Abstract
Mesothelioma is diagnosed in approximately 2,500 patients in the United States every year, most often arising in the pleural space, but also occurring as primary peritoneal mesothelioma. The vast majority of patients with mesothelioma die from their disease within 3 years. We developed a new mouse model of mesothelioma by bladder or intra-peritoneal injection of adenovirus Cre into mice with conditional alleles of each of Tp53 and Tsc1. Such mice began to develop malignant ascites about 6 months after injection, which was due to peritoneal mesothelioma, based on tumor morphology and immunohistochemical staining. Mesothelioma cell lines were established which showed loss of both Tsc1 and Tp53, with mTORC1 activation. Treatment of mice with malignant ascites due to mesothelioma with rapamycin led to a marked reduction in ascites, extended survival, and a 95–99% reduction in mesothelioma tumor volume, in comparison to vehicle-treated mice. To see if TSC1/TSC2 loss was a common genetic event in human mesothelioma, we examined 9 human mesothelioma cell lines, and found that 4 of 9 showed persistent activation of mTORC1 though none had loss of TSC1 or TSC2. A tissue microarray analysis of 198 human mesothelioma specimens showed that 33% of cases had reduced TSC2 expression and 60% showed activation of mTOR, indicating that mTOR activation is common in human mesothelioma and suggesting that it is a potential therapeutic target.
Keywords: TSC1, TSC2, mesothelioma, rapamycin, mTOR
Introduction
Mesothelioma is estimated to occur in about 2,500 people in the United States every year, most often as malignant pleural mesothelioma (MPM), but also arising in the peritoneum, pericardium, and tunica vaginalis testis.1,2 MPM presents mainly in men over 60 years of age, and is strongly associated with asbestos exposure. Surgery is associated with improved survival in selected early stage patients with MPM. However, the vast majority of patients with mesothelioma experience progressive disease, and median overall survival of patients with MPM is about 1 year. Thus, there is an urgent clinical need for more effective therapy. The first-line regimen of pemetrexed-cisplatin is considered standard treatment for fit patients, and was reported to increase survival by ~3 months, from 9.3 to 12.1 months, in comparison to cisplatin alone in a randomized trial.3 However, relapses are invariable, and second-line therapies are generally of limited benefit, although multiple novel therapeutic approaches are being pursued including PI3K inhibitors.
Tuberous sclerosis (TSC) is an autosomal dominant tumor suppressor gene syndrome, due to mutations in either TSC1 encoding the protein harmartin, or TSC2 encoding the protein tuberin.4 Although classic malignancy is not a feature of this disease, there are several progressive tumor conditions that occur in TSC, and can be fatal, including cardiac rhabdomyoma, cerebral giant cell astrocytomas, renal angiomyolipomas, and pulmonary lymphangioleiomyomatosis. In addition, TSC1 or TSC2 mutations have recently been identified at significant frequency in pancreatic neuroendocrine tumors,5 bladder cancer,6 PEComa,7,8 and are seen rarely in a variety of malignancies analyzed in the TCGA, e.g. squamous cell lung cancer9. Both TSC-associated and TSC-independent cancers with mutation in TSC1 or TSC2 have shown dramatic responses to everolimus treatment in some cases.8,10,11
We have recently discovered that concurrent loss of Tsc1 and Tp53 from the abdominal peritoneum in mice leads to consistent development of peritoneal mesothelioma, which is dramatically responsive to treatment with rapamycin. We then analyzed a set of mesothelioma cell lines and a mesothelioma TMA to find that although there were no cell lines with loss of TSC1 or TSC2, a sizeable fraction of mesothelioma samples had reduced TSC2 expression with concordant activation of mTORC1, similar to previous studies.12,13
These observations suggest that mTOR inhibitors may have therapeutic benefit in mesothelioma.
Results
Loss of Tsc1 and Tp53 in mice by bladder injection leads to peritoneal mesothelioma
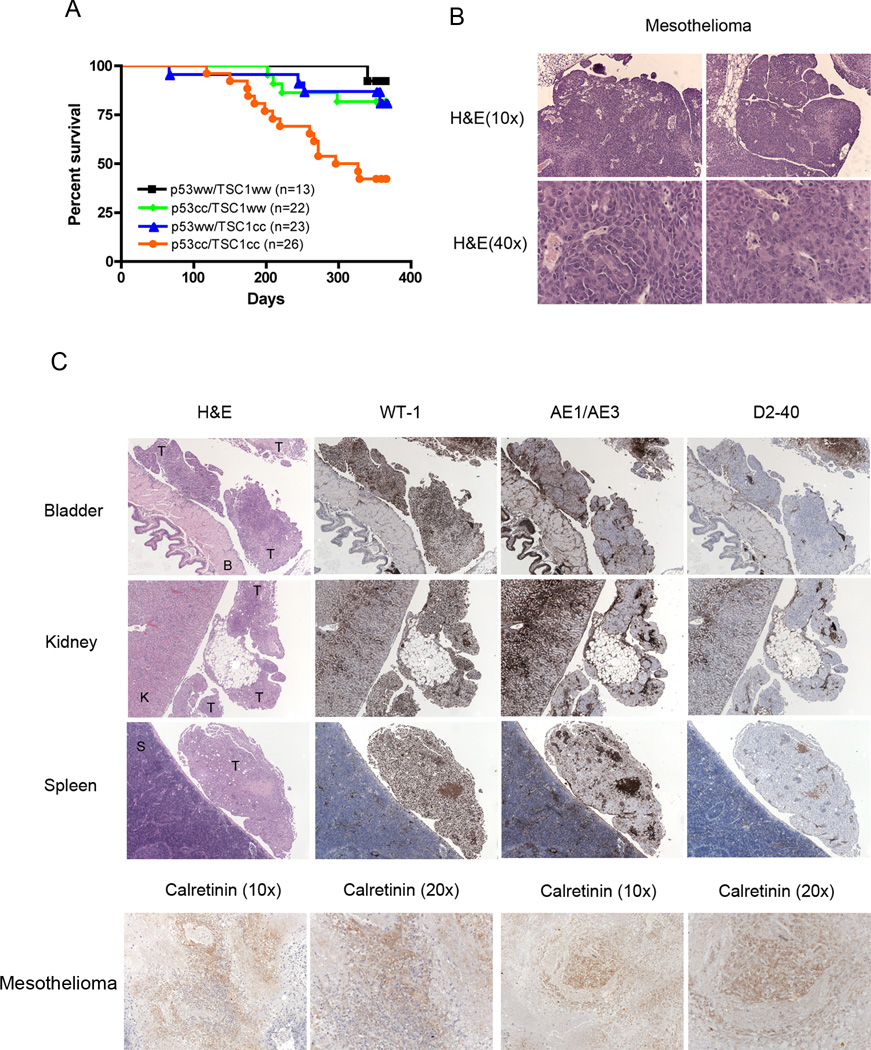
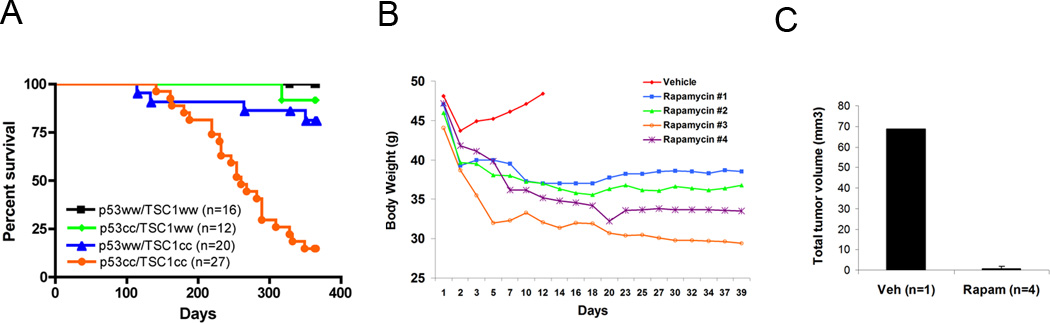
In our initial experiments, we sought to induce a Tsc1-mutant bladder cancer model in mice, since TSC1 is known to be mutated in about 10% of bladder cancers, by injection of Adenovirus Cre into the bladders of mice that had various combinations of homozygous conditional alleles of each of Tsc1 and Tp53: wild type, Tsc1cc, Tp53cc, Tsc1ccTp53cc. Following injection of adenovirus Cre (2×109 pfu) at 2–3 months of age, Tsc1ccTp53cc mice did well for several months, but then started to die at 6 months after AdCre injection with a median survival of 10.2 months (Figure 1A). Although some of these mice died suddenly, others were noted to have abdominal swelling with intraperitoneal fluid (ascites) for 1–2 weeks prior to death or humane euthanasia. Many fewer mice of the other three genotypes displayed early mortality (p=0.0003), and none developed ascites prior to sudden death (Figure 1A and Table 1).
Figure 1. Survival and mesothelioma development in Tsc1ccTp53cc mice in response to bladder injection of adenovirus Cre.
A. Survival curve of mice of various genotypes following bladder injection of adenovirus Cre. Note markedly reduced survival of Tsc1ccTp53cc mice in comparison to other genotypes (p=0.0003).
B. Histology of mesotheliomas generated in Tsc1ccTp53cc mice.
C. IHC staining of mesothelioma seen on the peritoneal surface of various organs using antibodies against WT-1, AE1/AE3, D2-40, and Calretinin.
Table 1.
Histopathology of mice with AdCre bladder injection.
| AdCre Bladder | p53ww/TSC1ww | p53cc/TSC1cc | p53ww/TSC1cc | p53cc/TSC1ww |
|---|---|---|---|---|
| Total | 13 | 26 | 23 | 22 |
| Death | 1 (8%) | 15 (58%) | 4 (17%) | 4 (18%) |
| Mesothelioma | 0 | 16 (73%)** | 0 | 0 |
| Unknown cause of death* | 1 | 4 | 4 | 4 |
Note that many mice that died were not found soon after death, so that necropsy could not be performed to determine a cause of death.
16 (73%) reflects the fraction of mice in which the presence of mesothelioma could be assessed (22).
Necropsy analysis of the Adenovirus Cre-injected Tsc1ccTp53cc mice demonstrated that they had no significant pathology in the bladder, but rather often had hemorrhagic ascites along with uneven deposits of apparent cancer on multiple peritoneal surfaces. Pathologic analysis demonstrated that there was a malignant tumor unevenly distributed on all of the peritoneal surfaces (including bladder, kidney, spleen, liver, and intestines), without evidence of a primary site, and limited local invasion of other abdominal structures. Histologically the tumors looked most similar to epithelioid mesothelioma (Figure 1B). Immunohistochemistry (IHC) staining with mesothelioma markers (Calretinin, WT-1, AE1/AE3, and D2-40) showed multiple regions of positivity in the tumors strongly suggesting that these tumors are peritoneal mesothelioma (Figure 1C).
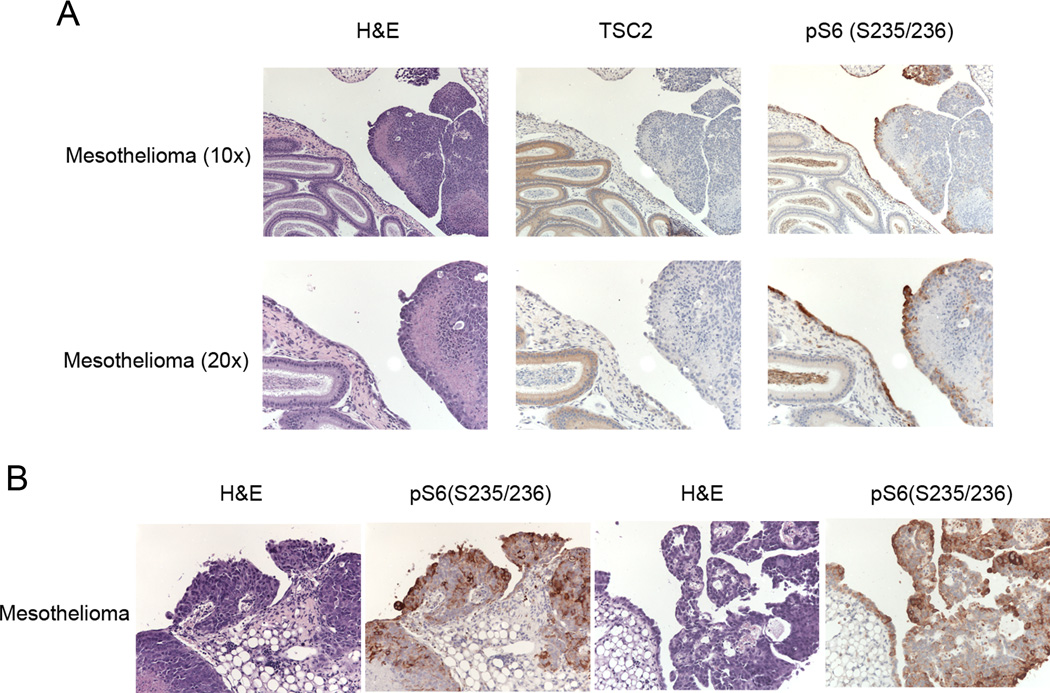
We sought to confirm that these mesotheliomas had occurred due to concurrent loss of Tsc1 and Tp53. TSC1 normally occurs in cells in a complex with TSC2 and TSC1 loss generally leads to markedly reduced TSC2 protein levels. Since a reliable Tsc1 antibody for IHC is lacking, we performed Tsc2 IHC staining, which we have shown previously is consistently reduced when Tsc1 expression is lost14. Tsc2 expression was absent in these mesothelioma, in contrast to normal structures, while phospho-S6(S235/236) staining was strong in the mesothelioma (Figure 2A and B), as seen in many other pathologic conditions with loss of TSC1/TSC2 leading to high level activation of mTORC1.
Figure 2. Immunohistochemistry (IHC) analysis of TSC2 and pS6 in Cre-induced mesothelioma of Tsc1ccTp53cc mice.
A–B. H&E sections and IHC staining of TSC2 and pS6(S235/S236) in mesothelioma and adjacent organs are shown at 10× and 20×.
Hence our tentative conclusion at this stage was that the Tsc1ccTp53cc mice which had received bladder injection of adenovirus Cre had developed mesothelioma after a delay of 6–12 months, likely due to leakage of small amounts of virus from the bladder at the time of the surgical injection procedure, causing recombination and loss of both Tsc1 and Tp53.
Tumor cell lines generated from the malignant ascites express mesothelioma markers
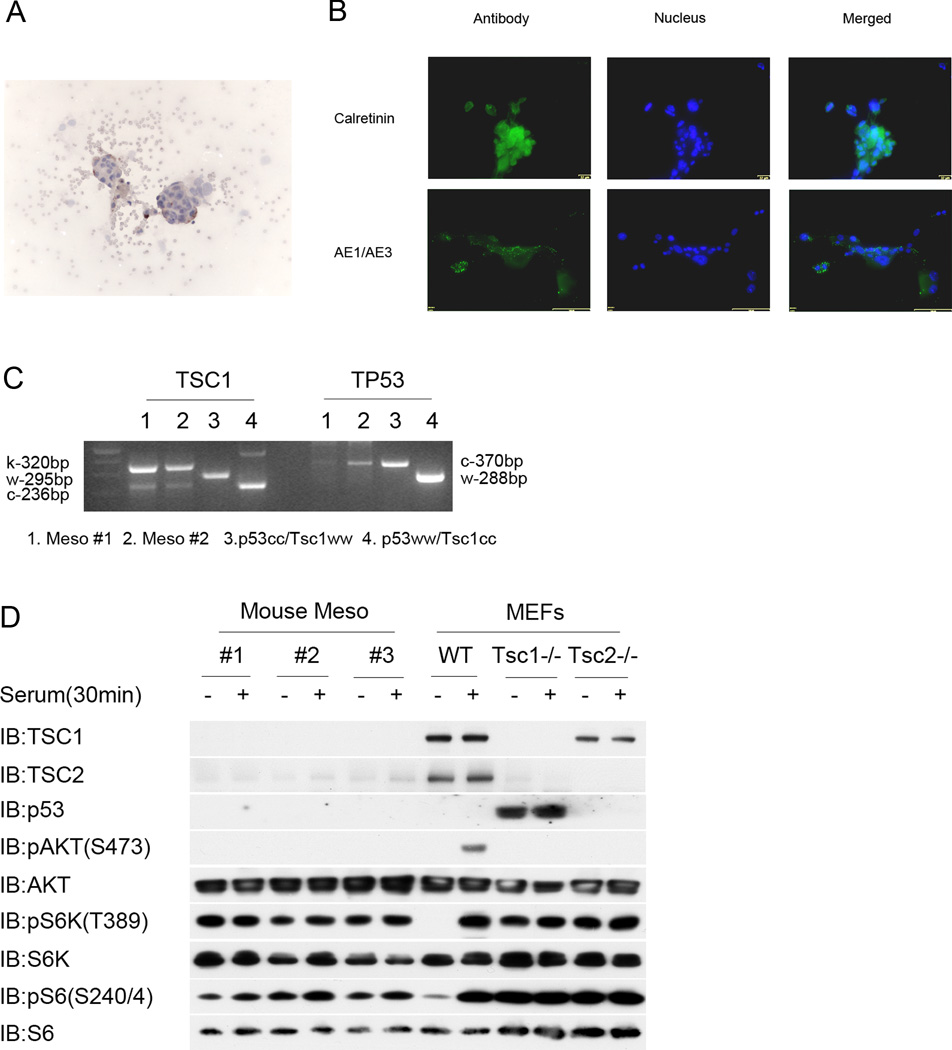
Examination of aspirates of ascites from Adenovirus-Cre-injected Tsc1ccTp53cc mice demonstrated the presence of tumor cell clusters (Figure 3A). Tumor cell lines were derived from several ascites samples, and showed reactivity with antibodies against calretinin and AE1/AE3 (Figure 3B), providing further evidence that the tumors were mesothelioma. Immunoblot analysis of 3 individual mesothelioma cell lines demonstrated absence of Tsc1 and Tp53 expression, reduced expression of Tsc2, and activation of mTORC1 as shown by persistent expression of pS6(S240/244) and pS6K(T389) in the absence of serum (Figure 3D). The three mesothelioma cell lines also showed a lack of AKT activation (reduced pAKT(S473)) after serum stimulation, consistent with negative feedback inhibition due to chronic mTORC1 activation. These findings are similar to what we and others have seen previously seen in cultures of murine embryo fibroblasts derived from Tsc1−/− and Tsc2−/− embryos (Figure 3D, right). We also confirmed that the cultured mesothelioma cells had undergone recombination at the Tsc1 and Tp53 loci (Figure 3C).
Figure 3. Analysis of mesothelioma cell lines generated from malignant ascites of Cre-treated Tsc1ccTp53cc mice.
A. Cytology preparations demonstrate mesothelioma cell clusters in malignant ascites of Cre-treated Tsc1ccTp53cc mice. Cytospins were stained for pS6(S235/S236) (brown), and counterstained with hematoxylin (blue).
B. Expression of Calretinin and AE1/AE3 by mouse mesothelioma cell lines generated from ascites.
C. Genotyping at the Tsc1 and Tp53 loci on two mouse mesothelioma cell lines and two control DNA samples. Note predominance of the k or knockout allele in the Tsc1 genotyping, and marked reduction in the conditional allele in the Tp53 genotyping, for each of the mesothelioma cell lines, indicative of recombination at both genes.
D. Immunoblot analysis of 3 mouse mesothelioma cell lines in comparison to widltype (WT), Tsc1−/−, or Tsc2−/− murine embryo fibroblast (MEF) cell lines after 48 h of serum starvation (−) or 30 min after serum addback following serum starvation (+). Note absence of Tsc1 and Tp53 in the mesothelioma cell lines; reduced expression of Tsc2; persistent high levels of pS6K(T389) and pS6(S240/S244) without serum; and reduced pAKT(S473) levels following serum addback for the mesothelioma lines. The WT MEF line shows a normal signaling pattern after serum addback.
Rapamycin inhibited mesothelioma tumor growth in vitro and in vivo
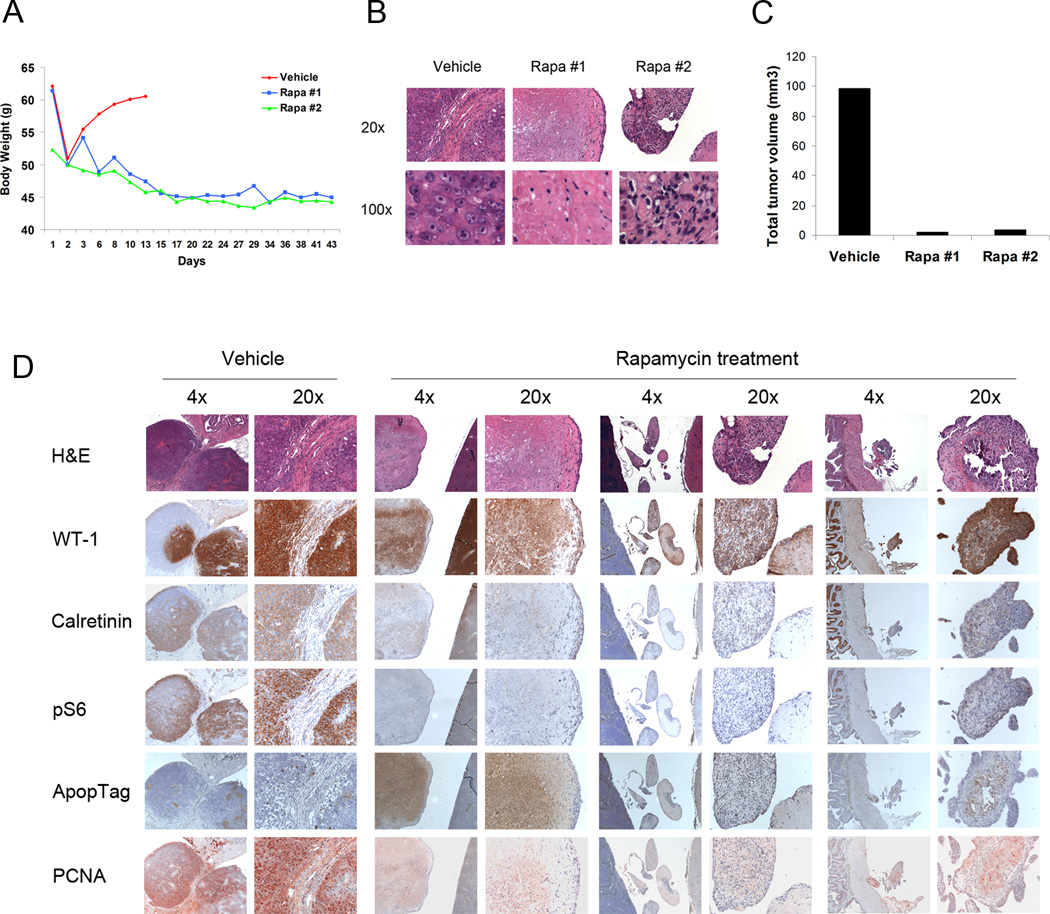
Since rapamycin allosterically inhibits mTORC1 through FKBP12, and is active in multiple murine model systems of tumors in which Tsc1 or Tsc2 is lost,15,16 we examined the therapeutic benefit of rapamycin for the mesotheliomas developing in this model. Three Tsc1ccTp53cc mice that were identified with malignant ascites were subjected to IP fluid removal, and cytology analysis to confirm that they had developed mesothelioma. Two were treated with rapamycin IP at 3mg/kg given three times per week for 5 weeks, starting the day after ascites was removed and mesothelioma diagnosed (Figure 4A). The third mouse was treated with vehicle injections on the same schedule. Ascites quickly recurred with weight gain in the vehicle-treated mouse, which died of mesothelioma within two weeks (Figure 4A). However, the body weight of mice treated with rapamycin declined through 5 weeks of treatment. The two treated mice were examined at necropsy. They showed no ascites and limited amounts of residual tumor nodules which were largely fibrotic (Figure 4B). Quantitative assessment based on histology showed a > 95% reduction in mesothelioma volume in the treated mice.
Figure 4. Rapamycin rescued survival and reduced malignant ascites in Cre-induced mesothelioma of Tsc1ccTp53cc mice.
A. Body weight of Cre-treated Tsc1ccTp53cc mice following aspiration of ascites on day 1. Two mice were treated with Rapamycin 3mg/kg IP 3 days per week (Rapa #1, Rapa #2); while one mouse received vehicle.
B. H/E stains of residual mesothelioma nodules in vehicle- and rapamycin- treated mice.
C. Mesothelioma tumor volume in vehicle- and rapamycin- treated mice.
D. H/E and IHC stains for WT-1, Calretinin, pS6(S235/236), Apoptag, and PCNA for vehicle-and rapamycin- treated mice.
IHC analysis demonstrated that the residual mesothelioma tumor nodules from the treated mice had persistent expression of WT-1, but markedly reduced expression of Calretinin, pS6(S235/236) and PCNA; and evidence of apoptosis in some cases (Figure 4D).
Thus, overall, rapamycin was dramatically effective in rescuing survival, eliminating ascites, and reducing mesothelioma extent and viability when given to the Adenovirus-Cre-injected Tsc1ccTp53cc mice by IP injection 3 days per week.
Intraperitoneal (IP) injection of adenovirus Cre in Tsc1ccTp53cc mice also leads to mesothelioma
As described above, we hypothesized that mesothelioma developed in these mice due to leakage of Adenovirus-Cre at the time of bladder injection. To examine this hypothesis, we performed IP injection of Adenovirus-Cre at 50% of the bladder injection dose (109 pfu) into a separate cohort of mice with similar combinations of individual and both conditional alleles of Tsc1 and Tp53. We observed a similar time course of survival and development of ascites, as what was seen after bladder injection in Tsc1ccTp53cc mice (Figure 5A). Despite using a lower dose of Adenovirus-Cre, the median survival was 8.5 months (1.7 months less than bladder-injected mice), and a larger proportion of Tsc1ccTp53cc mice (89%) died during one year of follow-up after IP injection in comparison to bladder injection. Mesothelioma was documented in 85% of the Cre IP injected Tsc1ccTp53cc mice (Figure 5A, Supplemental Figure 1, Table 2). Hence these observations provide strong support for our hypothesis for the mechanism of mesothelioma induction following bladder injection.
Figure 5. Survival and response to rapamycin treatment of Tsc1ccTp53cc mice following Intraperitoneal (IP) injection of adenovirus Cre.
A. Survival curve of mice of various genotypes following IP injection of adenovirus Cre. Note markedly reduced survival of Tsc1ccTp53cc mice in comparison to other genotypes (p<0.0001).
B. Body weight of IP Cre-treated Tsc1ccTp53cc mice following aspiration of ascites on day 1. Four mice were treated with Rapamycin 3mg/kg IP 3 days per week (Rapa #1 – Rapa #4); while one mouse received vehicle.
C. Mesothelioma tumor volume in vehicle- and rapamycin- treated mice. The average and SEM are shown for the four rapamycin-treated mice.
Table 2.
Histopathology of mice with AdCre IP injection.
| AdCre IP | p53ww/TSC1ww | p53cc/TSC1cc | p53ww/TSC1cc | p53cc/TSC1ww |
|---|---|---|---|---|
| Total | 18 | 27 | 23 | 12 |
| Death | 0 | 24 (89%) | 4 (17%) | 1 (8%) |
| Mesothelioma | 0 | 17 (85%)** | 0 | 0 |
| Unknown cause of death* | 0 | 7 | 4 | 1 |
Note that many mice that died were not found soon after death, so that necropsy could not be performed to determine a cause of death.
17 (85%) reflects the fraction of mice in which the presence of mesothelioma could be assessed (20).
We also repeated the rapamycin treatment experiments on the Tsc1ccTp53cc mice with mesothelioma due to IP injection of Adenovirus-Cre. Five Tsc1ccTp53cc mice that developed ascites were tapped for drainage and cytology analysis to confirm presence of mesothelioma. As before, the single mouse that received vehicle injection after the initial drainage rapidly regained weight with evidence of ascites, and survived for only two weeks. In contrast, all the mice treated with rapamycin IP 3mg/kg 3 days per week showed persistent and continuing loss of weight, indicative of therapeutic effect (Figure 5B). In addition, when analyzed after 5 weeks of treatment, there was a dramatic reduction in tumor volume, with only small residual foci with extensive fibrosis, with an overall reduction in tumor volume of about 98% (Figure 5C, Supplemental Figure 1).
Therefore, the IP injection model also displays marked in vivo sensitivity to rapamycin treatment.
Role of TSC1/TSC in human mesothelioma
The above observations indicated an important role for Tsc1 (and likely Tsc2 as well, since they function as a complex) in the development of mesothelioma in the mouse. Therefore, we investigated whether TSC1/TSC2 loss occurs in human mesothelioma.
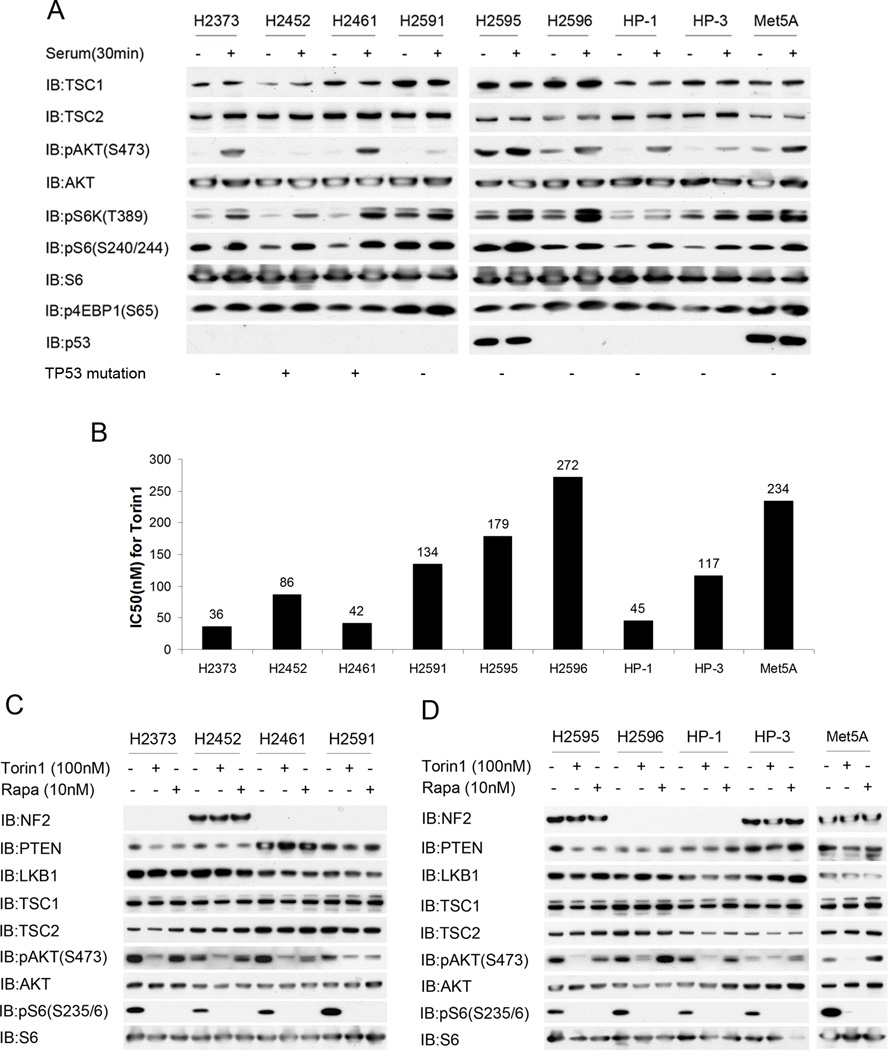
We first analyzed 9 mesothelioma cell lines by immunoblotting of cell lysates. All 9 cell lines demonstrated expression of both TSC1 and TSC2. However, there was evidence of constitutive activation of mTORC1 in the absence of serum in 4 of the 9 cell lines: H2591, H2595, H2596 and Met5A; with high levels of pS6K(T389), pS6(S240/244), and p4EBP1(S65). In addition, 3 of these 4 cell lines also showed evidence of AKT activation in the absence of serum with persistent pAKT(S473). These results suggested that the growth of these cell lines might be sensitive to inhibition of mTOR. For this purpose we used the ATP-competitive inhibitor Torin1, as it effectively blocks the kinase activity of both mTORC1 and mTORC2 and thereby reduces AKT activation.17 All 9 of the cell lines showed sensitivity to Torin1 with IC50’s < 300nM, and for 3 of the lines the IC50 was < 50 nM. Since loss of other tumor suppressor genes are also known to regulate AKT and mTOR activation in cells through upstream mechanisms,18 we also investigated expression of NF2, PTEN and LKB1. PTEN and LKB1 were expressed at similar levels in all cell lines (Figure 6C). Five of 9 cell lines showed no expression of NF2, consistent with previous results.19,20 However, lack of NF2 expression did not correlate either with mTORC1 activation, AKT activation, or Torin1 sensitivity (Figure 6C). We also examined the prevalence of TP53 mutation in these 9 lines, and found that two of them, H2452 and H2461, had mutations in TP53, c.817delCGTGTTTG and c.C817T, p.R273C, respectively. This did not correlate with levels of expression of TP53 as determined by immunoblotting (Figure 6A).
Figure 6. AKT and mTORC1 activation, and response to Torin1 in human mesothelioma cell lines.
A. Immunoblot analysis of human mesothelioma cell lines after 48 h of serum starvation (−) or 30 min after serum addback following serum starvation (+), for multiple components of the AKT-mTOR signaling pathway. Note high pS6K(T389), pS6(S240/244), and p4EBP1(S65) levels in serum starvation conditions for lines H2591, H2595, H2596, and Met5A. Presence or absence of mutation in TP53 as assessed by PCR sequencing of exons 5 to 9 is shown at bottom. H2452 had c.817delCGTGTTTG, and H2461 had c.C817T, p.R273C.
B. IC50 concentration of mTOR inhibitor Torin1 for 9 human mesothelioma cell lines.
C–D. Immunoblot analysis of human mesothelioma cell lines following treatment with mTOR inhibitors 10nM Rapamycin or 100nM Torin1 for 24 h. Note reduction in pAKT(S473) in all lines when treated with Torin1; and absence of NF2 in 5 of the lines.
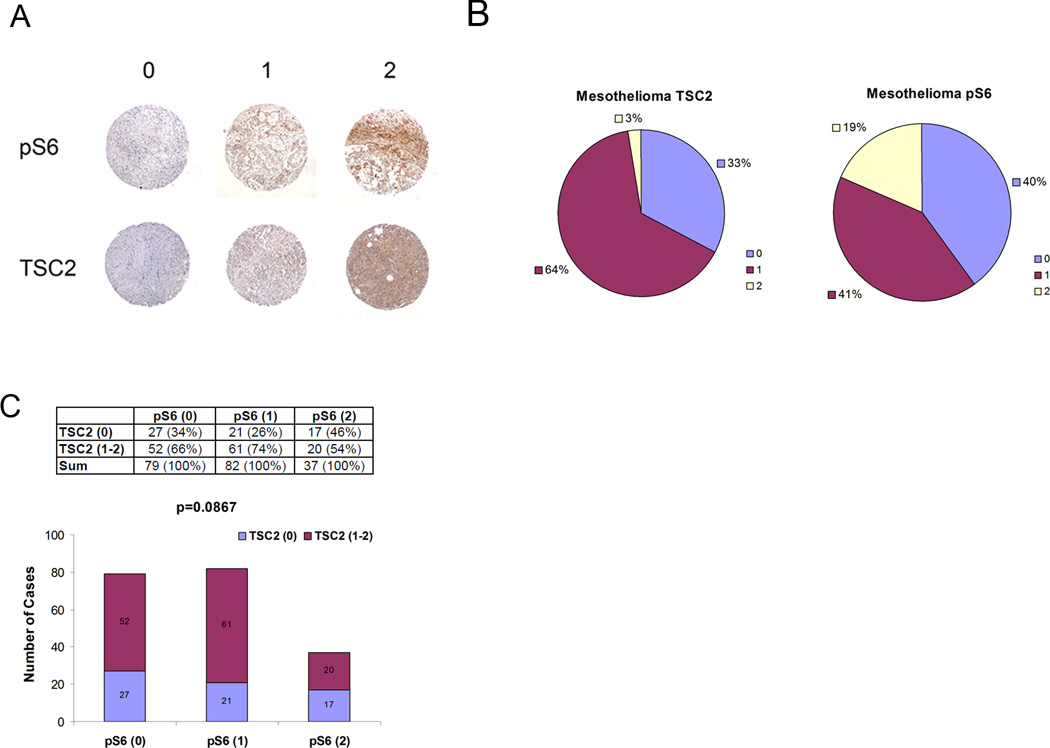
Since this analysis was performed on a relatively small number of mesothelioma cell lines, we then performed IHC analysis of a tissue microarray (TMA) of 198 mesothelioma cases from our institution (BWH). As described above, we used the antibody against TSC2 to identify cases in which there was reduced expression/inactivation of either TSC1 or TSC2. We screened for mTORC1 activation by analysis of pS6(S235/236) levels.18 The IHC intensity for each sample was determined by a blinded observer (LC) on a qualitative scale of 0–2; with 0 as undetectable, 1 as weak positive staining; and 2 as positive staining (Figure 7A). Reduced IHC staining for TSC2 was seen in 33% of cases, while positive staining for pS6(S235/236) was present in 60% of cases (Figure 7B). There was a trend toward an association of high pS6(S235/S236) staining with reduced TSC2 staining (p = 0.0867) suggesting that there was the predicted relationship between those two phenomena in vivo in these tumors prior to resection. Furthermore, the reduced expression of TSC2 that was seen suggests that genetic, epigenetic, or other mechanisms may lead to reduced TSC1/TSC2 expression in vivo in mesothelioma and contribute to its pathogenesis. The frequent (19%) high level expression of pS6(S235/S236) also suggests that mTORC1 activation is present in vivo in mesothelioma.
Figure 7. TSC2 loss and mTOR activation in a human mesothelioma TMA.
A. Examples of the three grades of staining (0, 1, 2) seen for TSC2 and pS6(S235/236) by IHC on a mesothelioma TMA.
B. Pie graph of TSC2 and pS6 staining scores on mesothelioma TMA.
C. Bar graph showing relative enrichment for reduced TSC2 staining among mesotheliomas with high pS6(S235/S236), p = 0.0867.
Discussion
Several previous studies have delineated an important role for AKT signaling in mesothelioma development. An initial study by JR Testa and lab members showed that pAKT(S473) levels were detectible in 17 of 26 (65%) human mesothelioma specimens by IHC staining, and correlated with p-mTOR(S2448) levels.13 Further, pAKT(S473) levels were also shown to be increased in both murine and human mesothelioma cell lines under standard culture conditions, but were sharply reduced with overnight serum withdrawal, suggesting that growth factor stimulation was required for AKT activation and that it was not due to some intrinsic or genetic defect in AKT regulation or in upstream regulation, such as PIK3CA mutation or PTEN loss.13 Several studies have characterized the activation of AKT and expression of PTEN by mesothelioma cell lines. In these studies in aggregate, PTEN loss is seen in about 10% of mesothelioma cell lines.13,21,22 Although other studies have reported a higher loss of PTEN expression (62% of 206 cases) in mesothelioma by IHC,23 it is possible that fixation or other artifacts explain this discordancy.
Furthermore, several studies have shown that HGF-MET, EGFR, and IGF1R signaling are all important upstream stimulators of AKT activation in mesothelioma.13,21,24,25 However, mesothelioma spheroid studies have shown that tumor resistance to apoptosis induction and chemotherapy was significantly reduced by co-treatment with rapamcyin and not PI3K inhibitors.26 This was interpreted as suggesting that mTOR was a critical mediator of survival signaling in mesothelioma, and independent of AKT.
Here we demonstrate that concurrent loss of Tp53 and Tsc1 in mesothelial cells leads to consistent development of malignant peritoneal mesothelioma in mice. This cancer is aggressive, invading local structures widely in the peritoneum, causes development of malignant ascites in about half of cases, and is associated with premature mortality (Figure 1). Although our initial experiments were done with bladder injection of Adenovirus Cre in an attempt to generate a Tsc1 mutant model of bladder cancer, we then showed that similar and somewhat more consistent pathology and clinical phenotype were seen when AdenoCre was delivered directly to the peritoneum by IP injection. Notably, loss of either Tp53 or Tsc1 alone by similar means led to a much lower rate of early mortality, and none of the mice with single gene loss developed malignant ascites, suggesting that mortality may have been due to another cause, possibly a different type of malignancy.
Rapamycin showed striking benefit in the treatment of this malignant mesothelioma with near complete response following 5 weeks of treatment. We used a treatment schedule that was well-tolerated and which we have shown previously gives persistent therapeutic levels.27 This dramatic response is consistent with the response to rapamycin seen in multiple other tumor models involving loss of either Tsc1 or Tsc2, in which a similar near 99% reduction in tumor volume is seen.15,16 In addition, rapamycin is highly effective in a variety of brain models of TSC in which Tsc1 or Tsc2 is lost in various brain cells, with marked improvement in phenotype, elimination of seizures, and improved survival. Similarly, dramatic responses to rapamycin or everolimus have been reported in a small number of human PEComas,8,10 in which TSC2 mutations are very common, and in a single case of a bladder cancer with a TSC1 mutation.11
Our studies on human mesothelioma cell lines demonstrated that there is robust AKT phosphorylation in response to serum in 7 of 9 lines, and that there is significant persistent AKT phosphorylation in the absence of serum for 48 hours in 3 of 9 lines. Eight of these lines were derived from human mesothelioma directly, while the Met5A line was derived by SV40 transduction into primary mesothelial cells.28 This constitutive AKT activation suggests that there is some pathologic driver abnormality (genetic or otherwise) in these three cell lines. However, our immunoblotting experiments suggest that this is not due to loss of TSC1, TSC2, PTEN, LKB1, or NF2. Furthermore, activation of mTORC1 is seen in the absence of serum in four of the nine cell lines, and also does not correlate with loss of any of the tumor suppressor genes listed above. It is particularly striking that there is no apparent correlation between NF2 loss and mTORC1 activation in these cell lines, in contrast to a previous report in mesothelioma and studies in meningeal cells.29,30 This may be due to the complex pattern of genetic changes present in these mesothelioma cell lines, which is as yet unknown. Further investigation is needed. However, note that previous studies have also reported a high frequency of mTORC1 activation in human mesothelioma cell lines.22
Overall our studies suggest that loss of Tp53 and Tsc1 are critical gatekeeper mutations that enable mesothelial proliferation and mesothelioma development in the mouse. Although we found no direct evidence of loss of TSC1 or TSC2 in the 9 human mesothelioma cell lines available to us, we did find reduced TSC2 expression in our mesothelioma TMA which matched partially with high pS6(S235/S236) levels, evidence of mTORC1 activation. These observations suggest that activation of this pathway occurs frequently in vivo in mesothelioma. Furthermore, temsirolimus, a rapamycin analogue, was reported to have major benefit in the treatment of a human mesothelioma xenograft model system in immune-deficient mice, with a reduction of median tumor volume of 79% after 38 days of treatment.22 The ATP competitive inhibitor Torin1 showed strong activity in the 9 mesothelioma cell lines studied (Figure 6B), and it is possible that inhibitors of that pharmacologic class will exhibit superior in vivo efficacy in comparison to the rapamycin family of allosteric mTOR inhibitors. In addition, combination therapy directed at other signaling pathways, or in combination with conventional chemotherapy agents may also have greater benefit.
Materials and Methods
Human samples
Pleural diffuse mesothelioma samples were available as discard samples from patients undergoing surgical resection at the Brigham and Women’s Hospital. Consent was obtained from each patient, and the study was approved by the Partners Human Research Committee.
Mice and drug treatments
All procedures were carried out in accordance with the Guide for the Humane Use and Care of Laboratory Animals, and the study was approved by the Animal Care and Use Committee of Children's Hospital, Boston. Tsc1 conditional allele mice, denoted Tsc1cc were originally derived in this laboratory.31 Tp53cc mice were provided by Yuko Fujiwara and Stuart Orkin, and were described in.32 Mice were euthanized when they displayed massive abdominal swelling, weight loss, inability to move or eat, or signs of distress.
Adenovirus Cre was purchased from University of Iowa Vector Core Facility. Bladder injection was done as described.33 Briefly, following anesthesia and shaving, a 1-cm midline incision was made in the lower anterior abdomen of male mice of age 2 – 3 months. The bladder was identified, and urine was removed by syringe. 10ul of an adenovirus Cre mixture containing 2×109 pfu was injected into bladder. The syringe was removed, the abdominal wall incision was closed with 5-0 silk sutures, and the skin was closed with wound clips.
Rapamycin was purchased from LC laboratories (Woburn, MA). A 20 mg/ml stock was made using ethanol, and mixed daily for injection with sterile vehicle (0.25% PEG-200, 0.25% Tween-80). Mice were treated with rapamycin by intraperitoneal (IP) injection at 3 mg/kg three times per week for up to five weeks. Control mice received the vehicle solution IP on the same schedule.
Reagents
Torin1 was generously provided by Nathanael Gray (Dana Farber Cancer Institute, Boston, MA). Cell culture media DMEM was from Cellgro (Manassas, VA) and supplements were from Invitrogen (Carlsbad, CA).
Cell culture
Nine human mesothelioma cell lines (H2373, H2452, H2461, H2591, H2595, H2596, HP-1, HP-3 and Met5A) were described previously,34 and were grown in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin-amphotericin B (PSA), in an incubator at 37°C in 5% CO2. For serum starvation, cells were cultured in the absence of serum for 48 h.
Mouse mesothelioma cell lines were derived from malignant ascites from mice following bladder injection of adenovirus Cre. When mice gained several grams of weight within 2 days and abdominal distention was obvious, ascites fluid was removed by aspiration with a 27G gauge needle. The ascites fluid was plated on a 10cm cell culture dish with medium (DMEM with 10%FBS and 1% PSA) overnight. The next day, cells were washed twice with sterile DPBS and fresh medium was added, and cultures of mesothelioma grew without further manipulation.
Immunoblotting
Cells were harvested in lysis buffer consisting of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA and a cocktail of protease inhibitors (Sigma-Aldrich, St. Louis, MO). Cell lysates were clarified by centrifugation for 5 min and the protein concentration of the supernatants was determined using a modified Bradford assay (Bio-Rad, Hercules, CA). For immunoblotting, 20 µg of protein was loaded in each lane, and was separated by SDS-PAGE on 4–12% gradient gels (Invitrogen, Carlsbad, California), transferred to PVDF membranes and detected by immunoblotting with the following primary antibodies: TSC1, p53, pAKT(S473), AKT, pS6(S240/244), S6, p4EBP1(S65), 4EBP1, NF2, PTEN, LKB1, pS6K(T389), S6K (Cell Signaling Technology); TSC2 (Santa Cruz Biotechnology, Santa Cruz, CA). Goat anti-mouse and anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) conjugated to horseradish peroxidase were used at a 1:3000 dilution and immunoreactive bands were detected by chemiluminescence (SuperSignal, Pierce, Rockford, IL) and film (Denville Scientific, South Plainfield, NJ).
IC50 determination
2,000 cells were plated per well in 96-well plates and cultured overnight. One day later Torin1 was added in serial dilutions to FC 10microM to 1.5 nM. After 48 h of culture, 20 µL CellTiter-Glo® (1:1 in PBS) (Promega, Wisconsin) was added, the plates were shaken, and read in a Synergy HT Multi-Mode Microplate Reader (BioTek) with software Gen5 1.09 at room temperature. The IC50 was determined utilizing XLfit4.0 software from IDBS.
Histology and tumor assessment in mice
Microscopic mesothelioma tumor volumes were determined in a semi-quantitative fashion by a single blinded observer (YG). Whole mounts of the abdominal contents of mice were prepared in the histology lab by a technician without knowledge of the study. H&E-stained 8 micron sections were prepared, and each tumor nodule was measured to determine its length and width. These measurements were converted into a measurement of tumor volume per lesion using the following formula: volume = 0.86 * (tumor length * tumor width)**1.5.15 The total tumor volume per mouse was then equal to the sum of the tumor volume of each lesion identified. Comparisons between sets of mice for tumor measurements were made using the non-parametric Mann-Whitney test in Prism (GraphPad Software, Inc., v4.0a).
Immunohistochemistry (IHC)
Eight micrometer paraffin sections were deparaffinized with xylene and alcohol series, treated with Target Retrieval Solution pH 6.1 (Dako, Carpinteria, CA), blocked with 3% H2O2 in methanol, and then put in 5% normal goat serum in 0.1% Triton X in PBS. Sections were incubated with primary antibodies overnight at 4°C, washed, and incubated with secondary antibody conjugated with horseradish peroxidase (HRP). DAB Chromogen solution (Envision+System Dako) was then applied to generate a color reaction. Slides were then counterstained with hematoxylin (Dako). Antibodies used for staining were: anti-pS6 (S235/236) (1:100, Cell Signaling), TSC2 (1:100, Cell Signaling, Beverly, MA), Calretinin (1:100, Abgent, San Diego, CA), WT-1 (1: 200, Santa Cruz Biotech, Ssnta Cruz, CA), and AE1/AE3 (cytokeratin 5, 6) (1:100, EMD Millipore, Billerica, A). PCNA staining was done according to the protocol of HistoMouse-Plus kit (Life Technologies Invitrogen, Grand Island, NY). Hematoxylin was used as a counterstain and an adjacent section was stained with hematoxylin and eosin.
Tissue-microarray analysis
A mesothelioma tissue microarray (TMA) was generated as described previously using tumor and normal specimens from resected patients with pleural mesothelioma as well as control tissues35. IHC scores of the TMA after staining for expression of TSC2 and pS6 were determined in a semi-quantitative fashion by a pulmonary oncologic pathologist (LRC). The intensity of the staining was determined according to the intensity of staining, as 0 is undetectable; 1 is weak positive staining; 2 is strong positive staining.
Statistical analysis
Quantitative data are reported as the mean ± standard error of the mean (SEM) from at least three independent experiments. The means for various treatment groups were compared using analysis of variance (ANOVA) and Dunnett’s post-hoc test.
Supplementary Material
A. Three different mesotheliomas that developed in Tsc1ccTp53cc mice after IP injection of adenovirus Cre are shown at two magnifications.
B. Response of mesotheliomas in Tsc1ccTp53cc mice after Intraperitoneal injection of adenovirus Cre to treatment with rapamycin. Three different residual mesothelioma nodules in different Cre-treated Tsc1ccTp53cc mice are shown.
Acknowledgments
We thank Dr. Nathanael Gray (Dana Farber Cancer Institute, MA) for kindly providing mTOR inhibitor Torin1 used in this study.
Grant Support: This work was supported by grants 1P01CA120964 and 2P50CA090578 from the National Institutes of Health, and the Chip Thayer Fund.
Footnotes
Disclosure of Potential Conflicts of Interest
We have no potential conflicts of interest to disclose.
References
- 1.Ettinger DS, Akerley W, Borghaei H, Chang A, Cheney RT, Chirieac LR, et al. Malignant pleural mesothelioma. J Natl Compr Canc Netw. 2012;10:26–41. doi: 10.6004/jnccn.2012.0006. [DOI] [PubMed] [Google Scholar]
- 2.Tsao AS, Wistuba I, Roth JA, Kindler HL. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–2090. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatkowski DJ, Thiele E, Whittemore VH. Tuberous sclerosis complex. Weinheim, Germany: Wiley-VCH; 2010. pp. 3–7. [Google Scholar]
- 5.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–6017. doi: 10.1158/1078-0432.CCR-09-0898. [DOI] [PubMed] [Google Scholar]
- 7.Qin W, Bajaj V, Malinowska I, Lu X, MacConaill L, Wu CL, et al. Angiomyolipoma have common mutations in TSC2 but no other common genetic events. PLoS One. 2011;6:e24919. doi: 10.1371/journal.pone.0024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammerman PS, Hayes DN, Wilkerson MD, Schultz N, Bose R, Chu A, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: Clinical and molecular correlates. Int J Cancer. 2012 doi: 10.1002/ijc.27800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer G, Hanrahan AJ, Milowsky MI, Al-Ahmadie H, Scott SN, Janakiraman M, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menges CW, Sementino E, Talarchek J, Xu J, Chernoff J, Peterson JR, et al. Group I p21-activated kinases (PAKs) promote tumor cell proliferation and survival through the AKT1 and Raf-MAPK pathways. Mol Cancer Res. 2012;10:1178–1188. doi: 10.1158/1541-7786.MCR-12-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altomare DA, You H, Xiao GH, Ramos-Nino ME, Skele KL, De Rienzo A, et al. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24:6080–6089. doi: 10.1038/sj.onc.1208744. [DOI] [PubMed] [Google Scholar]
- 14.Goto J, Talos DM, Klein P, Qin W, Chekaluk YI, Anderl S, et al. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci U S A. 2011;108:E1070–E1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auricchio N, Malinowska I, Shaw R, Manning BD, Kwiatkowski DJ. Therapeutic trial of metformin and bortezomib in a mouse model of tuberous sclerosis complex (TSC) PLoS One. 2012;7:e31900. doi: 10.1371/journal.pone.0031900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang MC, Ma J, Chen L, Kozlowski P, Qin W, Li D, et al. TSC1 loss synergizes with KRAS activation in lung cancer development in the mouse and confers rapamycin sensitivity. Oncogene. 2010;29:1588–1597. doi: 10.1038/onc.2009.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–S51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi AB, Mitsunaga SI, Cheng JQ, Klein WM, Jhanwar SC, Seizinger B, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A. 1995;92:10854–10858. doi: 10.1073/pnas.92.24.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekido Y, Pass HI, Bader S, Mew DJ, Christman MF, Gazdar AF, et al. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 1995;55:1227–1231. [PubMed] [Google Scholar]
- 21.Suzuki Y, Murakami H, Kawaguchi K, Tanigushi T, Fujii M, Shinjo K, et al. Activation of the PI3K-AKT pathway in human malignant mesothelioma cells. Mol Med Report. 2009;2:181–188. doi: 10.3892/mmr_00000081. [DOI] [PubMed] [Google Scholar]
- 22.Hoda MA, Mohamed A, Ghanim B, Filipits M, Hegedus B, Tamura M, et al. Temsirolimus inhibits malignant pleural mesothelioma growth in vitro and in vivo: synergism with chemotherapy. J Thorac Oncol. 2011;6:852–863. doi: 10.1097/JTO.0b013e31820e1a25. [DOI] [PubMed] [Google Scholar]
- 23.Opitz I, Soltermann A, Abaecherli M, Hinterberger M, Probst-Hensch N, Stahel R, et al. PTEN expression is a strong predictor of survival in mesothelioma patients. Eur J Cardiothorac Surg. 2008;33:502–506. doi: 10.1016/j.ejcts.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Brevet M, Shimizu S, Bott MJ, Shukla N, Zhou Q, Olshen AB, et al. Coactivation of receptor tyrosine kinases in malignant mesothelioma as a rationale for combination targeted therapy. J Thorac Oncol. 2011;6:864–874. doi: 10.1097/jto.0b013e318215a07d. [DOI] [PubMed] [Google Scholar]
- 25.Jagadeeswaran R, Ma PC, Seiwert TY, Jagadeeswaran S, Zumba O, Nallasura V, et al. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res. 2006;66:352–361. doi: 10.1158/0008-5472.CAN-04-4567. [DOI] [PubMed] [Google Scholar]
- 26.Wilson SM, Barbone D, Yang TM, Jablons DM, Bueno R, Sugarbaker DJ, et al. mTOR mediates survival signals in malignant mesothelioma grown as tumor fragment spheroids. Am J Respir Cell Mol Biol. 2008;39:576–583. doi: 10.1165/rcmb.2007-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddel RR, Malan-Shibley L, Gerwin BI, Metcalf RA, Harris CC. Tumorigenicity of human mesothelial cell line transfected with EJ-ras oncogene. J Natl Cancer Inst. 1989;81:945–948. doi: 10.1093/jnci/81.12.945. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29:4235–4249. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 32.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 33.Seager C, Puzio-Kuter AM, Cordon-Cardo C, McKiernan J, Abate-Shen C. Mouse models of human bladder cancer as a tool for drug discovery. Curr Protoc Pharmacol. 2010;Chapter 14(Unit14):14. doi: 10.1002/0471141755.ph1414s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penno MB, Askin FB, Ma H, Carbone M, Vargas MP, Pass HI. High CD44 expression on human mesotheliomas mediates association with hyaluronan. Cancer J Sci Am. 1995;1:196–203. [PubMed] [Google Scholar]
- 35.Burt BM, Rodig SJ, Tilleman TR, Elbardissi AW, Bueno R, Sugarbaker DJ. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 2011;117:5234–5244. doi: 10.1002/cncr.26143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Three different mesotheliomas that developed in Tsc1ccTp53cc mice after IP injection of adenovirus Cre are shown at two magnifications.
B. Response of mesotheliomas in Tsc1ccTp53cc mice after Intraperitoneal injection of adenovirus Cre to treatment with rapamycin. Three different residual mesothelioma nodules in different Cre-treated Tsc1ccTp53cc mice are shown.