Abstract
Background
The purpose of this study was to identify trends in survival and chemotherapy use for individuals with small-cell lung cancer (SCLC) in England using the National Lung Cancer Audit (NLCA).
Methods
We used data from the NLCA database to identify people with histologically proven SCLC from 2004–2011. We calculated the median survival by stage and assessed whether patient characteristics changed over time. We also assessed whether the proportion of patients with records of chemotherapy and/or radiotherapy changed over time.
Results
18,513 patients were diagnosed with SCLC in our cohort. The median survival was 6 months for all patients, 1 year for those with limited stage and 4 months for extensive stage. 69% received chemotherapy and this proportion changed very slightly over time (test for trends p = 0.055). Age and performance status of patients remained stable over the study period, but the proportion of patients staged increased (p-value<0.001), mainly because of improved data completeness. There has been an increase in the proportion of patients that had a record of receiving both chemotherapy and radiotherapy each year (from 19% to 40% in limited and from 9% to 21% in extensive stage from 2004 to 2011). Patients who received chemotherapy with radiotherapy had better survival compared with any other treatment (HR 0.24, 95% CI 0.23–0.25).
Conclusion
Since 2004, when the NLCA was established, the proportion of patients with SCLC having chemotherapy has remained static. We have found an upward trend in the proportion of patients receiving both chemotherapy and radiotherapy which corresponded to a better survival in this group, but as it only applied for a small proportion of patients, it was not enough to change the overall survival.
Introduction
Small-cell lung cancer (SCLC) accounted for 20% of all lung cancer cases diagnosed over a decade ago [1] but this proportion has decreased and currently only accounts for approximately 10%. [2], [3], [4], [5] SCLC is responsive to chemotherapy [6] (and combination chemo-radiotherapy) [7] and this is the main treatment recommended by the National Institute of Health and Clinical Excellence (NICE). [3] However, despite the sometimes dramatic response to chemotherapy, many patients relapse and die within 6 months of diagnosis. [8] Furthermore, survival from SCLC is poor in England compared with other European and North American countries, [9], [10], [11] with only 5% of the patients surviving for at least 5 years. [12].
The National Lung Cancer Audit (NLCA) was established in 2004 to measure the outcomes and quality of care for patients with lung cancer provided by the National Health Services (NHS) and in doing so to improve the quality of the service. [13] The audit has been used to set standards of care, such as 80% of patients should be seen by lung cancer nurse specialists, 75% of patients should have histological confirmation, and 95% patients should be discussed by a multi-disciplinary team (MDT). These standards are designed to make the treatment and care given in England more comparable to other European and North American countries. We used the NLCA database linked with the Hospital Episode Statistic (HES) database to assess the impact of NLCA on the English lung cancer population by studying the trends in chemotherapy use, survival and changing features of patients with SCLC since the audits introduction in 2004.
Methods
Data Source and Study Population
The NLCA database is a longitudinal database consisting of anonymous computerised records of individuals with a diagnosis of primary lung cancer. It has collected data on demographics, tumour features and treatment since 2004 via 157 English NHS hospitals responsible for managing and treating patients with lung cancer. Data is usually entered by members of lung cancer MDT. Using the NLCA database, we identified all English cases with histologically proven SCLC diagnosed between 1st January 2004 and 31st December 2011. The NLCA dataset has been analysed previously as part of a validation process [13], and currently the case ascertainment is in excess of 90% [4], [5]. We used linked data from Hospital Episode Statistics (HES), a mandatory national database collecting data on all in-patient diagnoses, consultant referrals and treatment procedure performed, to provide additional information on co-morbidity and treatment and the Office for National Statistics (ONS) which collects data from death certificates, the registration of which is a legal requirement in the United Kingdom (UK).
Covariates
For this study, we restricted our analyses to those patients with a histologically proven diagnosis of SCLC based on the recorded Systematised Nomenclature of Medicine (SNOMED) codes in the NLCA database (M-8041/3). Our initial dataset included information on age at diagnosis, sex, performance status (PS) and stage. PS was classified according to the World Health Organisation (WHO) definition and the stage was recorded using the Veteran’s Administration Lung Study Group system (limited or extensive). For a few cases where the most recent Tumour Node Metastases staging system (TNM) was used, we converted this to limited (T1-4, N0-3, M0) or extensive (M1a/b) as appropriate. [14] We defined socio-economic status (SES) using the Townsend deprivation Index, which uses a composite score of four variables (unemployment, overcrowding, non-car ownership and non-home ownership), and is split into 5 categories of deprivation. However due to more than 90% of missing data on SES from 2004–2005, we performed a separate analysis for 2006–2011.
To determine overall survival, we created a start date which was the date of diagnosis. In the absence of a date of diagnosis, a pseudo start date was generated using the median number of days (for the whole cohort) between date of diagnosis and the following dates in this order: (1) date first seen, (2) date of referral, or (3) date discussed by MDT. An end date for each patient was created using either the date of death (provided by ONS) or the date of the last ONS cross-check for death dates (31st March 2013). Therefore every patient had a minimum of 15 months of follow up for the survival analysis.
Chemotherapy and Radiotherapy
We used the NLCA and HES dataset to determine whether an individual in our cohort had received chemotherapy. From HES in-patient hospital episodes for each patient, we used International Classification of Diseases (ICD) codes Z51.1 & Z51.2 allocated for ‘chemotherapy session for neoplasm’ and ‘other chemotherapy’ to indicate chemotherapy provision. We also identified Office of Population Censuses and Survey Classification of Intervention (OPCS-4) codes for chemotherapy from the HES database. Presence of either one or both of the ICD/OPCS-4 codes from the HES database was taken as evidence of receiving chemotherapy. We also identified patients who had chemotherapy from the NLCA database as HES does not record chemotherapy given during an out-patient admission. Patients were classified as not receiving chemotherapy if there was no date of chemotherapy in the NLCA or HES. Patients were included in our study if their date of diagnosis was in our study period. We excluded patients from our cohort who had received their first dose of chemotherapy 1 month prior or 6 months after the date of diagnosis of lung cancer. This step was done to minimise the skewing of overall survival time in either direction by excluding patients who may have received chemotherapy for some other cancer prior to being diagnosed with SCLC or received chemotherapy for a slow growing cancer which had been misclassified as SCLC (6 months after date of diagnosis).
As the NLCA does not collect detailed information on radiotherapy treatment type and intent, it is difficult to know whether the radiotherapy was given for curative or palliative purpose. However, we used the NLCA database to identify patients who received radiotherapy using the date of radiotherapy given. It was also difficult to identify patients who received concurrent chemotherapy and radiotherapy as the NLCA records only the first dose of either treatment. Only 404 patients (2%) had clear evidence of concurrent chemotherapy and radiotherapy.
Statistical Analysis
All data management and statistical analyses were performed using Stata version 12 (StataCorp, Texas). Initially, we calculated the median age of diagnosis and median survival in days by the year in which a patient was diagnosed with SCLC. The first two years, 2004 and 2005, were grouped together to create a comparator group of adequate size. We also looked at the patient features at the time of diagnosis by year and performed the Cuzick’s non-parametric test. A p-value of <0.05 was considered as significant. We also looked at the proportion of patients receiving chemotherapy and the proportion who received both chemotherapy and radiotherapy. We performed Cox regression analysis to calculate the hazard ratios (HRs) depending on the type of treatment received after the diagnosis compared with no treatment received after diagnosis of SCLC, adjusted for patient features and years.
Ethics
The data was obtained from the Heathcare Quality Improvement Partnership (HQIP). Ethical approval from the University of Nottingham medical school research ethnics committee was obtained by the researchers to work on a linked HES and NLCA dataset (RU943 177570-MV6J3). The NLCA has Ethics and Confidentiality Committee (ECC) approval to use patient information from the National Health Services (NHS). Finally for this specific set of work, we also obtained approval from HQIP who commission the audit and HSCIC caldicott guardian signed off the data sharing agreement [IG Reference: IC381DS]. The data was anonymised in the linked dataset by the HSCIC personal prior to be given to the researchers.
Results
There were a total of 178,427 individuals diagnosed with lung cancer in the NLCA between 1st January 2004 and 31st December 2011. We restricted our analyses to only those individuals who had a histologically proven SCLC (n = 18,513 (10.3%)). Table 1 shows the patient features by year of diagnosis.
Table 1. Changing features of patients with small-cell lung cancer over the duration of the NLCA (n = 18,513).
| Year of Diagnosis | ||||||||
| 2004/2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | test for trends | |
| Number of patients N | 1867 | 1836 | 2081 | 2697 | 3208 | 3325 | 3499 | |
| Median Survival in days | ||||||||
| Whole cohort (n = 18513) | 179 | 179 | 190 | 190 | 179 | 186 | 190 | |
| Limited stage (n = 4830) | 358 | 321 | 343 | 362 | 332 | 358 | 339 | |
| Extensive stage (n = 9874) | 102 | 120 | 139 | 124 | 113 | 124 | 124 | |
| Stage Unknown (n = 772) | 168 | 135 | 183 | 219 | 128 | 223 | 256 | |
| Stage uncertain (n = 933) | 226 | 208 | 208 | 194 | 237 | 201 | 285 | |
| Stage missing (2104) | 168 | 164 | 164 | 161 | 142 | 142 | 168 | |
| Median age at diagnosis | 67.9 | 68.2 | 68.3 | 68.1 | 68.3 | 68.6 | 68.7 | |
| Sex (%) | ||||||||
| Female | 879 (47.08) | 843 (45.92) | 926 (44.50) | 1248 (46.27) | 1537 (47.91) | 1616 (48.60) | 1741 (49.76) | |
| Male | 988 (52.92) | 993 (54.08) | 1155 (55.50) | 1449 (53.73) | 1671 (52.09) | 1709 (51.40) | 1758 (50.24) | <0.001 |
| Age n (%) | ||||||||
| <65 | 668 (35.78) | 634 (34.33) | 709 (34.07) | 940 (34.85) | 1105 (34.45) | 1121 (33.71) | 1144 (32.70) | |
| 65–75 | 607 (32.51) | 612 (33.33) | 710 (34.12) | 901 (33.41) | 1103 (34.38) | 1124 (33.80) | 1219 (34.84) | |
| >75 | 592 (31.71) | 590 (32.14) | 662 (31.81) | 856 (31.74) | 1000 (31.17) | 1080 (32.48) | 1136 (32.47) | 0.101† |
| Performance Status (%) | ||||||||
| 0 | 223 (11.94) | 252 (13.73) | 261 (12.54) | 338 (12.53) | 456 (14.21) | 503 (15.13) | 550 (15.72) | |
| 1 | 395 (21.16) | 454 (24.73) | 533 (25.61) | 746 (27.66) | 948 (29.55) | 1039 (31.25) | 1229 (35.12) | |
| 2 | 304 (16.28) | 327 (17.81) | 411 (19.75) | 506 (18.76) | 648 (20.20) | 736 (22.14) | 747 (21.35) | |
| 3 | 188 (10.07) | 182 (9.91) | 221 (10.62) | 351 (13.01) | 478 (14.90) | 480 (14.44) | 510 (14.58) | |
| 4 | 67 (3.59) | 60 (3.27) | 78 (3.75) | 91 (3.37) | 132 (4.11) | 148 (4.45) | 152 (4.34) | 0.877† |
| missing | 690 (36.96) | 561 (30.56) | 577 (27.73) | 665 (24.66) | 546 (17.02) | 419 (12.60) | 311 (8.89) | |
| Charlson Index (%) | ||||||||
| 0 | 815 (43.65) | 736 (40.09) | 811 (38.97) | 912 (33.82) | 1035 (32.26) | 954 (28.69) | 948 (27.09) | |
| 1 | 321 (17.19) | 344 (18.74) | 383 (18.40) | 486 (18.02) | 581 (18.11) | 595 (17.89) | 603 (17.23) | |
| 2–3 | 191 (10.23) | 218 (11.87) | 238 (11.44) | 346 (12.83) | 415 (12.84) | 436 (13.11) | 471 (13.46) | |
| 4+ | 540 (28.92) | 538 (29.30) | 649 (31.19) | 953 (35.34) | 1177 (36.69) | 1340 (40.30) | 1477 (42.21) | <0.001† |
| Stage (%) | ||||||||
| SCLC-Limited | 449 (24.05) | 451 (24.56) | 480 (23.07) | 643 (23.84) | 893 (27.84) | 887 (26.68) | 1027 (29.35) | |
| SCLC-Extensive | 794 (42.53) | 824 (44.88) | 951 (45.70) | 1356 (50.28) | 1693 (52.77) | 2020 (60.75) | 2236 (63.90) | |
| SCLC-Unknown | 177 (9.48) | 246 (13.40) | 156 (7.50) | 90 (3.34) | 62 (1.93) | 24 (0.72) | 17 (0.49) | |
| SCLC-Uncertain | 37 (1.98) | 57 (3.10) | 245 (11.77) | 285 (10.57) | 193 (6.02) | 93 (2.80) | 23 (0.66) | |
| Stage missing | 410 (21.96) | 258 (14.05) | 249 (11.97) | 323 (11.98) | 367 (11.44) | 301 (9.05) | 196 (5.60) | <0.001† |
| Chemotherapy n (%) | 1236 (66.20) | 1266 (68.95) | 1483 (71.26) | 1878 (69.63) | 2188 (68.20) | 2282 (68.63) | 2478 (70.82) | 0.055 |
| Socio-economic status (%) | ||||||||
| 1 (Most Affluent) | 234 (12.75) | 314 (15.09) | 395 (14.65) | 494 (15.40) | 458 (13.77) | 487 (13.92) | ||
| 2 | 312 (16.99) | 383 (18.40) | 469 (17.39) | 582 (18.14) | 600 (18.05) | 657 (18.78) | ||
| 3 | 348 (18.95) | 413 (19.85) | 546 (20.24) | 620 (19.33) | 615 (18.50) | 716 (20.46) | ||
| 4 | 381 (20.75) | 455 (21.86) | 575 (21.32) | 738 (23.00) | 711 (21.38) | 789 (22.55) | ||
| 5 (Least Affluent) | 499 (27.18) | 515 (24.75) | 698 (25.88) | 722 (24.06) | 753 (22.65) | 817 (23.35) | 0.015† | |
| Missing | 62 (3.38) | 1 (0.05) | 14 (0.52) | 2 (0.06) | 188 (5.65) | 33 (0.94) | ||
Cuzick’s non-parametric test for trends otherwise chi-square test for trends.
The median age at diagnosis remained the same at 69 years (interquartile range (IQR) 62–75) from 2004–2011. A total of 12,811 (69.2%) patients received chemotherapy in our cohort and this proportion had increased very slightly over the study period showing a borderline significant trend (test for trends 0.055). Age and PS did not change over the years. There was a significant change in recording of stage (test for trends <0.001), with a decreasing proportion of patients with unknown, uncertain and missing stage and increasing proportions of limited and extensive stage SCLC. There was also a significant change in the distribution of co-morbidity over the years, with more patients having a Charlson index of 4 or more in more recent years (test for trend across Charlson score groups p<0.001).
Table 2 represents the multivariate logistic regression analyses for patients diagnosed between 2006 and 2011 (2004/2005 excluded because of high level of missing data on socioeconomic status). The odds of receiving chemotherapy reduced with increasing age, PS and co-morbidity (χ2 p-value for trends <0.001). We also observed a significant association between receiving chemotherapy and SES (ptrends<0.001), where patients from least affluent areas were 13% less likely to receive chemotherapy compared with patients from the most affluent areas after adjusting for confounders (adjusted OR 0.87, 95% CI 0.77–0.99).
Table 2. Results for multivariate logistic regression analysis for patients diagnosed between 2006–2011 (n = 16,646).
| Number ofpatients n (%) | Proportion of patientswho receivedchemotherapy n (%) | Unadjusted OR(95% CI) | Adjusted OR(95% CI)‡ | p-value | |
| Sex | |||||
| Female | 7911 (48) | 5533 (70) | 1 | 1 | |
| Male | 8735 (52) | 6042 (69) | 0.96 (0.90–1.03) | 0.98 (0.91–1.06) | 0.74† |
| Age | |||||
| <65 | 5653 (34) | 4670 (83) | 1 | 1 | |
| 65–75 | 5669 (34) | 4170 (74) | 0.58 (0.53–0.64) | 0.64 (0.58–0.71) | |
| >75 | 5324 (32) | 2735 (51) | 0.22 (0.20–0.24) | 0.25 (0.23–0.28) | <0.001 |
| Performance Status | |||||
| 0 | 2360 (14) | 2107 (89) | 1 | 1 | |
| 1 | 4949 (30) | 4220 (85) | 0.69 (0.59–0.80) | 0.88 (0.75–1.03) | |
| 2 | 3375 (20) | 2289 (68) | 0.25 (0.21–0.29) | 0.39 (0.33–0.46) | |
| 3 | 2222 (13) | 899 (40) | 0.08 (0.06–0.09) | 0.13 (0.11–0.15) | |
| 4 | 661 (4) | 80 (12) | 0.01 (0.01–0.02) | 0.02 (0.02–0.03) | <0.001 |
| missing | 3079 (19) | 1980 (64) | 0.21 (0.18–0.25) | 0.30 (0.25–0.35) | |
| Stage | |||||
| SCLC-Limited | 4381 (26) | 3522 (80) | 1 | 1 | |
| SCLC-Extensive | 9080 (55) | 5952 (66) | 0.46 (0.42–0.50) | 0.67 (0.60–0.74) | |
| SCLC-others | 3185 (19) | 2101 (66) | 0.47 (0.42–0.52) | 0.65 (0.58–0.74) | <0.001† |
| Charlson Index | |||||
| 0 | 5396 (32) | 4363 (81) | 1 | 1 | |
| 1 | 2992 (18) | 2211 (74) | 0.67 (0.60–0.74) | 0.81 (0.72–0.92) | |
| 2–3 | 2124 (13) | 1432 (67) | 0.48 (0.43–0.54) | 0.67 (0.59–0.74) | |
| 4+ | 6134 (37) | 3569 (58) | 0.32 (0.30–0.35) | 0.50 (0.45–0.55) | <0.001 |
| Socio-economic status | |||||
| 1 (Most affluent) | 2382 (14) | 1664 (70) | 1 | 1 | |
| 2 | 3003 (18) | 2133 (71) | 1.05 (0.94–1.19) | 1.00 (0.87–1.15) | |
| 3 | 3258 (20) | 2278 (70) | 1.00 (0.89–1.12) | 0.99 (0.86–1.13) | |
| 4 | 3649 (22) | 2526 (69) | 0.97 (0.86–1.08) | 0.84 (0.74–0.96) | |
| 5 | 4054 (24) | 2828 (70) | 0.99 (0.89–1.11) | 0.87 (0.77–0.99) | 0.001 |
| Missing | 300 (2) | 146 (49) | 0.40 (0.32–0.52) | 0.44 (0.33–0.59) | |
‡Adjusted for all other variables in the table.
†Log-likelihood ratio p-value - otherwise χ2 p-value.
Chemotherapy and Radiotherapy
Table 3 shows the proportion of SCLC patients receiving chemotherapy alone, radiotherapy alone and chemotherapy and radiotherapy, stratified by stage. The proportion of patients who received chemotherapy remained stable for all stages over the years; however there was an increase in the proportion of patients with recorded chemotherapy and radiotherapy for all stages. In limited stage it increased from 19% to 40% and in extensive stage from 10% to 21%. There was also an increase of recorded chemotherapy and radiotherapy for unknown, uncertain and missing stage. The use of radiotherapy alone, regardless of stage, had also increased over the years. Patients who received chemo-radiotherapy had a better median survival (335 days) compared with chemotherapy alone (235 days), radiotherapy alone (82 days) and no therapy (24 days) (data not shown). It was also observed that patients who received chemo-radiotherapy were younger, with less co-morbidity and had better PS compared with patients who received chemotherapy or radiotherapy alone (table S1).
Table 3. Proportion of people receiving chemotherapy and radiotherapy by stage (n = 18513).
| Year | Total patientsreported n (%) | Total patients withrecorded chemotherapyn (%) | Patients with recordedchemotherapy + radiotherapy n (%) | Recorded radiotherapy only n (%) |
| Limited stage small-cell (n = 4830) | ||||
| 2004/2005 | 449 | 363 (80.85) | 87 (19.38) | 16 (3.56) |
| 2006 | 451 | 356 (78.94) | 50 (11.09) | 15 (3.33) |
| 2007 | 480 | 387 (80.63) | 88 (18.33) | 26 (5.42) |
| 2008 | 643 | 501 (77.92) | 172 (26.75) | 37 (5.75) |
| 2009 | 893 | 714 (79.96) | 331 (37.07 | 52 (5.82) |
| 2010 | 887 | 714 (80.50) | 332 (37.43) | 46 (5.19) |
| 2011 | 1027 | 850 (82.77) | 411 (40.02) | 43 (4.19) |
| Extensive stage small-cell (n = 9874) | ||||
| 2004/2005 | 794 | 481 (60.58) | 77 (9.70) | 40 (5.04) |
| 2006 | 824 | 548 (66.50) | 69 (8.37) | 43 (5.22) |
| 2007 | 951 | 638 (67.09) | 102 (10.73) | 44 (4.63) |
| 2008 | 1356 | 910 (67.11) | 217 (16.00) | 86 (6.34) |
| 2009 | 1693 | 1068 (63.08) | 292 (17.25) | 139 (8.21) |
| 2010 | 2020 | 1311 (64.90) | 424 (20.99) | 141 (6.98) |
| 2011 | 2236 | 1477 (66.06) | 474 (21.20) | 129 (5.77) |
| Unknown stage small-cell (n = 772) | ||||
| 2004/2005 | 177 | 108 (61.02) | 22 (12.43) | 11 (6.21) |
| 2006 | 246 | 164 (66.67) | 19 (7.72) | 13 (5.28) |
| 2007 | 156 | 111 (71.15) | 28 (17.95) | 4 (2.56) |
| 2008 | 90 | 65 (72.22) | 14 (15.56) | 4 (4.44) |
| 2009 | 62 | 42 (67.74) | 15 (24.19) | 5 (8.06) |
| 2010 | 24 | 14 (58.33) | 2 (8.33) | 3 (12.50) |
| 2011 | 17 | 12 (70.59) | 4 (23.53) | 0 (0.00) |
| Uncertain stage (n = 922) | ||||
| 2004/2005 | 37 | 23 (62.16) | 4 (10.81) | 2 (5.41) |
| 2006 | 57 | 36 (63.16) | 5 (8.77) | 1 (1.75) |
| 2007 | 245 | 178 (72.65) | 19 (7.76) | 14 (5.71) |
| 2008 | 285 | 190 (66.67) | 62 (21.75) | 22 (7.72) |
| 2009 | 193 | 139 (72.02) | 50 (25.91) | 11 (5.70) |
| 2010 | 93 | 62 (66.67) | 20 (21.51) | 6 (6.45) |
| 2011 | 23 | 15 (65.22) | 5 (21.74) | 2 (8.70) |
| Stage missing (n = 2104) | ||||
| 2004/2005 | 410 | 261 (63.66) | 25 (6.10) | 18 (4.39) |
| 2006 | 258 | 162 (62.79) | 17 (6.59) | 12 (4.65) |
| 2007 | 249 | 169 (67.87) | 21 (8.43) | 7 (2.81) |
| 2008 | 323 | 212 (65.63) | 50 (15.48) | 20 (6.19) |
| 2009 | 367 | 225 (61.31) | 60 (16.35) | 20 (5.45) |
| 2010 | 301 | 181 (60.13) | 58 (19.27) | 22 (7.31) |
| 2011 | 196 | 124 (63.40) | 45 (22.96) | 12 (6.12) |
Survival
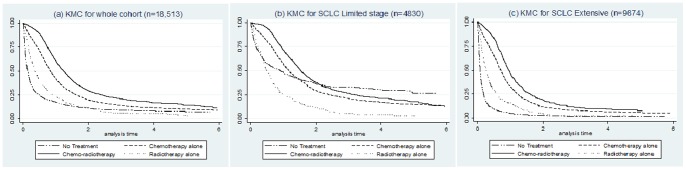
Median survival (MS) from the time of diagnosis for all the patients (N = 18,513) was 6 months (IQR 1.5–12.4). This was 11.4 months (5.5–21.1) and 4 months (1.0–9.0) for patients with limited and extensive stage disease (table 1). Kaplan-Meier survival curves (Figure 1) also showed a difference in overall hazard ratios (HR) based on the treatment received having adjusted for patient features. Compared with patients who received no treatment (MS = 0.72 months), patients who received chemotherapy had an adjusted HR of 0.33, 95% CI 0.32–0.34 (MS = 7.6 months) and patients who had records of radiotherapy and chemotherapy had an adjusted HR of 0.24, 95% CI 0.23–0.25 (MS = 11.6 months). We also looked at the hazard ratios by limited and extensive stage which showed a similar improved survival from receiving any treatment compared with no treatment. However in limited stage disease, there was only a small difference in survival for patients receiving no treatment and those having radiotherapy alone.
Figure 1. Kaplan-Meier curve estimates adjusted for age, sex, PS and co-morbidity by treatments received: (a) for whole cohort, (b) for SCLC limited and (c) for SCLC Extensive.
Discussion
Principal Findings
Histologically proven SCLC accounted for 10% of the total lung cancer cases diagnosed in the NLCA between 2004 and 2011. Survival in SCLC is poor and our results demonstrate that the median survival has not changed in the 8 year period since the audit began. The proportion of patients receiving chemotherapy has increased very slightly over the years and although there has been an increase in the proportion of patients receiving chemotherapy and radiotherapy we observed no change in the overall survival. One of the reasons for this could be the increase in radiotherapy and chemo-radiotherapy observed in our study was actually due to an increased use of prophylactic cranial irradiation (PCI). This treatment is recommended in the NICE (2011) updated guidelines [3], and has shown survival benefits in studies. [15] However, it could not change our survival estimates as the doubling proportion only accounted for a 20% increase in chemo-radiotherapy from 2004–2011, which is still lower than other comparable countries. In our study, there has been little change in the patient demographics from 2004–2011. However, there has been an increase in the proportions classified as extensive and limited stage small-cell lung cancer and an increase in the proportion of patients with more co-morbid illness, which may lead to fewer patients being considered for curative treatment. There was also inequality in chemotherapy use by socioeconomic status where patients from least affluent areas were less likely to receive chemotherapy.
Our results depict a low proportion of patients receiving chemo-radiotherapy, especially for limited stage SCLC. However our results are a reflection of the true treatment patterns and attitude towards treatment in England. It should be noted that even patients with limited stage SCLC can be considered too frail to receive chemo-radiotherapy and in our cohort, one-third of the limited stage SCLC patients had a PS of greater than or equal to 2 and almost the same proportion had a Charlson Index of greater than or equal to 2.
Our results showed that patients who had recorded chemotherapy and radiotherapy had a longer median survival and lower hazard ratio of death, which may reflect treatment efficiency or selection bias such as immortal time bias. Immortal time bias here refers to the fact that some patients, with an inherently better prognosis would receive treatment whereas those with a poor prognosis (associated with more advance disease and co-morbidity) would not. The treatment may be having no effect on prognosis and the apparent better survival may be simply a result of more favourable biological factors.
Strengths and Limitations
The main strength of our study is the large sample size. As far as we know, this is the largest study looking at the trends in chemotherapy use and features of patients with SCLC in England over the duration of the NLCA. Although the NLCA is non-mandatory, it has been validated and found to be representative of the population of lung cancer patients in England. [13] The NLCA provides more data compared with databases used by other international studies (in which case-mix adjustment is not possible). [9] Therefore we believe our results are likely to demonstrate real changes in chemotherapy practice in England. The cases identified in our study were all pathologically confirmed. Within the NLCA missing or unknown pathology is coded as non-small cell lung cancer. Thus some of these cases could have been small cell lung cancer. It is unlikely that this is a significant proportion and most unlikely that this would affect our conclusions. The proportion of small-cell cases observed in our study is in accordance with a study using the Thames Cancer Registry data. [2] A second limitation is the high proportion of patients with stage missing, uncertain and unknown, although this is decreasing year on year. We did not find any evidence of a change in the ratio of limited to extensive stage disease which suggests that the stage distribution did not change of the years and therefore the comparisons of survival by stage over the years was valid. It is unlikely that those patients with missing or uncertain stage were misclassified because of poorer prognosis, as we found their survival was better than those with extensive disease.
We were able to validate records of chemotherapy by using a combination of variables from NLCA and HES, but as inpatient HES data does not capture the majority of radiotherapy episodes, we might have underestimated the total proportion of patients who had radiotherapy. In addition, due to the limited data on radiotherapy, we were unable identify whether the radiotherapy was given with curative or palliative intent.
We have shown an increase in the proportion of patients receiving both chemotherapy and radiotherapy however our data were unable to differentiate radical intent chemo-radiotherapy from radiotherapy given for purely palliative purposes. Chemo-radiotherapy is associated with fairly big survival benefits in clinical trials and in our study there was some evidence of this. However, our group receiving chemotherapy and radiotherapy are likely to include a significant proportion of patients who had both for palliative purposes and therefore any survival benefits of chemo-radiotherapy would have been diluted. This probably explains why the overall survival did not change with the increase in the proportion of patients receiving chemotherapy and radiotherapy.
Comparison with other Studies
We used a combination of databases and definitions to identify patients who had received chemotherapy on an in-patient and out-patient basis, and found that almost 70% of our cohort had received chemotherapy which was 10% higher than an earlier study based on the NLCA database. [8] Previous studies including one study conducted in the Netherlands [16] and one study using the Surveillance Epidemiologic and End Results (SEERs) database [17] showed survival improvement for patients with SCLC, which we did not observe. However, the findings of the SEERs study of a 3% annual change in survival in both limited and extensive stage SCLC were over a longer timeframe (1973–2002) than in the present study. [17] Over this period treatment is likely to have changed much more than from 2004 to 2011. Chemotherapy regimens, supportive care and the use of combined chemo-radiotherapy have changed to a greater extent based on evidence from clinical trial in 1987 [18] and multiple meta-analysis in the 1990s [19], [20] showing chemo-radiotherapy to have better outcomes to chemotherapy alone promoting the use of newer therapies. To our knowledge, there have been no new changes in the treatment regimen of SCLC since 2004 and this is the likely explanation for the lack of an annual overall survival improvement in our study. Our study shows the inequality in chemotherapy use in older patients (i.e. 65–75 & >75 years) even after adjusting for several other patient features, which is similar to the results found by other studies. [8], [21], [22] A similar association was seen in patients from more deprived areas who were less likely to receive chemotherapy, which is similar to the findings of other studies. [8], [23] We also observed that the proportion of patients with comorbidities increased over the years, which is not seen in previous studies. The reason for this is unknown but could be due to better recording of comorbidities in the HES database.
Clinical Relevance
The overall survival of patients with SCLC has not changed since the audit initiated in 2004, probably due to the fact that there has been little change in the type of treatment offered or in the stage at presentation. The only improvement we observed over the years was an increase in the proportion of patients who received radiotherapy in addition to chemotherapy which corresponded to a better survival compared with other treatment regimens but as this applied to only small proportion of the patients as a whole, it was not enough to change the overall survival. The reasons for the unchanged survival are unknown but this could be due to the relatively modest advances in treatment for a small proportion of patients (mostly chemo-radiotherapy for limited stage). It is concerning that only a relatively small proportion of patients receive chomo-radiotherapy, as this is the established best standard of care, and clearly associated with better survival. The therapy does not require patients to have a reasonable level of fitness and co-morbidity rates in the UK can be high. Nevertheless it is important for clinicians to reflect on our findings and to consider whether more patients might be offered this apparently more effective treatment.
The UK radiotherapy database and Systemic Anti-cancer Therapy dataset (SACT) started to collect data in April 2009 and April 2012, [24] and in due course, analysis of these data would provide us with a more detailed picture of the impact of increasing radiotherapy use on survival for patients with SCLC.
Supporting Information
Baseline characteristics of patients receiving chemo-radiotherapy, chemotherapy alone or radiotherapy alone (n = 18,513).
(DOCX)
Acknowledgments
The National Lung Cancer Audit is commissioned by the Healthcare Quality Improvement Partnership (HQIP). The authors are very grateful to the wider NLCA steering group and all the clinicians, nurses and administrators who have entered the data. We would also like to acknowledge the Health and Social Care Information Centre (Arthur Yelland and Xanthe Hannah) who provided data.
Funding Statement
RBH has a grant provided by the British Lung foundation chair of respiratory epidemiology and has a minor consultancy role with GSK concerning people with lung fibrosis where he was paid £350. He is also a co-applicant on a grant for £500,000 from GSK to look at biomarkers for lung fibrosis. AK is also doing his PhD from the University of Nottingham on a studentship provided by the British Lung Foundation. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
References
- 1.National Institute for Health and Care Excellence (Feburary 2005) The Diagnosis and Treatment of Lung Cancer: Methods, evidence & guidance.
- 2. Riaz SP, Luchtenborg M, Coupland VH, Spicer J, Peake MD, et al. (2012) Trends in incidence of small cell lung cancer and all lung cancer. Lung Cancer 75: 280–284. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Clinical Excellence (April 2011) Lung Cancer: The diagnosis and treatment of lung cancer.
- 4.Health and Social Care Information Centre (2010) National Lung Cancer Audit 2010.
- 5.Health and Social Care Information Centre (2011) National Lung Cancer Audit Report 2011.
- 6. Green RA, Humphery E, Close H, Patno ME (1969) Alkylating agents in bronchogenic carcinoma. The American Journal of Medicine 46: 516–525. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Dai C-H, Chen P, Wu J-N, Bao Q-L, et al. (2010) Survival and prognostic factors in small cell lung cancer. Medical Oncology 27: 73–81. [DOI] [PubMed] [Google Scholar]
- 8. Rich AL, Tata LJ, Free CM, Stanley RA, Peake MD, et al. (2011) How do patient and hospital features influence outcomes in small-cell lung cancer in England? British Journal of Cancer 105: 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woolhouse I (2011) Variation in lung cancer outcomes in the UK and Europe. Clinical Medicine 11: 110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleman MP, Forman D, Bryant H, Butler J, Rachet B, et al. (2011) Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 377: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walters S, Maringe C, Coleman MP, Peake MD, Butler J, et al. (2013) Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax 68: 551–564. [DOI] [PubMed] [Google Scholar]
- 12.CRUK (2012) Statistics and outlook for lung cancer. Cancer Research UK.
- 13. Rich AL, Tata LJ, Stanley RA, Free CM, Peake MD, et al. (2011) Lung cancer in England: information from the National Lung Cancer Audit (LUCADA). Lung Cancer 72: 16–22. [DOI] [PubMed] [Google Scholar]
- 14.Union for International Cancer Control (2009) TNM Classification of Malignant Tumours, 7th Edition. In: Sobin LH, Gospodarowicz MK, Wittekind C, editors: Wiley-Blackwell.
- 15. Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, et al. (2007) Prophylactic Cranial Irradiation in Extensive Small-Cell Lung Cancer. New England Journal of Medicine 357: 664–672. [DOI] [PubMed] [Google Scholar]
- 16. Janssen-Heijnen MLG, Maas HAAM, Siesling S, Koning CCE, Coebergh JWW, et al. (2012) Treatment and survival of patients with small-cell lung cancer: small steps forward, but not for patients >80. Annals of Oncology 23: 954–960. [DOI] [PubMed] [Google Scholar]
- 17. Govindan R, Page N, Morgensztern D, Read W, Tierney R, et al. (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of Clinical Oncology 24: 4539–4544. [DOI] [PubMed] [Google Scholar]
- 18. Perry MC, Eaton WL, Propert KJ, Ware JH, Zimmer B, et al. (1987) Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. New England Journal of Medicine 316: 912–918. [DOI] [PubMed] [Google Scholar]
- 19. Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, et al. (1992) A Meta-Analysis of Thoracic Radiotherapy for Small-Cell Lung Cancer. New England Journal of Medicine 327: 1618–1624. [DOI] [PubMed] [Google Scholar]
- 20. Warde P, Payne D (1992) Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. Journal of Clinical Oncology 10: 890–895. [DOI] [PubMed] [Google Scholar]
- 21. Brown JS, Eraut D, Trask C, Davison AG (1996) Age and the treatment of lung cancer. Thorax 51: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludbrook JJS, Truong PT, MacNeil MV, Lesperance M, Webber A, et al. (2003) Do age and comorbidity impact treatment allocation and outcomes in limited stage small-cell lung cancer? a community-based population analysis. International Journal of Radiation Oncology, Biology, Physics 55: 1321–1330. [DOI] [PubMed] [Google Scholar]
- 23. Crawford SM, Sauerzapf V, Haynes R, Zhao H, Forman D, et al. (2009) Social and geographical factors affecting access to treatment of lung cancer. British Journal of Cancer 101: 897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Clinical Analysis and Specialised Application Team (NATCANSAT) (2013) National Radiotherapy Dataset - RTDS.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of patients receiving chemo-radiotherapy, chemotherapy alone or radiotherapy alone (n = 18,513).
(DOCX)


