Abstract
Maintaining proper energy balance in mammals entails intimate crosstalk between various tissues and organs. These inter-organ communications are mediated, to a great extent, by secreted hormones that circulate in blood. Regulation of the complex metabolic networks by secreted hormones (e.g., insulin, glucagon, leptin, adiponectin, FGF21) constitutes an important mechanism governing the integrated control of whole-body metabolism. Disruption of hormone-mediated metabolic circuits frequently results in dysregulated energy metabolism and pathology. As part of an effort to identify novel metabolic hormones, we recently characterized a highly conserved family of fifteen secreted proteins, the C1q/TNF-related proteins (CTRP1–15). While related to adiponectin in sequence and structural organization, each CTRP has its own unique tissue expression profile and non-redundant function in regulating sugar and/or fat metabolism. Here, we summarize the current understanding of the physiological functions of CTRPs, emphasizing their metabolic roles. Future studies using gain-of-function and loss-of-function mouse models will provide greater mechanistic insights into the critical role CTRPs play in regulating systemic energy homeostasis.
Keywords: adipokine, obesity, type 2 diabetes, insulin resistance, gluconeognesis, fat oxidation
Introduction
Mammals use complex central and peripheral mechanisms to maintain proper energy balance (1, 2). Among peripheral mechanisms, secreted hormones play a particularly important role (2–5). They mediate inter-organ communication and tissue crosstalk to coordinate the integrated control of whole-body metabolism. Circulating levels of many secreted hormones, such as insulin, leptin, adiponectin, and FGF21, are dynamically regulated in response to short-term changes in nutritional status or long-term changes in metabolic state (2–6). Sustained metabolic perturbations due to excess caloric intake, as in obesity, frequently disrupt the signaling pathways regulated by these circulating hormones. Consequently, hormonal axis deregulation is mechanistically linked to common metabolic disorders such as obesity, insulin resistance, and type 2 diabetes (7).
Adiponectin and the C1q family
Among peripheral tissues, adipose fulfills two critical roles—as the major storage depot for triglycerides and as an endocrine organ that secretes active polypeptide hormones into circulation (8). Adipose-derived secretory proteins are collectively called adipokines and act in an autocrine, paracrine, and/or endocrine manner to modulate insulin sensitivity and glucose and fatty acid metabolism (7). Adipokines may also influence whole-body metabolism by indirectly modulating inflammatory processes in adipose and other metabolic tissues (9). Adiponectin is a pleotropic insulin-sensitizing adipokine that has been the subject of extensive genetic and correlative studies in humans and mechanistic studies in transgenic and knockout (KO) mice (10, 11). Despite the widely described anti-diabetic, antiatherogenic, and anti-inflammatory properties of adiponectin (10–12), adiponectin KO mice exhibit highly variable phenotypes (13–17). Three independent adiponectin KO mouse lines develop some degree of insulin resistance when fed a high-fat diet, while another line exhibits enhanced fat oxidation; all four KO mouse lines have mild or undetectable metabolic abnormalities when fed a standard chow diet (13–16). The generally mild metabolic phenotypes of adiponectin KO mice have been partially attributable to enhanced leptin sensitivity (18). However, despite attempts to provide a unifying mechanism of action for adiponectin (19, 20), a clear understanding of the pleiotropic functions of adiponectin has been confounded by conflicting studies concerning its role in glucose metabolism (21), atherosclerosis (17), food intake (22, 23), inflammation (24–26), and tumor angiogenesis (27, 28). Additional mechanisms may partially account for the variable and mild metabolic alterations of adiponectin KO mice (29, 30).
Adiponectin belongs to the larger C1q protein family, defined by the presence of a C-terminal globular domain with sequence homology to the immune complement protein C1q (31). Human and mouse genomes encode >30 C1q domain-containing proteins (31, 32). These include the founding immune complement C1q (A-, B-, and C-chain) (33), as well as multimerins (34), emilins (35), C1q/TNF-related proteins (CTRPs) (36), cerebellins (37), adiponectin (38), otolin (39), C1q-related factor (CRF) (40), C1qDC1/caprin-2 (41), and nonfibrillar collagen VIII (42, 43) and collagen X (44). With the exception of cytosolic C1qDC1 (41), all C1q family members are secreted proteins that likely evolved from a common C1q domain-containing ancestral protein. C1q proteins play diverse roles in mammalian physiology, including immunity, development, and metabolism (31, 34, 35, 37, 45, 46). Of the C1q family members, CTRP1-15 share strikingly similar structural organization and biochemical properties with adiponectin (29, 36, 47–55) Here, we highlight the unique and shared characteristics between CTRPs and adiponectin; overlapping metabolic functions with CTRPs could account for the mild phenotypes of adiponectin KO mice (30).
CTRP characteristics
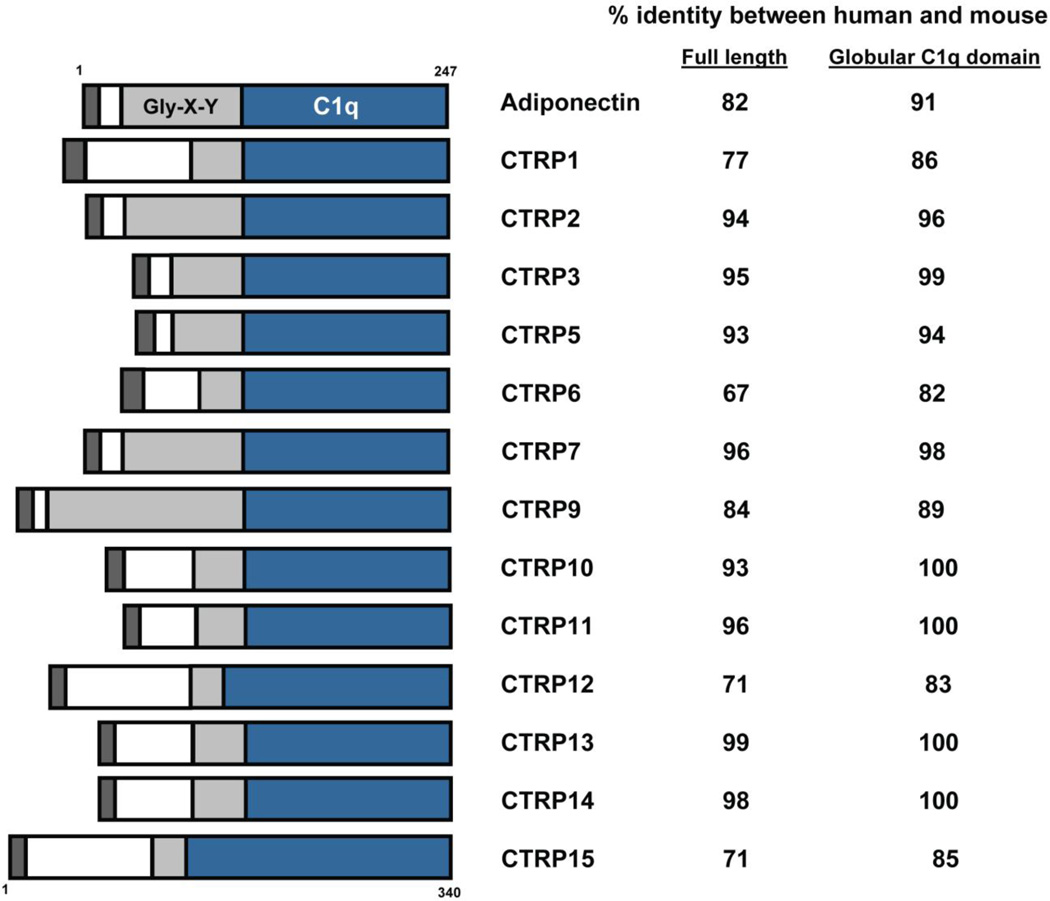
CTRPs were identified by sequence homology with the globular domain of adiponectin (36). Further, the overall CTRP domain organization is similar to adiponectin; every CTRP contains a signal peptide to direct protein secretion, an N-terminal domain with one or more conserved Cys residues, a collagen-like domain with variable numbers of Gly-X–Y repeats, and a C-terminal globular C1q domain (Fig. 1). All CTRPs are highly conserved throughout vertebrate evolution, and clear orthologs can be identified from zebrafish, frog, mouse, and human genomes. Of the CTRPs, CTRP9 shares the highest degree of sequence identity (54%) with adiponectin at the presumed functional globular domain (47). Unlike adiponectin, whose transcript is expressed almost exclusively by adipocytes (38), CTRPs are widely expressed in human and mouse tissues (29, 36, 47–55). Although adipose predominantly expresses CTRP1, CTRP2, CTRP3, CTRP5, CTRP7, CTRP9, CTRP12, and CTRP13, each CTRP has a unique tissue expression profile that may reflect unique functions (29, 47, 50, 51).
Figure 1. Schematic of CTRPs.
With the exception of CTRP4, every CTRP consists of four domains: a signal peptide for secretion (dark grey), an N-terminal domain with one or more conserved Cys residues (white), a collagen domain with varying numbers of Gly-X–Y repeats (light gray), and a C-terminal globular domain homologous to the immune complement C1q (blue). The numbers on the right refer to the percent amino acid identity between human and mouse orthologs when comparing the full-length protein (first column) or the C-terminal globular domain (second column). CTRP4 and CTRP8 are omitted—the former consists of only two tandem C1q domains, while the latter is absent in the mouse genome.
CTRP structural organization
All characterized C1q members, including CTRPs, form trimers (56–61). Adiponectin trimers can be further assembled into higher-order hexameric and octadecameric complexes (62– 67). Assembly of these structures requires the conserved N-terminal Cys-39 residue and is achieved with the help of endoplasmic reticulum proteins such as ERp44 and DsbA-L (68, 69). CTRP3, CTRP5, CTRP6, CTRP9, CTRP10, CTRP12, CTRP13, and CTRP15/myonectin also form multimeric complexes that are assembled through the conserved N-terminal Cys residues (29, 47, 50–53). CTRP11 forms multimeric complexes through an interaction with the oxidoreductase ERp44, but conserved N-terminal Cys-28 and Cys-32 residues are not required for assembly (52). While different adiponectin oligomers may have distinct signaling properties (62, 70, 71) that correlate with insulin sensitivity in humans (66, 72–74), the functional significance of CTRP oligomers remains largely undefined, with the exception of CTRP12 (75).
CTRP posttranslational modifications
When secreted from mammalian cells, CTRP1, CTRP2, CTRP6, CTRP12, and CTRP15/myonectin contain N-linked glycans, but CTRP3, CTRP5, CTRP9, CTRP10, CTRP11, and CTRP13 contain other carbohydrate moieties (29, 47, 50–53). Mass spectrometry revealed that proline residues within the Gly-X-P repeats and lysine residues within the consensus GXKG(E/D) motif in the N-terminal collagen domain of CTRP9 are hydroxylated and glycosylated, respectively (47). Proline hydroxylation and lysine glycosylation within the collagen domain affect the stability, function and biological potency of adiponectin (76–78). One or more GXKG(E/D) motifs are present in the collagen domain of all CTRPs except CTRP4, CTRP12, and CTRP15/myonectin, suggesting these modifications may occur in other CTRPs to affect protein stability and/or function. Adiponectin also contains sialic acids important for stability (79, 80), although it is presently unknown whether any CTRPs are modified with sialic acid. The potential influence of posttranslational modifications on assembly of higher-order CTRP structures underscores the importance of using recombinant CTRPs produced in mammalian expression systems to conduct functional studies. Recombinant proteins produced in mammalian cells, rather than bacteria, are likely to possess native posttranslational modifications and higher-order structures to confer biological activity.
CTRPs in circulation
When specific antibodies are available, endogenous CTRPs can be detected circulating in the blood (29, 47, 50, 53). Although adiponectin circulates in human plasma at high concentrations (10–30 μg/mL), CTRPs circulate at 1–2 orders of magnitude less than adiponectin (81–84). In both humans and mice, sex and genetic background influence metabolic hormone levels and signaling pathways, and thus variably influence the development of obesity, insulin resistance, and type 2 diabetes (85–88). Females have significantly higher circulating adiponectin levels (63, 89, 90), which may be due to the ability of testosterone to suppress adiponectin (89–91). Likewise, the expression and circulating levels of some CTRPs also exhibit sexually dimorphic patterns, with female mice expressing higher levels of CTRP5, CTRP9, CTRP11, and CTRP13 relative to males (29, 47, 51, 52). However, it is unclear whether sex hormones directly modulate CTRP expression levels. Alterations in metabolic state also affect circulating CTRP levels consistent with metabolic function (47–51, 53). Adiponectin expression levels are consistently reduced in obesity and type 2 diabetes (92–97); similarly, circulating levels of CTRP1, CTRP3, CTRP9, CTRP12, and CTRP15/myonectin are also reduced in diet-induced obese (DIO) mouse models (48– 50, 53).
CTRP metabolic functions
The metabolic function and regulation of adiponectin has been extensively studied in the past decade (10, 11); however, much less is known about the regulation and function of CTRPs. Here, we summarize current understanding of CTRP metabolic functions (Table 1), as non-metabolic roles have been reviewed elsewhere (45).
Table 1.
Summary of CTRPs and their potential metabolic functions.
| CTRP | Tissue distribution | Regulation | Signaling specificity |
Sites of action | Physiological function | Reference |
|---|---|---|---|---|---|---|
| CTRP1 | Primarily adipose tissue and placenta | Decreased expression and circulating levels in DIO mice; increased expression in adiponectin KO mice | AMPK 44/42-MAPK | Skeletal muscle | Promotes glucose uptake and skeletal muscle fat oxidation; decrease fat mass and enhances insulin sensitivity in transgenicmice fed a high-fat diet | 29, 36, 48 |
| CTRP2 | Primarily adipose tissue; lower levels in lung, liver, testis, uterus | Increased expression in the adipose tissue of ob/ob mice | AMPK | Skeletal muscle ? | Promotes fatty acid oxidation in cultured C2C12 myotubes | 36 |
| CTRP3 | Adipose tissue, kidney, testis, uterus, bone | Decreased expression and circulating levels in DIO mice; increased expression in overnight fasted mice | AKT | Liver | Suppresses hepatic gluconeogenic gene expression and glucose output; ameliorates diet-induced hepatic steatosis by inhibiting liver triglyceride synthesis | 36, 49 99, 109, 112–116 |
| p44/42-MAPK p38-MAPK | Endothelial cells | Inceases migration and proliferation of endothelial cells in vitro | ||||
| AKT | Heart | Increases survival and decreases infarct size following myocardial infarction | ||||
| ? | Monocytes, adipocytes and colonic fibroblasts | Decreases LPS-induced inflammatory response | ||||
| CTRP5 | Adipose tissue, eye, testis skeletal muscle, brain spleen, uterus | Circulating levels of CTRP5 are increased in db/db and ob/ob mice, and OLETF rats | AMPK | Skeletal muscle ? | Inreases glucose uptake and fatty acid oxidation in mitochondriadepleted myotubes in vitro | 29, 36, 84, 117–123 |
| Eye | S163R mutation results in late-onset retinal maculardegeneration in humans | |||||
| CTRP6 | Primarily placenta; much lower levels in spleen, lung, testis, prostate, uterus, adipose tissue | Increased expression in the adipose tissue of ob/ob mice; rosiglitazone treament decrease expression in the adipose tissue | ? | ? | ? | 29, 36 |
| CTRP7 | Primarily adipose tissue and lung | ? | ? | ? | ? | 29, 36 |
| CTRP9 | Primarily adipose tissue | Decreased circulating levels in DIO mice; Increased expression and circulating levels in fasted/re-fed mice | AMPK | Skeletal muscle | Promotes fat oxidation | 47, 124, 130–133, |
| AMPK/eNOS | Endothelial cells | Vasorelaxation | ||||
| AMPK | Heart | Improved cardiac function following ischemia/reperfusion injury | ||||
| p44/42-MAPK PKA | Vascular smooth muscle cells | Increased migration and cell proliferation; reduces neointima formation | ||||
| CTRP11 | Primarily adipose tissue and testis; lower levels in brain and kidney | Increased expression in the adipose tissue of fasted/re-fed mice | p44/42-MAPK | Pre-adipocytes | Inhibit adipogenesis | 52 |
| CTRP12 | Primarily adipose tissue in humans; primarily testis, adipose, kidney, spleen, uterus in mice | Decreased expression and circulating levels in ob/ob and DIO mice | AKT | Adipose tissue | Promotes glucose uptake in adipocytes | 50, 54,75, 141 |
| AKT | Liver | Suppresses hepatic gluconeogenic gene expression and glucose output | ||||
| ? | Pancreatic β-cells ? | Enhances insulin secretion inlean but not DIO mice; increases glucose-induced insulin secretion in INS-1 cells | ||||
| ? | Adipose tissue | Suppresses adipose tissue inflammation | ||||
| CTRP13 | Primarily adipose, brain, and kidney | Increased expression in the adipose tissue of ob/ob ice and in adipocytes treated with rosiglitazone | AMPK | Adipocytes, myotubes, and hepatocytes | Promotes glucose uptake in cultured adipocytes, myotubes, and hepatocytes | 51, 142 |
| AMPK | Liver ? | Suppressed gluconeogenic gene expression and glucose production in cultured H4IIEhepatocytes | ||||
| ? | Liver ? | Decreased palmitate-induced insulin resistance in cultured hepatocytes by suppressing JNK signaling | ||||
| ? | Hypothalamus | Suppressed food intake in mice by inhibiting orexigenic neuropeptide (NPY) expression | ||||
| Myonectin (CTRP15) | Primarily skeletal muscle | Decreased expression and circulating levels in fasted mice and strikingly induced in fasted/re-fed mice | ? | Adipose tissue and liver | Promotes lipid uptake by increasing the expression of molecules (FATP, FABP) involved in fatty acid uptake | 53 |
CTRP1
CTRP1 is predominantly expressed by adipose tissue (29). Circulating CTRP1 levels decrease in DIO (48) and increase in adiponectin KO mice or mice given daily injections of the anti-diabetic drug rosiglitazone (29), suggesting that increased circulating CTRP1 levels may have a positive metabolic effect. Concordantly, administration of physiological amounts of recombinant CTRP1 to wild-type mice acutely and substantially lowers blood glucose (29). Further, a two-fold increase in circulating CTRP1 levels modestly improves insulin sensitivity and decreases high-fat diet-induced weight gain in transgenic mouse models (48). Reduced weight gain in response to high-fat feeding is primarily due to increased energy expenditure from enhanced fat oxidation in skeletal muscle (48). These in vivo effects are mediated by the highly conserved AMP-activated protein kinase (AMPK). In skeletal muscles of transgenic mice, AMPKα and its downstream target acetyl-CoA carboxylase (ACC) are hyperphosphorylated (48). Phosphorylation of AMPKα at Thr-172 activates the kinase, whereas AMPK phosphorylation of ACC at Ser-79 inactivates the carboxylase (98). Inactivation of ACC reduces malonyl CoA levels to promote fatty acyl-CoA import into mitochondria for β-oxidation (98), reflecting a direct action of CTRP1 on AMPK signaling in muscle cells. In addition, recombinant CTRP1 can recapitulate increases in skeletal muscle AMPKα (Thr-172) and ACC (Ser-79) phosphorylation in wild-type mice (48). These studies collectively indicate that CTRP1 is a novel secreted regulator of skeletal muscle fat oxidation.
CTRP3
CTRP3, also known as CORS26/cartducin (99), is expressed by adipocytes (100–102) and adipose stromal cells (29) and in other tissues (29, 100, 103–105). Overnight fasting increases circulating CTRP3 levels relative to mice fed ad libitum, while high-fat feeding reduces CTRP3 levels by ~50% (49). In humans, circulating CTRP3 levels positively correlate with adiponectin and negatively correlate with waist circumference, blood pressure, fasting glucose, triglycerides, and cholesterol (82). Additionally, CTRP3 regulates glucose metabolism. Increased circulating CTRP3 levels substantially lower blood glucose in wildtype and insulin-resistant, leptin-deficient obese ob/ob mice (49). These results suggest CTRP3 influences metabolism independently of insulin. CTRP3 targets liver hepatocytes to suppress gluconeogenic gene expression (G6Pase and PEPCK) and gluconeogenesis via the activation of protein kinase B/Akt signaling (49). This differs from the mechanism of adiponectin in liver which activates AMPK to suppress hepatic glucose output (106, 107), although the role of AMPK in this process has been recently challenged (108).
We generated transgenic mice with elevated circulating CTRP3 levels (109). When challenged with a high-fat diet, CTRP3 transgenic mice exhibit remarkable resistance to the development of hepatic steatosis despite similarities in body weight, food intake, and energy expenditure (109). Further, DIO mice administered daily physiological amounts of recombinant CTRP3 for five consecutive days exhibit reduced hepatic triglyceride content, confirming the direct action of CTRP3 in the liver (109). Mechanistically, CTRP3 reduces hepatic triglyceride content by inhibiting the expression of enzymes involved in triglyceride synthesis (GPATs, AGPATs, and DGATs) (109); it has no appreciable effect on hepatic fat oxidation, unlike adiponectin (110). Adiponectin also improves alcoholic and non-alcoholic fatty liver phenotypes, although triglyceride synthesis enzymes were not examined (110).
Given the importance of chronic inflammation in obesity (111), it is interesting to note that CTRP3 may also modulate metabolism indirectly through anti-inflammatory processes (45). Recombinant CTRP3 reduces lipopolysaccharide-induced inflammation in monocytes (112), adipocytes (113) and colonic fibroblasts (114); however, the physiological relevance of these in vitro studies awaits in vivo confirmation.
CTRP3 also has non-metabolic functions in the vasculature and heart. Recombinant CTRP3 induces endothelial cell proliferation and migration in vitro by activating ERK1/2 and p38 MAPK signaling (115), implying a potential role of CTRP3 in regulating angiogenesis. Myocardial infarction induced by coronary artery occlusion substantially reduces CTRP3 expression in adipose tissue and in circulation in mice, although reconstitution of CTRP3 expression significantly restores cardiac function and survival rates (116). CTRP3 activation of Akt, but not AMPK, signaling attenuates cardiomyocyte apoptosis, suppresses interstitial fibrosis, and increases revascularization following myocardial infarction (116). Together, these studies highlight a novel and important role for CTRP3 in modulating metabolic, immune, and cardiovascular functions.
CTRP5
CTRP5 is widely expressed, with highest levels detected in the eye and adipose tissue (29). Prolonged mitochondrial depletion induces CTRP5 expression in rat L6 myocytes (117). Further, expression of recombinant GST-tagged CTRP5 or an un-tagged C-terminal globular head stimulates AMPK signaling to translocate GLUT4 and enhances fat oxidation via the AMPK-ACC pathway in vitro (117). These studies imply an autocrine function for CTRP5 in muscle in response to reduced mitochondrial content. CTRP5 is also expressed by cultured mouse and human adipocytes and circulates in human serum (84). Saturated fatty acids upregulate CTRP5 expression in adipocytes, where CTRP5 acts in an autocrine fashion to reduce adiponectin and resistin secretion (84). While the physiological relevance of CTRP5 in metabolism remains uncertain, a point mutation (S163R) impairs folding and secretion of human CTRP5 to cause an autosomal dominant form of late-onset retinal macular degeneration (L-ORD) (118–121). S163R knock-in mouse models of L-ORD have yielded conflicting results—one model successfully recapitulates the retinal degeneration phenotype (122), while another does not (123).
CTRP9
CTRP9 is the closest paralog of adiponectin and shares the highest degree of amino acid identity (54%) at the globular domain. Adipose predominantly expresses CTRP9 (47). In leptin-deficient obese ob/ob mice, CTRP9 expression is elevated in adipose tissue and in circulation at 8 weeks of age relative to lean controls, but subsequently normalizes by 12 weeks of age (47). This observation suggests a possible compensatory response in young ob/ob mice prior to developing severe obesity and insulin resistance. Concordantly, circulating CTRP9 levels are significantly reduced in DIO mice, a model more closely resembling the common form of human obesity induced by excess caloric intake (Peterson and Wong, unpublished data). A modest (~40%) increase in circulating CTRP9 levels by adenoviral-mediated expression acutely reduces serum glucose levels in ob/ob mice without altering body weight and plasma insulin levels (47). In transgenic mouse models where circulating CTRP9 levels are chronically elevated, animals have reduced caloric intake and increased metabolic rate in response to high-fat feeding (124). Consequently, CTRP9 transgenic mice are strikingly resistant to weight gain when challenged with a high-fat diet for 12 weeks (124). The observed reduction in adiposity is due to chronic activation of AMPK in the skeletal muscle, increased mitochondrial content and up-regulated expression of genes that mediate fat oxidation. As expected, lean CTRP9 transgenic mice fed a high-fat diet exhibit improved systemic insulin sensitivity and fail to develop hepatic steatosis (124). These observations suggest CTRP9 enhances fat oxidation in an AMPK-dependent manner.
The metabolic roles of CTRP9 functionally overlap with those of adiponectin. Adiponectin, in particular its globular domain, enhances skeletal muscle fat oxidation by activating the AMPK signaling pathway (107, 125, 126). Over-expression of adiponectin also improves insulin function in rat skeletal muscle (127); however, other studies concluded that liver, not skeletal muscle, is the predominant adiponectin target in vivo (106, 128). It remains to be confirmed, using skeletal muscle-specific adiponectin receptor KO mice, whether adiponectin acts directly on skeletal muscle to exert some of its whole-body metabolic effects (129).
CTRP9 also controls cardiac and endothelial cell functions. In human umbilical vein endothelial cells and in freshly isolated vascular rings, recombinant CTRP9 increases nitric oxide production to induce vasodilatation through the AMPK/eNOS pathway (130). CTRP9 also benefits cardiac function in response to stress. Overexpression of CTRP9 reduces myocardial infarct size and hypoxia-induced apoptosis of cardiac myocytes following myocardial ischemia/reperfusion through AMPK signaling (131). Expression of only the C-terminal globular domain of CTRP9 also attenuates oxidative stress and myocardial infarct size induced by ischemia/reperfusion injury and enhances cardiac output in DIO mice (132). In another model of femoral artery injury, adenoviral CTRP9 overexpression substantially reduces neointimal formation by suppressing vascular smooth muscle cell proliferation and migration through the cAMP/PKA/ERK pathway (133). Since obesity and insulin resistance are risk factors for vascular and cardiac dysfunction (134–136), these studies highlight the potential protective roles of CTRP9 in the heart in response to stress.
CTRP11
CTRP11 is predominantly expressed by white and brown adipose tissues (52). Sequence alignment indicates a striking degree of amino acid identity between CTRP11 of different vertebrate species (52). Within white adipose tissue, CTRP11 is primarily produced by stromal vascular cells (52). CTRP11 expression is acutely regulated by changes in metabolic state, as overnight fasting followed by re-feeding upregulates expression (52). The lack of a CTRP11-specific antibody precludes analysis of the endogenous protein; hence, it remains uncertain whether CTRP11 also circulates in blood. Functional studies suggest a role for CTRP11 in regulating adipogenesis (52). CTRP11 suppresses 3T3-L1 mouse cell differentiation into mature adipocytes by inhibiting the expression of PPAR-γ and C/EBP-α, two major transcriptional regulators that drive adipogenesis (137–139). Further, CTRP11 inhibits mitotic clonal expansion of 3T3-L1 pre-adipocytes (52), a process essential for adipocyte differentiation in culture (140). Suppression of adipogenesis is specific, as expression of other structurally-related proteins such as CTRP1, CTRP10, and adiponectin do not influence 3T3-L1 differentiation. These in vitro studies indicate that CTRP11 likely acts in a paracrine manner to mediate crosstalk between adipocytes and cells of the stromal vascular compartment to maintain adipose tissue homeostasis (52).
CTRP12
Sequence alignments between CTRP12 and other members of the C1q family indicate only modest sequence identity at the globular C1q domain (50, 54). Human adipose tissue predominantly expresses CTRP12, although expression is more widespread in mice (50). Unlike other CTRPs, endogenous CTRP12 exists in two differently sized isoforms—a full-length and a cleaved globular isoform. Furin/PCSK3, a member of the proprotein convertase family, cleaves CTRP12 at Lys-91 within the N-terminal KKXR motif (75). The two CTRP12 isoforms differ in oligomeric structure and signaling specificity; full-length protein preferentially activates Akt signaling, whereas the globular gCTRP12 isoform preferentially activates p44/42-MAPK and p38-MAPK signaling (75). While CTRP12 expression in the adipose tissue of DIO mice is significantly reduced (50, 54), its expression in cultured adipocytes is acutely induced by insulin or rosiglitazone treatment (50, 75). Cleavage of CTRP12 appears to be enhanced in DIO mice (141).
A modest increase in circulating CTRP12 levels by adenoviral expression or recombinant protein administration is sufficient to lower blood glucose and improve insulin sensitivity in three different mouse models—lean wild-type, DIO, and leptin-deficient ob/ob mice (50). These metabolic improvements are mediated by enhanced insulin signaling in the liver and adipose tissue, but not in skeletal muscle (50). Independent of insulin, recombinant CTRP12 suppresses gluconeogenesis in cultured hepatocytes and promotes glucose uptake in adipocytes. Thus, CTRP12 improves metabolic function through both insulin-dependent and -independent mechanisms. Adipose tissue inflammation is dampened in obese mice overexpressing CTRP12, suggesting the anti-inflammatory action of CTRP12 is responsible for whole-body insulin sensitivity improvements (54). In contrast, adenoviral overexpression of CTRP12 does not alter adipose tissue histomorphology or inflammatory gene expression (e.g., IL-1β, IL-6, TNF-α) despite remarkable improvements in systemic insulin sensitivity and glucose homeostasis (50). CTRP12 is thus a novel adipokine with beneficial anti-diabetic properties, although future studies using loss-of-function mouse models will help clarify its mechanism of action.
CTRP13
CTRP13 is preferentially expressed in adipose and brain tissues of mice and in adipose tissue of humans (51). Within mouse adipose tissue, CTRP13 is also mainly produced by cells of the stromal vascular compartment. In cultured adipocytes, myotubes, and hepatocytes, recombinant CTRP13 activates AMPK signaling to promote glucose uptake. CTRP13 also partially reverses lipid-induced insulin resistance in hepatocytes by suppressing SAPK/JNK stress signaling, which impairs insulin signaling (51). Further, CTRP13 activates AMPK signaling to reduce gluconeogenesis in H4IIE hepatocytes by inhibiting gluconeogenic enzymes G6Pase and PEPCK. However, the physiological relevance of CTRP13 in peripheral tissues remains to be determined in vivo.
In the brain, CTRP13 modulates food intake as an anorexigenic factor (142). Wild-type mice given restricted access to food downregulate hypothalamic CTRP13 expression, whereas this expression is upregulated in similarly-treated DIO mice. Further, recombinant CTRP13 administered via intracerebroventricular cannulae suppresses food intake and reduces body weight in mice (142). Interestingly, CTRP13 and the orexigenic neuropeptide AgRP reciprocally regulate each other’s expression in the hypothalamus; central CTRP13 delivery suppresses Agrp expression, while AgRP delivery increases Ctrp13 expression. Food-restricted mice have reduced CTRP13 and increased orexigenic neuropeptide (NPY and AgRP) expression in the hypothalamus. In contrast, when food restriction is coupled to enhanced physical activity in an activity-based anorexia mouse model, hypothalamic expression of both CTRP13 and AgRP increases (142). These results suggest that CTRP13 and AgRP form a hypothalamic feedback loop to modulate food intake and that this neural circuit may be disrupted in anorexia.
CTRP15/myonectin
Unlike other CTRPs, CTRP15 is a myokine expressed predominantly by mouse and human skeletal muscle (53). The term myonectin was used for CTRP5 in a recent study (143). To prevent confusion in nomenclature, CTRP5 retains its original designation (36, 117, 118), while CTRP15 is now referred to as myonectin (53). Expression and circulating levels of CTRP15/myonectin are upregulated upon re-feeding after fasting and significantly downregulated in DIO mice (53). Interestingly, CTRP15/myonectin expression is differentially regulated in different muscle fiber types. At basal levels, CTRP15/myonectin is expressed more in oxidative slow-twitch fibers compared to fast-twitch glycolytic fibers. Re-feeding after fasting increases CTRP15/myonectin expression ~80-fold in slow-twitch fibers but only ~4-fold in fast-twitch fibers (53). Administration of glucose or lipid after overnight fasting increased serum CTRP15/myonectin levels four-fold; likewise, nutrient-starved myotubes upregulated CTRP15/myonectin expression similarly to glucose or fatty acid supplementation. These observations suggest that CTRP15/myonectin is a postprandial hormone produced and secreted by skeletal muscle in response to nutrient flux. Consistent with this notion, administration of recombinant CTRP15/myonectin to mice significantly lowered circulating free fatty acids by promoting hepatocyte uptake through upregulation of CD36, fatty acid binding proteins, and fatty acid transport proteins (53).
CTRP receptors
Three adiponectin receptors—adipoR1, adipoR2, and T-cadherin—have been identified by expression cloning strategies (144, 145). An additional distinct macrophage receptor for adiponectin is thought to exist, although this receptor remains elusive (146). In addition, the calreticulin/CD91 complex may bind adiponectin on the plasma membrane of macrophages to facilitate the removal of apoptotic cells (147). Since T-cadherin is a GPI-anchored plasma membrane protein (148), it is unclear how T-cadherin transduces a signal in response to adiponectin binding. Nonetheless, KO mice demonstrate that T-cadherin largely mediates the cardioprotective effects of adiponectin (149). AdipoR1 and adipoR2 contain seven transmembrane domains with inverse GPCR topologies. APPL1 is an intracellular adaptor protein that couples adiponectin binding to adipoR1/2 to intracellular signal transduction (150–153). Mice lacking adipoR1 or adipoR2 show variable and opposing phenotypes (154–156). In general, the metabolic phenotypes of adipoR1/2 KO mice are more striking than the relatively mild metabolic phenotypes of adiponectin KO mice. This complex story is ongoing (20, 157).
No CTRP receptors have been definitively identified. AdipoR1 may partially mediate the effects of CTRP9 on vascular endothelial cells (130) and cardiomyocytes (131), although these studies relied entirely on RNA interference approaches. No evidence demonstrates that CTRP9 interacts with adipoR1 on the plasma membrane of intact cells. FACS analysis can monitor receptor-ligand interactions on live cells, suggesting these experiments are feasible. Since adipoR1 and adipoR2 belong to the 11-member PAQR family of transmembrane proteins (158), it is tempting to speculate that other PAQR family members may be CTRP receptors.
Concluding remarks
Recent studies have provided insight into the metabolic roles of CTRP proteins. Although much has been learned since CTRPs were initially described, many more questions remain to be addressed. CTRPs possess unique and shared functions supported by a high degree of vertebrate conservation. Future studies with gain-of-function and loss-of-function mouse models will reveal novel insights into the physiological function, mechanism of action, and possible redundancy of each CTRP in normal and disease states. Going forward, identifying CTRP receptors will remain a major challenge but will provide enormous insights into the signaling pathways controlled by each CTRP to mediate its unique biological function.
Acknowledgment
G.W.W. is supported by grants from the National Institute of Health (DK084171) and the American Heart Association (SDG2260721).
Abbreviations
- ACC
acetyl Co-A carboxylase
- AgRP
agouti-related protein
- AMPK
AMP-activated protein kinase
- CTRP
C1q/TNF-related protein
- C/EBP-α
CCAAT/enhancer binding protein alpha
- DIO
diet-induced obese
- Erk1/2
extracellular signal-regulated protein kinases 1 and 2
- eNOS
endothelial nitric oxide synthase
- FACS
fluorescent activated cell sorter
- GLUT4
glucose transporter 4
- G6Pase
glucose-6-phosphatase
- GPCR
G-protein coupled receptor
- GPI
Glycosylphosphatidylinositol
- JNK
c-Jun N-terminal kinase
- KO
knock-out
- LKB1
liver kinase B1
- L-ORD
late-onset retinal macular degeneration
- MAPK
mitogen activated protein kinase
- NPY
neuropeptide Y
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- PCSK
proprotein convertase subtilisin/kexin
- PEPCK
phosphoenolpyruvate carboxykinase
- SAPK
stress-activated protein kinase
Footnotes
Competing Interests
The authors have declared that no competing interests exist.
References
- 1.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 6.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 9.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 13.Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. Increased beta -oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 14.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 15.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 16.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 17.Nawrocki AR, Hofmann SM, Teupser D, Basford JE, Durand JL, Jelicks LA, Woo CW, Kuriakose G, Factor SM, Tanowitz HB, Hui DY, Tabas I, Scherer PE. Lack of association between adiponectin levels and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2010;30:1159–1165. doi: 10.1161/ATVBAHA.109.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano W, Kubota N, Itoh S, Kubota T, Awazawa M, Moroi M, Sugi K, Takamoto I, Ogata H, Tokuyama K, Noda T, Terauchi Y, Ueki K, Kadowaki T. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr J. 2008;55:515–522. doi: 10.1507/endocrj.k08e-093. [DOI] [PubMed] [Google Scholar]
- 19.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 20.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tullin S, Sams A, Brandt J, Dahl K, Gong W, Jeppesen CB, Krogh TN, Olsen GS, Liu Y, Pedersen AA, Petersen JM, Rolin B, Wahlund PO, Kalthoff C. Recombinant adiponectin does not lower plasma glucose in animal models of type 2 diabetes. PLoS One. 2012;7:e44270. doi: 10.1371/journal.pone.0044270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 23.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, Neumann E, Muller-Ladner U. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 25.Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G. Adiponectin Deficiency Protects Mice From Chemically Induced Colonic Inflammation. Gastroenterology. 2006;132:601–614. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Pini M, Sennello JA, Chan L, Fantuzzi G. Adiponectin deficiency does not affect the inflammatory response to endotoxin or concanavalin a in mice. Endocrinology. 2006;147:5019–5022. doi: 10.1210/en.2006-0855. [DOI] [PubMed] [Google Scholar]
- 27.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landskroner-Eiger S, Qian B, Muise ES, Nawrocki AR, Berger JP, Fine EJ, Koba W, Deng Y, Pollard JW, Scherer PE. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009;15:3265–3276. doi: 10.1158/1078-0432.CCR-08-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J. 2008;416:161–177. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis KE, Scherer PE. Adiponectin: no longer the lone soul in the fight against insulin resistance? Biochem J. 2008;416:e7–e9. doi: 10.1042/BJ20082033. [DOI] [PubMed] [Google Scholar]
- 31.Kishore U, Gaboriaud C, Waters P, Shrive AK, Greenhough TJ, Reid KB, Sim RB, Arlaud GJ. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 2004;25:551–561. doi: 10.1016/j.it.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Tom Tang Y, Hu T, Arterburn M, Boyle B, Bright JM, Palencia S, Emtage PC, Funk WD. The complete complement of C1q-domain-containing proteins in Homo sapiens. Genomics. 2005;86:100–111. doi: 10.1016/j.ygeno.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 34.Colombatti A, Spessotto P, Doliana R, Mongiat M, Bressan GM, Esposito G. The EMILIN/Multimerin family. Front Immunol. 2011;2:93. doi: 10.3389/fimmu.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombatti A, Doliana R, Bot S, Canton A, Mongiat M, Mungiguerra G, Paron-Cilli S, Spessotto P. The EMILIN protein family. Matrix Biol. 2000;19:289–301. doi: 10.1016/s0945-053x(00)00074-3. [DOI] [PubMed] [Google Scholar]
- 36.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101:10302–10307. doi: 10.1073/pnas.0403760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuzaki M. Cbln and C1q family proteins: new transneuronal cytokines. Cell Mol Life Sci. 2008;65:1698–1705. doi: 10.1007/s00018-008-7550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 39.Deans MR, Peterson JM, Wong GW. Mammalian Otolin: a multimeric glycoprotein specific to the inner ear that interacts with otoconial matrix protein Otoconin-90 and Cerebellin-1. PLoS One. 2010;5:e12765. doi: 10.1371/journal.pone.0012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berube NG, Swanson XH, Bertram MJ, Kittle JD, Didenko V, Baskin DS, Smith JR, Pereira-Smith OM. Cloning and characterization of CRF a novel C1q-related factor, expressed in areas of the brain involved in motor function. Brain Res Mol Brain Res. 1999;63:233–240. doi: 10.1016/s0169-328x(98)00278-2. [DOI] [PubMed] [Google Scholar]
- 41.Aerbajinai W, Lee YT, Wojda U, Barr VA, Miller JL. Cloning and characterization of a gene expressed during terminal differentiation that encodes a novel inhibitor of growth. J Biol Chem. 2004;279:1916–1921. doi: 10.1074/jbc.M305634200. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi N, Benya PD, van der Rest M, Ninomiya Y. The cloning and sequencing of alpha 1(VIII) collagen cDNAs demonstrate that type VIII collagen is a short chain collagen and contains triple-helical and carboxyl-terminal non-triple-helical domains similar to those of type X collagen. J Biol Chem. 1989;264:16022–16029. [PubMed] [Google Scholar]
- 43.Muragaki Y, Jacenko O, Apte S, Mattei MG, Ninomiya Y, Olsen BR. The alpha 2(VIII) collagen gene. A novel member of the short chain collagen family located on the human chromosome 1. J Biol Chem. 1991;266:7721–7727. [PubMed] [Google Scholar]
- 44.Thomas JT, Cresswell CJ, Rash B, Nicolai H, Jones T, Solomon E, Grant ME, Boot-Handford RP. The human collagen X gene. Complete primary translated sequence and chromosomal localization. Biochem J. 1991;280(Pt 3):617–623. doi: 10.1042/bj2800617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaffler A, Buechler C. CTRP family: linking immunity to metabolism. Trends Endocrinol Metab. 2012;23:194–204. doi: 10.1016/j.tem.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Yuzaki M. Synapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizers. Eur J Neurosci. 2010;32:191–197. doi: 10.1111/j.1460-9568.2010.07346.x. [DOI] [PubMed] [Google Scholar]
- 47.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23:241–258. doi: 10.1096/fj.08-114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson JM, Aja S, Wei Z, Wong GW. C1q/TNF-related protein-1 (CTRP1) enhances fatty acid oxidation via AMPK activation and ACC inhibition. J Biol Chem. 2012;287:1576–1587. doi: 10.1074/jbc.M111.278333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson JM, Wei Z, Wong GW. C1q/TNF-related Protein-3 (CTRP3), a Novel Adipokine That Regulates Hepatic Glucose Output. J Biol Chem. 2010;285:39691–39701. doi: 10.1074/jbc.M110.180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012;287:10301–10315. doi: 10.1074/jbc.M111.303651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem. 2011;286:15652–15665. doi: 10.1074/jbc.M110.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/Tumor Necrosis Factor-related Protein 11 (CTRP11), a Novel Adipose Stroma-derived Regulator of Adipogenesis. J Biol Chem. 2013;288:10214–10229. doi: 10.1074/jbc.M113.458711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N. Adipolin/C1qdc2/CTRP12 functions as an adipokine that improves glucose metabolism. J Biol Chem. 2011;286:34552–34558. doi: 10.1074/jbc.M111.277319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson JM, Wei Z, Wong GW. CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem Biophys Res Commun. 2009;388:360–365. doi: 10.1016/j.bbrc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 57.Bogin O, Kvansakul M, Rom E, Singer J, Yayon A, Hohenester E. Insight into Schmid metaphyseal chondrodysplasia from the crystal structure of the collagen X NC1 domain trimer. Structure. 2002;10:165–173. doi: 10.1016/s0969-2126(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 58.Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, Verger D, Fontecilla-Camps JC, Arlaud GJ. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003;278:46974–46982. doi: 10.1074/jbc.M307764200. [DOI] [PubMed] [Google Scholar]
- 59.Kvansakul M, Bogin O, Hohenester E, Yayon A. Crystal structure of the collagen alpha1(VIII) NC1 trimer. Matrix Biol. 2003;22:145–152. doi: 10.1016/s0945-053x(02)00119-1. [DOI] [PubMed] [Google Scholar]
- 60.Rety S, Salamitou S, Garcia-Verdugo I, Hulmes DJ, Le Hegarat F, Chaby R, Lewit-Bentley A. The crystal structure of the Bacillus anthracis spore surface protein BclA shows remarkable similarity to mammalian proteins. J Biol Chem. 2005;280:43073–43078. doi: 10.1074/jbc.M510087200. [DOI] [PubMed] [Google Scholar]
- 61.Tu X, Palczewski K. Crystal structure of the globular domain of C1QTNF5: Implications for late-onset retinal macular degeneration. J Struct Biol. 2012;180:439–446. doi: 10.1016/j.jsb.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 63.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki S, Wilson-Kubalek EM, Wert D, Tsao TS, Lee DH. The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 2007;581:809–814. doi: 10.1016/j.febslet.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Briggs DB, Jones CM, Mashalidis EH, Nunez M, Hausrath AC, Wysocki VH, Tsao TS. Disulfide-dependent self-assembly of adiponectin octadecamers from trimers and presence of stable octadecameric adiponectin lacking disulfide bonds in vitro. Biochemistry. 2009;48:12345–12357. doi: 10.1021/bi9015555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 67.Radjainia M, Wang Y, Mitra AK. Structural polymorphism of oligomeric adiponectin visualized by electron microscopy. J Mol Biol. 2008;381:419–430. doi: 10.1016/j.jmb.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proc Natl Acad Sci U S A. 2008;105:18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 73.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, Inukai T, Nakano Y. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–1960. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- 74.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 75.Wei Z, Lei X, Seldin MM, Wong GW. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem. 2012;287:35804–35814. doi: 10.1074/jbc.M112.365965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–19529. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, Hoo RC, Mak WW, Cooper GJ, Xu A. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 78.Richards AA, Stephens T, Charlton HK, Jones A, Macdonald GA, Prins JB, Whitehead JP. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol. 2006;20:1673–1687. doi: 10.1210/me.2005-0390. [DOI] [PubMed] [Google Scholar]
- 79.Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J Biol Chem. 2001;276:28849–28856. doi: 10.1074/jbc.M104148200. [DOI] [PubMed] [Google Scholar]
- 80.Peake PW, Hughes JT, Shen Y, Charlesworth JA. Glycosylation of human adiponectin affects its conformation and stability. J Mol Endocrinol. 2007;39:45–52. doi: 10.1677/JME-07-0030. [DOI] [PubMed] [Google Scholar]
- 81.Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, Lee KW, Nam MS, Park YS, Woo JT, Kim YS, Choi DS, Youn BS, Baik SH. C1q/TNF-related protein-3 (CTRP-) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes. 2012;61:2932–2936. doi: 10.2337/db12-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Choi DS, Baik SH, Bluher M, Youn BS, Choi KM. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One. 2013;8:e55744. doi: 10.1371/journal.pone.0055744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chalupova L, Zakovska A, Adamcova K. Development of a novel enzyme-linked immunosorbent assay (ELISA) for measurement of serum CTRP1: a pilot study: measurement of serum CTRP1 in healthy donors and patients with metabolic syndrome. Clin Biochem. 2013;46:73–78. doi: 10.1016/j.clinbiochem.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Schmid A, Kopp A, Aslanidis C, Wabitsch M, Muller M, Schaffler A. Regulation and Function of C1Q/TNF-related Protein-5 (CTRP-5) in the Context of Adipocyte Biology. Exp Clin Endocrinol Diabetes. 2013;121:310–317. doi: 10.1055/s-0032-1333299. [DOI] [PubMed] [Google Scholar]
- 85.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44:3–15. doi: 10.1007/s001250051573. [DOI] [PubMed] [Google Scholar]
- 86.O'Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005;307:370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 87.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 88.Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, Stannard B, Dietz KR, Le Roith D, Reitman ML. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–3264. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 89.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, Matsuda M, Kondo H, Furuyama N, Kihara S, Nakamura T, Tochino Y, Funahashi T, Matsuzawa Y. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 90.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, Zhang J, Chen B, Lam MC, Tse C, Cooper GJ, Lam KS. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 91.Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- 92.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 93.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 94.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 95.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 96.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Plasma adiponectin levels in overweight and obese Asians. Obes Res. 2002;10:1104–1110. doi: 10.1038/oby.2002.150. [DOI] [PubMed] [Google Scholar]
- 97.Asayama K, Hayashibe H, Dobashi K, Uchida N, Nakane T, Kodera K, Shirahata A, Taniyama M. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes Res. 2003;11:1072–1079. doi: 10.1038/oby.2003.147. [DOI] [PubMed] [Google Scholar]
- 98.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Maeda T, Abe M, Kurisu K, Jikko A, Furukawa S. Molecular cloning and characterization of a novel gene, CORS26, encoding a putative secretory protein and its possible involvement in skeletal development. J Biol Chem. 2001;276:3628–3634. doi: 10.1074/jbc.M007898200. [DOI] [PubMed] [Google Scholar]
- 100.Schaffler A, Ehling A, Neumann E, Herfarth H, Paul G, Tarner I, Gay S, Scholmerich J, Muller-Ladner U. Genomic organization, promoter, amino acid sequence, chromosomal localization, and expression of the human gene for CORS-26 (collagenous repeat-containing sequence of 26-kDa protein) Biochim Biophys Acta. 2003;1630:123–129. doi: 10.1016/j.bbaexp.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Schmid A, Kopp A, Hanses F, Bala M, Muller M, Schaffler A. The novel adipokine C1q/TNF-related protein-3 is expressed in human adipocytes and regulated by metabolic and infection-related parameters. Exp Clin Endocrinol Diabetes. 2012;120:611–617. doi: 10.1055/s-0032-1323803. [DOI] [PubMed] [Google Scholar]
- 102.Schaffler A, Weigert J, Neumeier M, Scholmerich J, Buechler C. Regulation and function of collagenous repeat containing sequence of 26-kDa protein gene product"cartonectin". Obesity (Silver Spring) 2007;15:303–313. doi: 10.1038/oby.2007.566. [DOI] [PubMed] [Google Scholar]
- 103.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Gay S, Scholmerich J, Muller-Ladner U. Genomic organization, chromosomal localization and adipocytic expression of the murine gene for CORS-26 (collagenous repeat-containing sequence of 26 kDa protein) Biochim Biophys Acta. 2003;1628:64–70. doi: 10.1016/s0167-4781(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 104.Yokohama-Tamaki T, Maeda T, Tanaka TS, Shibata S. Functional analysis of CTRP3/cartducin in Meckel's cartilage and developing condylar cartilage in the fetal mouse mandible. J Anat. 2011;218:517–533. doi: 10.1111/j.1469-7580.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maeda T, Jikko A, Abe M, Yokohama-Tamaki T, Akiyama H, Furukawa S, Takigawa M, Wakisaka S. Cartducin, a paralog of Acrp30/adiponectin, is induced during chondrogenic differentiation and promotes proliferation of chondrogenic precursors and chondrocytes. J Cell Physiol. 2006;206:537–544. doi: 10.1002/jcp.20493. [DOI] [PubMed] [Google Scholar]
- 106.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 107.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 108.Miller RA, Chu Q, Le Lay J, Scherer PE, Ahima RS, Kaestner KH, Foretz M, Viollet B, Birnbaum MJ. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest. 2011;121:2518–2528. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol. 2013 doi: 10.1152/ajpgi.00102.2013. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 112.Weigert J, Neumeier M, Schaffler A, Fleck M, Scholmerich J, Schutz C, Buechler C. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 2005;579:5565–5570. doi: 10.1016/j.febslet.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 113.Kopp A, Bala M, Buechler C, Falk W, Gross P, Neumeier M, Scholmerich J, Schaffler A. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology. 2010;151:5267–5278. doi: 10.1210/en.2010-0571. [DOI] [PubMed] [Google Scholar]
- 114.Hofmann C, Chen N, Obermeier F, Paul G, Buchler C, Kopp A, Falk W, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis. 2011;17:2462–2471. doi: 10.1002/ibd.21647. [DOI] [PubMed] [Google Scholar]
- 115.Akiyama H, Furukawa S, Wakisaka S, Maeda T. CTRP3/cartducin promotes proliferation and migration of endothelial cells. Mol Cell Biochem. 2007;304:243–248. doi: 10.1007/s11010-007-9506-6. [DOI] [PubMed] [Google Scholar]
- 116.Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 2012;125:3159–3169. doi: 10.1161/CIRCULATIONAHA.112.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park SY, Choi JH, Ryu HS, Pak YK, Park KS, Lee HK, Lee W. C1q tumor necrosis factor alpha-related protein isoform 5 is increased in mitochondrial DNA-depleted myocytes and activates AMP-activated protein kinase. J Biol Chem. 2009;284:27780–27789. doi: 10.1074/jbc.M109.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hayward C, Shu X, Cideciyan AV, Lennon A, Barran P, Zareparsi S, Sawyer L, Hendry G, Dhillon B, Milam AH, Luthert PJ, Swaroop A, Hastie ND, Jacobson SG, Wright AF. Mutation in a short-chain collagen gene, CTRP5, results in extracellular deposit formation in late-onset retinal degeneration: a genetic model for age-related macular degeneration. Hum Mol Genet. 2003;12:2657–2667. doi: 10.1093/hmg/ddg289. [DOI] [PubMed] [Google Scholar]
- 119.Ayyagari R, Mandal MN, Karoukis AJ, Chen L, McLaren NC, Lichter M, Wong DT, Hitchcock PF, Caruso RC, Moroi SE, Maumenee IH, Sieving PA. Late-onset macular degeneration and long anterior lens zonules result from a CTRP5 gene mutation. Invest Ophthalmol Vis Sci. 2005;46:3363–3371. doi: 10.1167/iovs.05-0159. [DOI] [PubMed] [Google Scholar]
- 120.Shu X, Tulloch B, Lennon A, Vlachantoni D, Zhou X, Hayward C, Wright AF. Disease mechanisms in late-onset retinal macular degeneration associated with mutation in C1QTNF5. Hum Mol Genet. 2006;15:1680–1689. doi: 10.1093/hmg/ddl091. [DOI] [PubMed] [Google Scholar]
- 121.Mandal MN, Vasireddy V, Reddy GB, Wang X, Moroi SE, Pattnaik BR, Hughes BA, Heckenlively JR, Hitchcock PF, Jablonski MM, Ayyagari R. CTRP5 is a membrane-associated and secretory protein in the RPE and ciliary body and the S163R mutation of CTRP5 impairs its secretion. Invest Ophthalmol Vis Sci. 2006;47:5505–5513. doi: 10.1167/iovs.06-0312. [DOI] [PubMed] [Google Scholar]
- 122.Chavali VR, Khan NW, Cukras CA, Bartsch DU, Jablonski MM, Ayyagari R. A CTRP5 gene S163R mutation knock-in mouse model for late-onset retinal degeneration. Hum Mol Genet. 2011;20:2000–2014. doi: 10.1093/hmg/ddr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shu X, Luhmann UF, Aleman TS, Barker SE, Lennon A, Tulloch B, Chen M, Xu H, Jacobson SG, Ali R, Wright AF. Characterisation of a C1qtnf5 Ser163Arg knock-in mouse model of late-onset retinal macular degeneration. PLoS One. 2011;6:e27433. doi: 10.1371/journal.pone.0027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2013 doi: 10.1152/ajpregu.00110.2013. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Satoh H, Nguyen MT, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM. Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes. 2005;54:1304–1313. doi: 10.2337/diabetes.54.5.1304. [DOI] [PubMed] [Google Scholar]
- 128.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karpe F. Insulin resistance by adiponectin deficiency: is the action in skeletal muscle? Diabetes. 2013;62:701–702. doi: 10.2337/db12-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, Sun Y, Lopez BL, Christopher TA, Peterson JM, Wong GW, Yu S, Yi D, Ma XL. C1q/TNF-Related Proteins, A Family of Novel Adipokines, Induce Vascular Relaxation Through the Adiponectin Receptor-1/AMPK/eNOS/Nitric Oxide Signaling Pathway. Arterioscler Thromb Vasc Biol. 2011;31:2616–2623. doi: 10.1161/ATVBAHA.111.231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kambara T, Ohashi K, Shibata R, Ogura Y, Maruyama S, Enomoto T, Uemura Y, Shimizu Y, Yuasa D, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. CTRP9 protein protects against myocardial injury following ischemia-reperfusion through AMP-activated protein kinase (AMPK)-dependent mechanism. J Biol Chem. 2012;287:18965–18973. doi: 10.1074/jbc.M112.357939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Su H, Yuan Y, Wang XM, Lau WB, Wang Y, Wang X, Gao E, Koch WJ, Ma XL. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFalpha-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res Cardiol. 2013;108:315–326. doi: 10.1007/s00395-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, Miyabe M, Kataoka Y, Murohara T, Ouchi N. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27:25–33. doi: 10.1096/fj.12-213744. [DOI] [PubMed] [Google Scholar]
- 134.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 135.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 136.Lamarche B, Lemieux S, Dagenais GR, Despres JP. Visceral obesity and the risk of ischaemic heart disease: insights from the Quebec Cardiovascular Study. Growth Horm IGF Res. 1998;8(Suppl B):1–8. doi: 10.1016/s1096-6374(98)80018-x. [DOI] [PubMed] [Google Scholar]
- 137.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 138.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 140.Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Enomoto T, Shibata R, Ohashi K, Kambara T, Kataoka Y, Uemura Y, Yuasa D, Murohara T, Ouchi N. Regulation of adipolin/CTRP12 cleavage by obesity. Biochem Biophys Res Commun. 2012;428:155–159. doi: 10.1016/j.bbrc.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 142.Byerly MS, Swanson R, Wei Z, Seldin MM, McCulloh PS, Wong GW. A Central Role for C1q/TNF-Related Protein 13 (CTRP13) in Modulating Food Intake and Body Weight. PLoS One. 2013;8:e62862. doi: 10.1371/journal.pone.0062862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lim S, Choi SH, Koo BK, Kang SM, Yoon JW, Jang HC, Choi SM, Lee MG, Lee W, Shin H, Kim YB, Lee HK, Park KS. Effects of Aerobic Exercise Training on C1q Tumor Necrosis Factor alpha-Related Protein Isoform 5 (Myonectin): Association with Insulin Resistance and Mitochondrial DNA Density in Women. J Clin Endocrinol Metab. 2012;97:E88–E93. doi: 10.1210/jc.2011-1743. [DOI] [PubMed] [Google Scholar]
- 144.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 145.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, Ohsugi M, Takamoto I, Yamashita S, Asahara H, Akira S, Kasuga M, Kadowaki T. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13:401–412. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 147.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ranscht B, Dours-Zimmermann MT. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron. 1991;7:391–402. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- 149.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120:4342–4352. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol. 2006;8:516–523. doi: 10.1038/ncb1404. [DOI] [PubMed] [Google Scholar]
- 151.Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, Liu DD, Torres JM, Jia W, Lechleiter JD, Liu F, Dong LQ. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009;284:22426–22435. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xin X, Zhou L, Reyes CM, Liu F, Dong LQ. APPL1 mediates adiponectin-stimulated p38 MAPK activation by scaffolding the TAK1-MKK3-p38 MAPK pathway. Am J Physiol Endocrinol Metab. 2011;300:E103–E110. doi: 10.1152/ajpendo.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Deepa SS, Zhou L, Ryu J, Wang C, Mao X, Li C, Zhang N, Musi N, DeFronzo RA, Liu F, Dong LQ. APPL1 mediates adiponectin-induced LKB1 cytosolic localization through the PP2A-PKCzeta signaling pathway. Mol Endocrinol. 2011;25:1773–1785. doi: 10.1210/me.2011-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 155.Bjursell M, Ahnmark A, Bohlooly YM, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Linden D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–593. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- 156.Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW, Sullivan JM, Reifel-Miller A. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683–692. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- 157.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 158.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]