic node negative bladder cancer. J Surg Oncol 102: 334–337. [DOI] [PubMed] [Google Scholar]
- 16. Kim DS, Cho KS, Lee YH, Cho NH, Oh YT, et al. (2010) High-grade hydronephrosis predicts poor outcomes after radical cystectomy in patients with bladder cancer. J Korean Med Sci 25: 369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ku JH, Moon KC, Kwak C, Kim HH (2010) Influence of stage discrepancy on outcome in patients treated with radical cystectomy. Tumori 96: 699–703. [DOI] [PubMed] [Google Scholar]
- 18. Manoharan M, Katkoori D, Kishore TA, Jorda M, Luongo T, et al. (2010) Lymphovascular invasion in radical cystectomy specimen: is it an independent prognostic factor in patients without lymph node metastases? World J Urol 28: 233–237. [DOI] [PubMed] [Google Scholar]
- 19. Palmieri F, Brunocilla E, Bertaccini A, Guidi M, Pernetti R, et al. (2010) Prognostic value of lymphovascular invasion in bladder cancer in patients treated with radical cystectomy. Anticancer Res 30: 2973–2976. [PubMed] [Google Scholar]
- 20. Shariat SF, Svatek RS, Tilki D, Skinner E, Karakiewicz PI, et al. (2010) International validation of the prognostic value of lymphovascular invasion in patients treated with radical cystectomy. BJU Int 105: 1402–1412. [DOI] [PubMed] [Google Scholar]
- 21. Stephenson AJ, Gong MC, Campbell SC, Fergany AF, Hansel DE (2010) Aggregate lymph node metastasis diameter and survival after radical cystectomy for invasive bladder cancer. Urology 75: 382–386. [DOI] [PubMed] [Google Scholar]
- 22. Font A, Taron M, Gago JL, Costa C, Sánchez JJ, et al. (2011) BRCA1 mRNA expression and outcome to neoadjuvant cisplatin-based chemotherapy in bladder cancer. Ann Oncol 22: 139–144. [DOI] [PubMed] [Google Scholar]
- 23. Kauffman EC, Ng CK, Lee MM, Otto BJ, Wang GJ, et al. (2011) Early oncological outcomes for bladder urothelial carcinoma patients treated with robotic-assisted radical cystectomy. BJU Int 107: 628–635. [DOI] [PubMed] [Google Scholar]
- 24. Park E, Ha HK, Chung MK (2011) Prediction of prognosis after radical cystectomy for pathologic node-negative bladder cancer. Int Urol Nephrol 43: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 25. Park J, Kim S, Jeong IG, Song C, Hong JH, et al. (2011) Does the greater number of lymph nodes removed during standard lymph node dissection predict better patient survival following radical cystectomy? World J Urol 29: 443–449. [DOI] [PubMed] [Google Scholar]
- 26. Gondo T, Nakashima J, Ozu C, Ohno Y, Horiguchi Y, et al. (2012) Risk stratification of survival by lymphovascular invasion, pathological stage, and surgical margin in patients with bladder cancer treated with radical cystectomy. Int J Clin Oncol 17: 456–461. [DOI] [PubMed] [Google Scholar]
- 27. Otto W, May M, Fritsche HM, Dragun D, Aziz A, et al. (2012) Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend Med 9: 481–489. [DOI] [PubMed] [Google Scholar]
- 28. Afonso J, Longatto-Filho A, Martinho O, Lobo F, Amaro T, et al. (2013) Low RKIP expression associates with poor prognosis in bladder cancer patients. Virchows Arch 462: 445–453. [DOI] [PubMed] [Google Scholar]
- 29. Eisenberg MS, Boorjian SA, Cheville JC, Thompson RH, Thapa P, et al. (2013) The SPARC (Survival Prediction After Radical Cystectomy) Score: A Multifactorial Outcome Prediction Model for Patients Undergoing Radical Cystectomy for Bladder Cancer. J Urol 190(6): 2005–10 doi:10.1016/j.juro.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 30. Lotan Y, Bagrodia A, Passoni N, Rachakonda V, Kapur P, et al. (2013) Prospective Evaluation of a Molecular Marker Panel for Prediction of Recurrence and Cancer-specific Survival After Radical Cystectomy. Eur Urol 64(3): 465–71 doi:10.1016/j.eururo.2013.03.043 [DOI] [PubMed] [Google Scholar]
- 31. Dicken BJ, Saunders LD, Jhangri GS, de Gara C, Cass C, et al. (2004) Gastric cancer: establishing predictors of biologic behavior with use of population-based data. Ann Surg Oncol 11: 629–635. [DOI] [PubMed] [Google Scholar]
- 32. Woo CS, Silberman H, Nakamura SK, Ye W, Sposto R, et al. (2002) Lymph node status combined with lymphovascular invasion creates a more powerful tool for predicting outcome in patients with invasive breast cancer. Am J Surg 184: 337–340. [DOI] [PubMed] [Google Scholar]
- 33. Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, et al. (2002) Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296: 1883–1886. [DOI] [PubMed] [Google Scholar]
- 34. Alitalo K, Mohla S, Ruoslahti E (2004) Lymphangiogenesis and cancer: meeting report. Cancer Res 64: 9225–9229. [DOI] [PubMed] [Google Scholar]
- 35. Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, et al. (2009) Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol 27: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kikuchi E, Horiguchi Y, Nakashima J, Hatakeyama N, Matsumoto M, et al. (2005) Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol 174: 2120–2123. [DOI] [PubMed] [Google Scholar]
- 37. Akao J, Matsuyama H, Yamamoto Y, Hara T, Kawai Y, et al. (2008) Clinical significance of lymphovascular invasion in upper urinary tract urothelial cancer. BJU Int 102: 572–575. [DOI] [PubMed] [Google Scholar]
- 38. Alexander-Sefre F, Singh N, Ayhan A, Salveson HB, Wilbanks G, et al. (2003) Detection of tumour lymphovascular space invasion using dual cytokeratin and CD31 immunohistochemistry. J Clin Pathol 56: 786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Capdet J, Martel P, Charitansky H, Lim YK, Ferron G, et al. (2009) Factors predicting the sentinel node metastases in T1 breast cancer tumor: an analysis of 1416 cases. Eur J Surg Oncol 35: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 40. Lee KB, Ki KD, Lee JM, Lee JK, Kim JW, et al. (2009) The risk of lymph node metastasis based on myometrial invasion and tumor grade in endometrioid uterine cancers: a multicenter, retrospective Korean study. Ann Surg Oncol 16: 2882–2887. [DOI] [PubMed] [Google Scholar]
- 41. Meier I, Merkel S, Papadopoulos T, Sauer R, Hohenberger W, et al. (2008) Adenocarcinoma of the esophagogastric junction: the pattern of metastatic lymph node dissemination as a rationale for elective lymphatic target volume definition. Int J Radiat Oncol Biol Phys 70: 1408–1417. [DOI] [PubMed] [Google Scholar]
- 42. Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, et al. (2002) Simplified staging for hepatocellular carcinoma. J Clin Oncol 20: 1527–1536. [DOI] [PubMed] [Google Scholar]
- 43. Albers P, Siener R, Kliesch S, Weissbach L, Krege S, et al. (2003) German Testicular Cancer Study Group. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol 21: 1505–1512. [DOI] [PubMed] [Google Scholar]
- 44. Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, et al. (1997) Language bias in randomised controlled trials published in English and German. Lancet 350: 326–9. [DOI] [PubMed] [Google Scholar]
- 45. Margulis V, Lotan Y, Montorsi F, Shariat SF (2008) Predicting survival after radical cystectomy for bladder cancer. BJU Int 102: 15–22. [DOI] [PubMed] [Google Scholar]
- 46. Chromecki TF, Bensalah K, Remzi M, Verhoest G, Cha EK, et al. (2011) Prognostic factors for upper urinary tract urothelial carcinoma. Nat Rev Urol 8: 440–447. [DOI] [PubMed] [Google Scholar]
- 47. Miyata Y, Kanda S, Ohba K, Nomata K, Eguchi J, et al. (2006) Tumor lymphangiogenesis in transitional cell carcinoma of the upper urinary tract: association with clinicopathological features and prognosis. J Urol 176: 348–353. [DOI] [PubMed] [Google Scholar]
- 48. Straume O, Jackson DG, Akslen LA (2003) Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res 9: 250–256. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tumor characteristics of the eligible studies.
(DOC)
Lymphovascular invasion according to pathological features.
(DOC)
Estimation of hazard ratio.
(DOC)
Subgroup analysis for recurrence-free survival.
(DOC)
Subgroup analysis for cancer-specific survival.
(DOC)
Subgroup analysis for overall survival.
(DOC)
PRISMA Checklist, page one.
(TIF)
PRISMA Checklist, page two.
(TIF)