Abstract
Biodegradable plates have been used extensively in fracture fixation since the 1960s. They rarely cause stress-protection atrophy or problems requiring secondary plate removal, common complications seen with metallic plates. However, aseptic foreign-body reactions have been reported, sometimes years after the original implantation. Both inadequate polymer degradation and debris accumulation have been implicated as causes. The current generation of commercial biodegradable plates is formulated to minimize this complication by altering the ratio of polylactic and polyglycolic acids. This in vivo study compares the degree of local foreign-body reaction of two commercially available resorbable plates in rabbits. Two types of biodegradable plates were examined: poly(D/L)lactide acid (PDLLA) and polylactide-co-glycolide acid (PLGA). Each plate was placed into a periosteal pericalvarial pocket created beneath the anterior or posterior scalp of a rabbit. Humane killing occurred at 3, 6, and 12 months postoperatively. Foreign-body reaction was evaluated histologically. The PDLLA plates demonstrated marked local foreign-body reactions within the implant capsule as early as 3 months after implantation, with presence of inflammatory cells and granulomatous giant cells in close association with the implant material. All local foreign-body reactions were subclinical with no corresponding tissue swelling requiring drainage. PLGA plates did not demonstrate any signs of inflammatory reactions. In addition, the PLGA plates did not appear to resorb or integrate at 12 months. Neither PDLLA nor PLGA plates demonstrated inflammation of the soft tissue or adjacent bone outside the implant capsule. In our study, the PDLLA plates demonstrated histological evidence of foreign-body reaction that is confined within the implant capsule, which was not seen with the PLGA plates. This finding may be attributable to the lack of significant resorption seen in the PLGA plates. Both PDLLA and PLGA plates were biocompatible with the rabbit tissue environment and should be considered for continued use in craniofacial, maxillofacial, and orthopedic reconstruction.
Keywords: resorbable plates, local foreign-body reaction, local inflammatory reaction, sterile abscess
Biodegradable plates have become a routine method in maxillofacial, craniofacial, and orthopedic reconstruction. These materials provide rigid fixation for bone healing, and degrade as the fractured bone regains strength. These characteristics may prove particularly ideal for the pediatric population, in which bone growth and turnover create potential problems for metallic plates.
Recently, there have been reports of aseptic foreign-body reactions, leading to sterile abscesses at implant fixation sites, sometimes years after the original placement of an implant.1 Previous literature postulated inadequate polymer degradation and debris accumulation as the primary causes.1 2 The main components of biodegradable plates, polylactic isomers, and/or polyglycolic acids (PGA), demonstrate differing inherent degradation properties and rates. The current generation of commercial biodegradable plates is formulated to balance degradation and stability by altering the ratio of these polymeric components. The polymer's ratio determines the mechanism and ultimate rate of degradation. These features may mediate the body's immunological response to this material.2 3 The purpose of this in vivo study is to compare the biocompatibility of two commonly used fixation plates, in an animal model, through the assessment of local foreign-body reactions. Given previous reports of delayed foreign-body reactions with poly-L-lactide materials,4 5 we hypothesize that the poly-lactide-glycolic acid (PLGA) plate, which has the higher ratio of L-lactide, will exhibit more significant foreign-body reactions than the poly(D/L)lactic acid (PDLLA) plates.
Materials and Methods
Two types of bone plates were used for implantation (Table 1). The PLGA plate is a copolymer composed of poly-L-lactide, D-lactide and glycolide in an 85/5/10 molecular ratio (Delta System, Stryker Osteosynthesis, Freiburg, Germany). The PDLLA plate is a copolymer of D and L isomers of lactide, in a 1:1 molecular ratio (Resorb-X System, KLS-Martin, Jacksonville, FL). Both plates were 1.5 × 1.5 cm in width and length. The PDLLA plate had a thickness of 2.0 mm and the PLGA plate had a thickness of 1.4 mm.
Table 1. Chemical composition of each plate used in this study.
| Plate | Chemical (%) | Composition |
|---|---|---|
| Poly(D/L)Lactic acid | 50 | D-lactic acid |
| 50 | L-lactic acid | |
| Poly-lactate-glycolic acid | 5 | D-lactic acid |
| 85 | L-lactic acid | |
| 10 | Glycolic acid |
Animal Study
A total of 15 mature male rabbits were used in this in vivo study. All research was performed in accordance with guidelines detailed by and with approval from the Institutional Animal Care and Use Committee. All procedures were performed under sterile conditions.
The rabbits were anesthetized with inhaled 1% isoflurane in conjunction with intramuscular drug enforcement administration (DEA) Schedule III medications (ketamine 42.8 mg, xylazine 8.2 mg, acepromazine 0.7 mg) at dosage of 0.5 to 0.7 mL/kg. After adequate anesthesia, the dorsum of head was shaved and prepared using chlorhexidine gluconate. Two 1-cm transverse skin incisions were made, one at the anterior aspect of the dorsal head, and one at the posterior aspect of the dorsal head. Tissue was dissected down to the calvarial periosteum, and a periosteal pocket, measuring 1.5 × 1.5 cm, was created at each incision site. A sterile PLGA plate was placed in the anterior pocket, and a sterile PDLLA plate was placed in the posterior pocket. The subcutaneous tissue and skin were then closed using 3–0 Monocryl (Ethicon, Somerville, NJ). At the conclusion of the procedure, 0.5cc of 1% bupivacaine was infused at the surgery site.
Of the 15 animals, 5 animals were humanely killed at each of the three time points—3, 6, and 12 months postoperatively. All animals were euthanized via overdose of beuthanasia (1 mL/4.55 kg, intravenous) under general anesthesia. The hair over the dorsal scalp was then shaved. The two regions of the skull implanted with plates were excised en bloc with overlying soft tissue and skin. The samples were fixed in 4% formaldehyde/1% glutaraldehyde.
Tissue sections were obtained from decalcified, formalin-fixed, paraffin-embedded tissue blocks following standardized tissue processing. The resultant tissue sections were stained with hematoxylin and eosin using a standardized automated system. Digital images were captured and compared among the treatment groups with original magnifications of ×40, ×100, ×200, and ×400. Qualitative assessment of cellular infiltration and plate resorption were based upon previously published criteria (Table 2).6
Table 2. Histological qualitative assessment scale used to classify degree of cellular infiltration and plate resorption.
| Cellular infiltration | + | Few single cells |
| ++ | Small conglomerates of cells | |
| +++ | Large conglomerates of cells | |
| ++ + + | Large confluents with inflammatory cells | |
| Resorption | + | Minor dissolution of edges, cracks, small fragments |
| ++ | Crack with larger fragments | |
| +++ | Several distinctive fragments | |
| ++ + + | Complete fragmentation |
Notes: Created based on De Jong et al. Tissue response to partially in vitro predegraded poly-L-lactide implants.6
Results
Histopathologic features of all specimens underwent qualitative evaluation by a board-certified anatomic pathologist (M.J.H.). Table 3 summarizes these results. All animals recovered uneventfully after implantation. There was no case of swelling, inflammation, or infection at the implantation sites in any of the animals before humane killing.
Table 3. Summary of results comparing PDLLA and PLGA plates.
| Local swelling, infection | Plate grossly visible | Plate grossly integrated | Plate resorption | Reaction within capsule | Reaction outside capsule | |
|---|---|---|---|---|---|---|
| PDLLA | ||||||
| 3 mo | – | + | + | +++ | +++ | – |
| 6 mo | – | – | + | ++ + + | ++ | – |
| 12 mo | – | – | + | ++ + + | ++ | – |
| PLGA | ||||||
| 3 mo | – | + | – | – | – | – |
| 6 mo | – | + | – | – | – | – |
| 12 mo | – | + | – | + | – | – |
Abbreviations: PDLLA, poly(D/L)lactide acid; PLGA, poly-lactide-glycolic acid.
Poly(D/L)Lactic Acid Plate
At 3 months, the PDLLA implants were no longer palpable. Gross examination revealed integration of the implant into the surrounding tissue with notably decreased plate visibility (Fig. 1). Microscopic examination revealed fragmentation of the implant material with local giant-cell reaction within an enclosed dense fibrous capsule, demonstrative of active resorption (Fig. 2). Higher magnification (×400) demonstrated a foreign-body giant cell reaction with inflammatory cell infiltration surrounding the implant fragments, but contained entirely within the capsule surrounding the implant material (Fig. 3). No inflammatory cell infiltration was noted outside the capsule. The scalp soft tissues and calvarial bone were normal microscopically.
Figure 1.
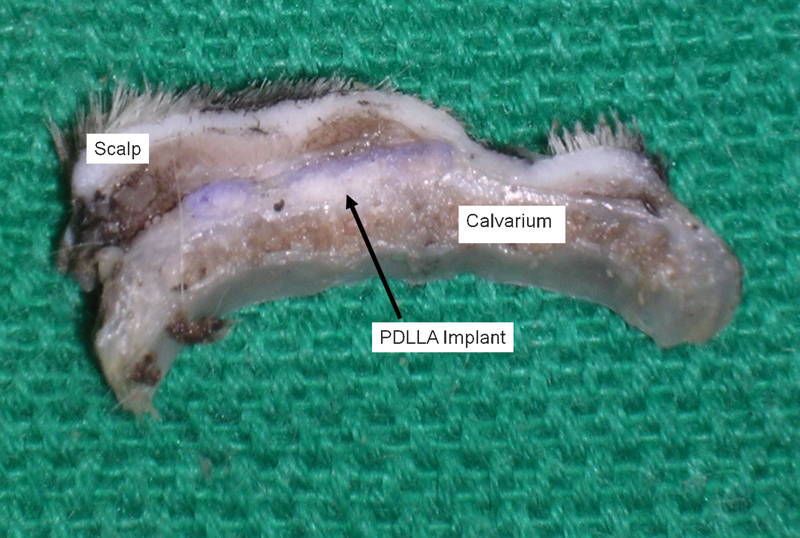
Gross appearance of poly(D/L)lactide acid plate at 3 months postimplantation. The implant was integrated and demonstrated notable degradation.
Figure 2.
Poly(D/L)lactide acid plate at 3 months postimplantation. The implant demonstrated fragmentation and giant cell infiltration into the implant. Surrounding loose connective tissue is normal in appearance without evidence of inflammation, cellulitis, or abscess formation. (light microscopy, hematoxylin and eosin stain, original magnification ×40).
Figure 3.
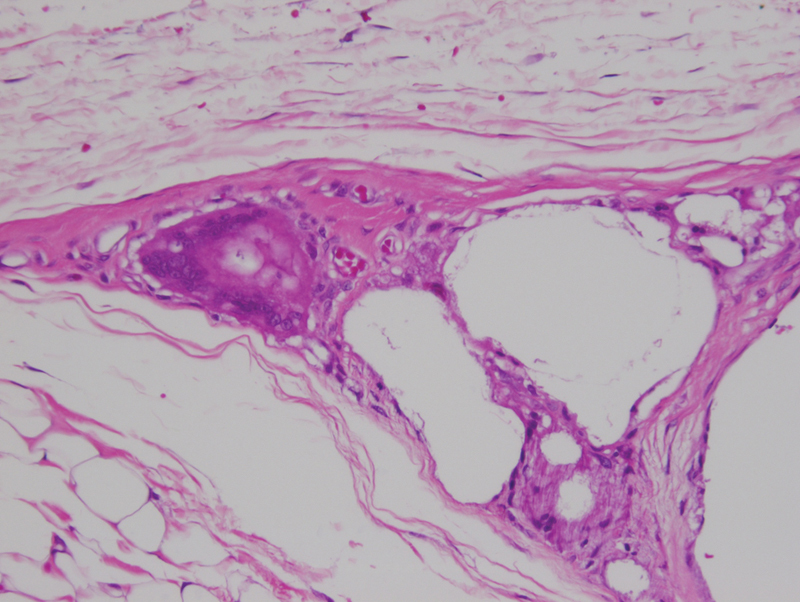
Poly(D/L)lactide acid plate at 3 months postimplantation, demonstrated small encapsulated implant fragments surrounding by giant cells, indicating active resorption. Note that foreign-body reaction is limited entirely within the implant capsule. (light microscopy, hematoxylin and eosin stain, original magnification ×400).
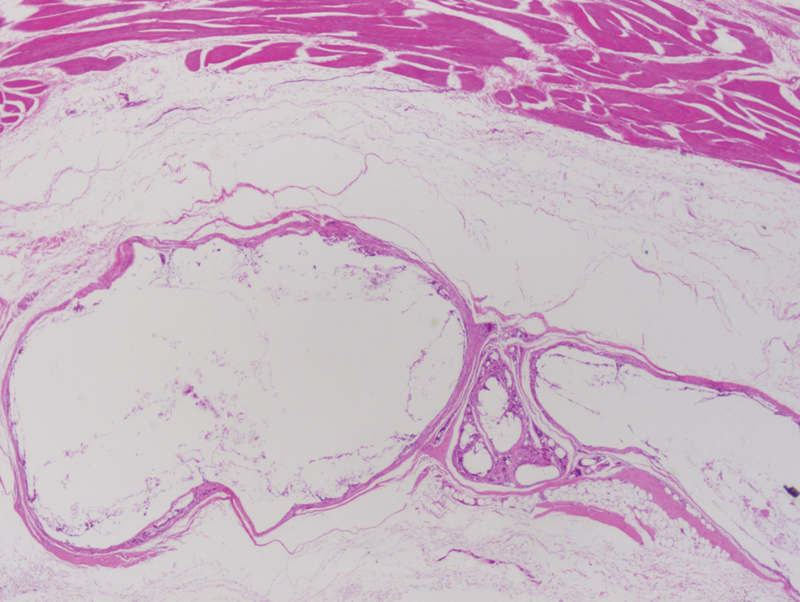
At 6 months, there was no residual implant upon gross visual examination (Fig. 4) or on microscopic examination at low power (×40 magnification [Fig. 5]). However, there was evidence of focal foreign-body reaction, which at ×400 magnification demonstrated remaining microscopic implant material undergoing active resorption (Fig. 6). As seen before, the reaction is well-contained within the fibrous capsule surrounding the implant material. The surrounding soft tissue and bone were unremarkable. Similar microscopic findings were seen with the 12 month samples.
Figure 4.
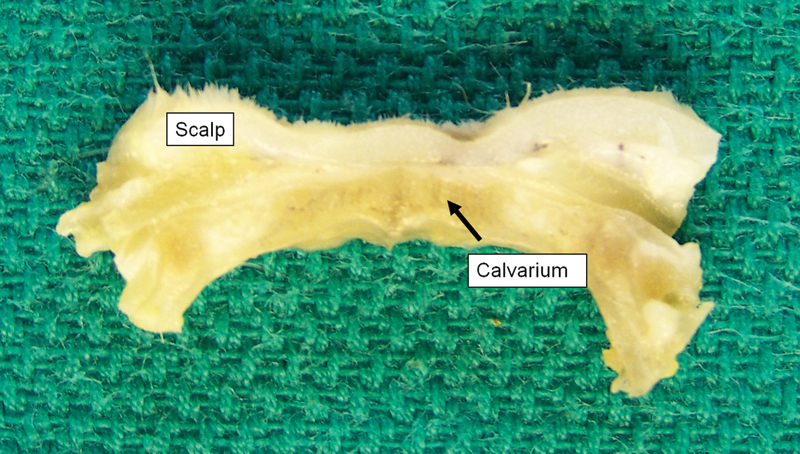
Gross appearance of Poly(D/L)lactide acid plate at 6 months postimplantation. There is no evidence of residual implant material.
Figure 5.

Poly(D/L)lactide acid plate at 6 months postimplantation No residual implant material was noted. Some dermal fibrosis was noted in the soft tissue of the scalp, due to scarring from prior surgical incision. Underlying calvarial bone was unremarkable. (light microscopy, hematoxylin and eosin stain, original magnification ×40).
Figure 6.
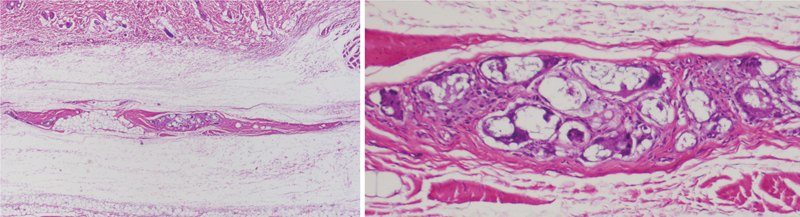
Poly(D/L)lactide acid plate at 6 months postimplantation demonstrated focal foreign-body reaction (left, light microscopy, hematoxylin and eosin stain, original magnification ×100). At ×400 magnification (right), microscopic residual implant material was visible with surrounding giant cells. Note that this reaction was contained within the fibrous capsule surrounding the implant. The surrounding connective tissue was unremarkable.
Poly-Lactide-Glycolic Acid Plate
At 3 months, the PLGA implant did not demonstrate any decrease in plate visibility or gross integration into the surrounding tissue (Fig. 7). The plate was covered by a thin layer of fibrous tissue suggestive of capsulization. The implant was too hard for scalpel sectioning, and as a result, histological evaluation could only be performed on the soft tissue and bone surrounding the implant material. Microscopic examination of this tissue demonstrated no evidence of inflammatory cell infiltration or foreign-body giant cell reaction (Fig. 8). The scalp soft tissue and the underlying bone were normal in appearance.
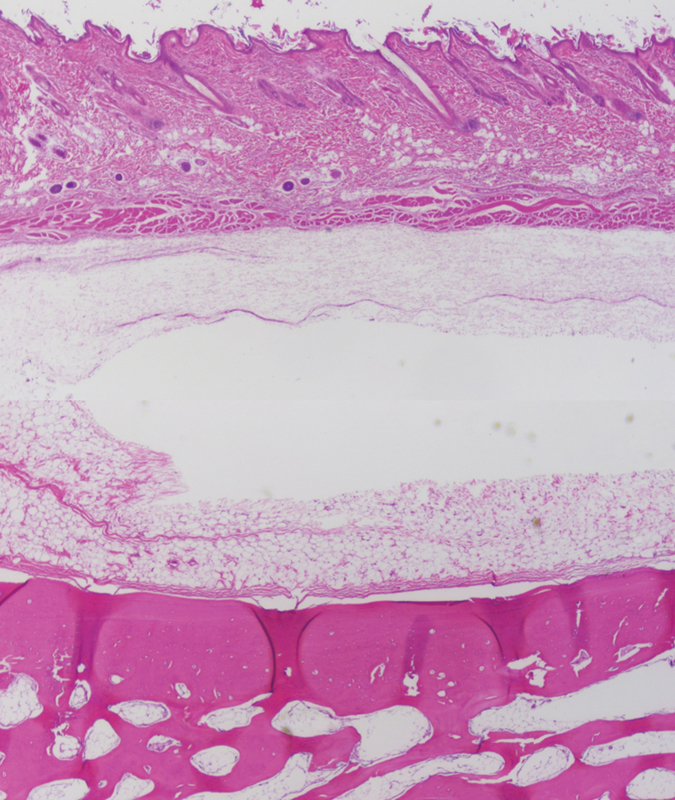
Figure 7.
Poly-lactide-glycolic acid plate at 3 months postimplantation. The plate was epithelialized, but did not integrate into surrounding tissue. Note existence of a soft-tissue cavity which contained the implant. (light microscopy, hematoxylin and eosin stain, original magnification ×40).
Figure 8.
Poly-lactide-glycolic acid plate at 3 months postimplantation (light microscopy, hematoxylin and eosin stain, original magnification ×40, composite image approximating location of implant cavity). There was no evidence of implant fragments or local foreign-body reactions. Surrounding soft tissue and bone are histologically unremarkable. These findings persist in 6-month and 12-month samples.
The microscopic findings at 6 and 12 months were similar to those for the 3-month period. There was no gross evidence of implant material integration. The surrounding soft tissues and bone were normal in appearance with no evidence of inflammation. At 12 months, the implant was more friable with some visible fracturing. The implant material was still grossly visible within the soft tissue periosteal pocket. In addition, there was more notable tissue indentation identified on gross examination, which may suggest increased adherence of the implant to the tissue lining the periosteal pocket (Fig. 9).
Figure 9.

Gross appearance of poly-lactide-glycolic acid plate at 12 months postimplantation. The implant remained separate from the tissue, but was more friable with some fracture lines along the edges. Note that the tissue cavity was more prominent, which may suggest better adherence.
Discussion
The development of biodegradable plates was prompted from multiple publications citing concerns over migration, need for removal, and growth restriction as a result of metallic plate used in children. Skin irritation, infection, palpability, and pain were cited as the most common reasons for plate removal.7 8 Intracranial migration of metallic plates poses a significant problem as well.9 Less clear are the effects of metallic plates on bone growth. Some animal models have suggested minimal and isolated restriction at the site of fixation, but its effect on humans is difficult to discern.8 10 These potential complications would be mitigated with the use of biodegradable plates.
The ideal biodegradable plate is mechanically strong, adaptable to allow healing, and undergoes resorption in a predictable time frame. In addition, the plate material needs to be biocompatible with the recipient environment, and causes minimal local inflammatory reactions. There are multiple factors influencing the occurrence of local foreign-body inflammatory reactions (Table 4). Among these, chemical composition has been studied the most.
Table 4. Factors influencing local foreign-body reactions.
| Implant | Chemical composition |
| Plate size | |
| Plate shape | |
| Surface texture | |
| Recipient environment | Blood supply |
| Temperature | |
| Genetic proclivity for hypersensitivity | |
| Plate placement | Subcutaneous |
| Epiperiosteal | |
| Subperiosteal |
Polylactic acid (PLA) and PGAs are the primary components of most biodegradable plates. The biomechanical and resorptive properties of these plates vary depending on the ratio of lactic acid isomers and/or glycolic acid.11 PLA degradation is biphasic. Early degradation occurs via hydrolysis of ester bonds.3 11 The resulting degradation product crystallizes and undergoes a secondary hydrolysis, which is the rate-limiting step.3 The final end products, CO2 and H2O, are ultimately metabolized by the liver.11 The rate of degradation depends upon the crystallinity and hydrophobicity of the early by-products. L-isomers form crystalline lattices that are highly hydrophobic and more resistant to degradation than the D-isomers.3 12 Different ratios of the two determine their polymer chain organization and orientation. Poly L-lactic acid crystalline particles have been identified within the cytoplasm of local phagocytic cells years after implantation.2
More amorphous polymers, such as PGA, are inherently less hydrophobic, undergo more rapid breakdown and are susceptible to bulk degradation or fragmentation of the material. PGA is more hydrophilic and undergoes resorption more quickly. PGA is typically used in conjunction with PLA to improve the structural integrity of an implant, as pure PGA does not offer the necessary strength for fracture fixation. These semicrystalline polymers typically exhibit degradation at the outer surface of the material, and are less likely to fragment or undergo bulk degradation.
Previous clinical studies have demonstrated that polylactic/glycolic acid implants are associated with up to a 22% prevalence of developing a local foreign-body inflammatory reaction, or “sterile abscess.”1 2 4 13 Bergsma et al reported that remnants from poly-L-lactide implants could lead to accumulation of localized sterile fluid up to 5.7 years after implantation, necessitating surgical drainage.4 These types of reactions result when the implant is either incompletely degraded or when there is a large volume of debris from degradation overwhelming metabolic clearance.1 Long-term follow-up studies suggest that poly-L-lactide implants are particularly prone to developing delayed foreign-body reactions even years after implantation; while poly-L-lactide-coglycolic acid implants demonstrate reactions significantly earlier, at 3 to 6 months following implantation depending on the polymeric ratios.2 11 In addition, delayed foreign-body reactions were encountered with ultrasound activated poly-D/L-lactide resorbable pins.5 These findings may suggest that the inherent properties of the materials may be altered by external factors, such as ultrasound.5
Our findings demonstrated that the PDLLA implant (50:50, D:L-lactide) fragmented and resorbed more quickly on visual inspection than PLGA material, with only microscopically visualized residual material at 6 and 12 months postimplantation. There was a large amount of inflammatory cell infiltrate during degradation; however, this reaction was contained within the implant capsule. There was no case of clinically obvious local induration or infection requiring intervention. We postulate that the higher ratio of D-lactide to L-lactide increased the degradability of the implant without overwhelming the clearance metabolism of the local tissue. Further investigation will be necessary to see if the microscopic residues persist in long-term follow-up after the 12-month-study period.
In contrast, the PLGA implant material studied (85:5:10, L-lactide:D-lactide:glycolic acid), on visual inspection, showed minimal signs of resorption at 3, 6, and 12 months. Previously published data by Landes et al report that PLGA implants (82:18 LactoSorb, Lorenz Surgical, Jacksonville, FL, and 85:15 PolyMax RAPID, Synthes, Oberdorf, Switzerland) fully degrade between 12 and 18 months in humans for maxillary and mandibular fracture fixation.2 This same study demonstrated a 5% prevalence of clinically significant local tissue reactions at 3 months.2 However, our study did not find any local tissue reactions beyond fibrous capsule formation. Histologically, the PLGA implants in this study were highly inert and stable with no evidence of inflammatory infiltration in the surrounding soft tissues or bone. Host species, the recipient environment, and differing chemical compositions may explain the differences in these studies. The face is typically better vascularized than the pericranium, and may respond more readily to a foreign body, leading to earlier and more robust responses. In the Landes et al study, the faster resorbing of the two plates demonstrated a significantly earlier local soft tissue reaction.2 This trend was seen in our study as well.
There are several limitations to our qualitative study. Although the primary goal of this study is to evaluate the foreign-body reaction, quantification of plate resorption using biomechanical testing would be beneficial for our data analysis. Within the setting of this study, the validity of our hypothesis could not be fully evaluated, as our study perimeters did not capture any local reactions to the PLGA implants, most likely because of lack of plate integration and resorption. A follow-up study with longer time span, beyond 12 months, is necessary. In addition, as the use of biodegradable plates increases, further studies will be required to determine how these models will extrapolate to humans.
Conclusion
The PDLLA implant demonstrated a moderate degree of foreign-body inflammatory reaction confined within the implant capsule as early as 3 months postimplantation. In contrast, there was no histological evidence of foreign-body reaction associated with the PLGA implants, which did not fragment or resorb by visual inspection at 12 months following implantation. Neither implant material caused any inflammatory infiltrate outside the capsule nor in adjacent soft tissue and bone. Both implant materials are biocompatible with no evidence of sterile abscess formation or local soft tissue reaction.
Footnotes
Financial Disclosures/Commercial Associations Financially supported by Stryker Craniomaxillofacial, Kalamazoo, MI. Dr. Larry H. Hollier Jr. is a medical consultant of Stryker Craniomaxillofacial.
References
- 1.Eppley B L Morales L Wood R et al. Resorbable PLLA-PGA plate and screw fixation in pediatric craniofacial surgery: clinical experience in 1883 patients Plast Reconstr Surg 20041144850–856., discussion 857 [DOI] [PubMed] [Google Scholar]
- 2.Landes C A, Ballon A, Roth C. Maxillary and mandibular osteosyntheses with PLGA and P(L/DL)LA implants: a 5-year inpatient biocompatibility and degradation experience. Plast Reconstr Surg. 2006;117(7):2347–2360. doi: 10.1097/01.prs.0000218787.49887.73. [DOI] [PubMed] [Google Scholar]
- 3.van der Elst M, Klein C P, de Blieck-Hogervorst J M, Patka P, Haarman H J. Bone tissue response to biodegradable polymers used for intra medullary fracture fixation: a long-term in vivo study in sheep femora. Biomaterials. 1999;20(2):121–128. doi: 10.1016/s0142-9612(98)00117-3. [DOI] [PubMed] [Google Scholar]
- 4.Bergsma J E, de Bruijn W C, Rozema F R, Bos R R, Boering G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials. 1995;16(1):25–31. doi: 10.1016/0142-9612(95)91092-d. [DOI] [PubMed] [Google Scholar]
- 5.Nkenke E, Vairaktaris E, Schwarz S. et al. Prospective assessment of complications associated with ultrasound activated resorbable pin osteosynthesis in pediatric craniofacial surgery: preliminary results. Neurocirugia (Astur) 2011;22(6):498–506. doi: 10.1016/s1130-1473(11)70105-1. [DOI] [PubMed] [Google Scholar]
- 6.De Jong W H, Eelco Bergsma J, Robinson J E, Bos R R. Tissue response to partially in vitro predegraded poly-L-lactide implants. Biomaterials. 2005;26(14):1781–1791. doi: 10.1016/j.biomaterials.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Rubin J P, Yaremchuk M J. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 1997;100(5):1336–1353. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 8.Orringer J S, Barcelona V, Buchman S R. Reasons for removal of rigid internal fixation devices in craniofacial surgery. J Craniofac Surg. 1998;9(1):40–44. doi: 10.1097/00001665-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Fearon J A Munro I R Bruce D A Observations on the use of rigid fixation for craniofacial deformities in infants and young children Plast Reconstr Surg 1995954634–637., discussion 638 [PubMed] [Google Scholar]
- 10.Eppley B L, Platis J M, Sadove A M. Experimental effects of bone plating in infancy on craniomaxillofacial skeletal growth. Cleft Palate Craniofac J. 1993;30(2):164–169. doi: 10.1597/1545-1569_1993_030_0164_eeobpi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 11.Bell R B, Kindsfater C S. The use of biodegradable plates and screws to stabilize facial fractures. J Oral Maxillofac Surg. 2006;64(1):31–39. doi: 10.1016/j.joms.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.van der Elst M, Dijkema A R, Klein C P, Patka P, Haarman H J. Tissue reaction on PLLA versus stainless steel interlocking nails for fracture fixation: an animal study. Biomaterials. 1995;16(2):103–106. doi: 10.1016/0142-9612(95)98270-o. [DOI] [PubMed] [Google Scholar]
- 13.Böstman O, Hirvensalo E, Mäkinen J, Rokkanen P. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers. J Bone Joint Surg Br. 1990;72(4):592–596. doi: 10.1302/0301-620X.72B4.2199452. [DOI] [PubMed] [Google Scholar]


