Abstract
Iron-sulfur [Fe-S] clusters are ubiquitous and critical cofactors in diverse biochemical processes. They are assembled by distinct [Fe-S] cluster biosynthesis pathways, typically in organelles of endosymbiotic origin. Apicomplexan parasites, including Plasmodium, the causative agent of malaria, harbor two separate [Fe-S] cluster biosynthesis pathways in the their mitochondrion and apicoplast. In this study, we systematically targeted the five nuclear-encoded sulfur utilization factors (SUF) of the apicoplast [Fe-S] cluster biosynthesis pathway by experimental genetics in the murine malaria model parasite Plasmodium berghei. We show that four SUFs, namely SUFC, D, E, and S are refractory to targeted gene deletion, validating them as potential targets for antimalarial drug development. We achieved targeted deletion of SUFA, which encodes a potential [Fe-S] transfer protein, indicative of a dispensable role during asexual blood stage growth in vivo. Furthermore, no abnormalities were observed during Plasmodium life cycle progression in the insect and mammalian hosts. Fusion of a fluorescent tag to the endogenous P. berghei SUFs demonstrated that all loci were accessible to genetic modification and that all five tagged SUFs localize to the apicoplast. Together, our experimental genetics analysis identifies the key components of the SUF [Fe-S] cluster biosynthesis pathway in the apicoplast of a malarial parasite and shows that absence of SUFC, D, E, or S is incompatible with Plasmodium blood infection in vivo.
Introduction
Iron-sulfur [Fe-S] clusters are small inorganic cofactors that are present in most organisms. Proteins containing [Fe-S] clusters are involved in numerous biological processes, ranging from mitochondrial oxidative phosphorylation [1] and photosynthesis [2] to DNA replication [3], DNA repair [4], ribosome biogenesis [5], and regulation of gene expression [6]. Accordingly, the list of [Fe-S] cluster-containing proteins is continuously expanding. Although early in vitro studies suggested a spontaneous assembly [7], [Fe-S] clusters are not formed spontaneously in living cells but rather assembled through distinct [Fe-S] biosynthesis pathways.
Bacteria harbor the sulfur utilization factor (SUF) and iron-sulfur cluster (ISC) systems for assembly of [Fe-S] clusters. In Escherichia coli, the ISC system is thought to mediate housekeeping functions, whereas the SUF system was shown to be especially important under stress conditions such as iron starvation [8], [9]. However, deletion of individual operons is not lethal [10], [11].
In eukaryotes, [Fe-S] biogenesis machineries are thought to have evolved from their bacterial counterparts that had been acquired by endosymbiosis [12]. Different [Fe-S] cluster assembly systems are required for biogenesis in distinct cellular compartments, namely the ISC system in the mitochondrion and the SUF system in plastids. The mitochondrial ISC proteins were found to supply [Fe-S] clusters to the mitochondrial [Fe-S] proteins and to [Fe-S] proteins in the cytosol [13], where the cytosolic iron-sulfur protein assembly (CIA) machinery is responsible for the maturation of cytosolic [Fe-S] proteins [14].
In spite of the differences between bacteria and eukaryotes, the basic principles of [Fe-S] clusters biogenesis seem to be conserved. First, the [Fe-S] cluster is assembled de novo onto a scaffold protein. For this step, sulfur is mobilized from cysteine by a cysteine desulfurase (SufS, IscS, NifS) [15]. The iron source is mostly unknown. Second, the [Fe-S] cluster is transferred from the scaffold protein to a target apoprotein and assembled into the polypeptide chain. The most common [Fe-S] clusters are rhombic [2Fe-2S] or cubic [4Fe-4S]. More complex structures have also been described, some of which include additional heavy metals [16].
The SUF system in E.coli, for instance, consists of six genes organized in the sufABCDSE operon [11]. SufS acts as cysteine desulfurase that provides the sulfur for the [Fe-S] cluster and SufE has been shown to interact with SufS to enhance its activity up to 50-fold [17], [18]. SufB, C, and D form a functional complex serving as a [Fe-S] scaffold [19], [20] and also enhancing SufS function [18]. SufC was shown to contain ATPase activity in Erwinia chrysanthemi [21] and the crystal structure of E. coli SufC confirmed it to be an ABC-type ATPase [22]. SufA interacts with the SufBCD complex to accept [Fe-S] clusters formed de novo [19].
In Plasmodium, a genus of eukaryotic, single cell parasites that are the causative agents of malaria, components of the SUF, ISC, and CIA system have been identified by bioinformatic analyses [23]–[25]. Like in other eukaryotes, the ISC system is predicted to localize to the mitochondrion and the SUF system to the apicoplast, a vestigial, non-photosynthetic plastid of red algal origin [24]. In Plasmodium, all components of the SUF system are nuclear-encoded, except for SUFB, which is encoded in the small circular apicoplast genome [26].
A biochemical study focusing on the P. falciparum plastid SUF system provided evidence that SUFC localizes to the apicoplast [27]. In bacteria and plants, SUFC interacts with SUFB [21], [28], [29], which was confirmed for the corresponding P. falciparum proteins [27]. In Arabidopsis thaliana, SUFB and SUFC both display ATPase activity [29], whereas the bacterial SUFB seems to lack this activity. ATPase activity of recombinant PfSUFB and PfSUFC proteins provided supporting evidence for the evolutionary conservation of the plastid SUF system between plants and apicomplexan parasites [27]. PfSUFE and PfSUFS have also been assigned to the apicoplast, while PfSUFS was shown to functionally complement E. coli deficient in SufS [30].
Data-mining and bioinformatic analysis of potential Plasmodium [Fe-S] cluster-containing proteins revealed at least 31 candidates, seven of which are predicted to localize to the apicoplast (Table 1). These proteins are involved in diverse pathways, such as mevalonate-independent isoprenoid biosynthesis, lipoic acid metabolism, and biogenesis of [Fe-S] clusters itself. Because some proteins are components of essential biosynthesis pathways, most notably the DOXP pathway of isoprenoid biosynthesis [31], plastid [Fe-S] cluster assembly is likely essential for parasite survival.
Table 1. Confirmed and predicted [Fe-S] cluster-containing proteins in Plasmodium.
| P. berghei a | P. falciparum a | Annotation | PlasmoAPb | ApicoAPb | PlasMitb | MitoProtIIb |
| APICOPLAST a | ||||||
| PBANKA_020870 | PF3D7_0104400 | IspH/LytB, 4-hydroxy-3-methylbut-2-enyl diphosphate reductase | −/− | no SP | possibly | 0.5404 |
| PBANKA_050700 | PF3D7_1022800 | IspG/GcpE, 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase | +/++ | ATP | non-mito | 0.8285 |
| PBANKA_070700 | PF3D7_0823600 | LipB, lipoate-protein ligase | +/++ | no SP | non-mito | 0.9748 |
| PBANKA_081190 | PF3D7_0910800 | nucleotide binding protein, putative | ++/++ | ATP | non-mito | 0.9760 |
| PBANKA_112110 | PF3D7_0622200 | radical SAM protein, putative | +/++ | ATP | non-mito | 0.9952 |
| PBANKA_135750 | PF3D7_1344600 | LipA, lipoyl synthase | ++/++ | ATP | non-mito | 0.6838 |
| PBANKA_141660 | PF3D7_1318100 | ferredoxin, putative | ++/++ | ATP | non-mito | 0.8117 |
| MITOCHONDRION a | ||||||
| PBANKA_061790 | PF3D7_0720400 | ferrodoxin reductase-like protein | −/++ | no SP | possibly | 0.8670 |
| PBANKA_082810 | PF3D7_0927300 | fumarat hydratase, putative | −/++ | no SP | non-mito | 0.8939 |
| PBANKA_090930 | PF3D7_1139700 | adrenodoxin reductase, putative | −/+ | no SP | possibly | 0.0610 |
| PBANKA_122950 | PF3D7_0614800 | endonuclease III homologue, putative | −/++ | no SP | non-mito | 0.5453 |
| PBANKA_130330 | PF3D7_1439400 | ubiquinol-cytochrome c reductase iron-sulfur subunit, putative | −/++ | no SP | possibly | 0.9782 |
| PBANKA_135520 | PF3D7_1342100 | aconitate hydratase | −/++ | no SP | possibly | 0.8460 |
| PBANKA_142880 | PF3D7_1212800 | iron-sulfur subunit of succinate dehydrogenase | −/++ | no SP | possibly | 0.1521 |
| PBANKA_143040 | PF3D7_1214600 | adrenodoxin-type ferredoxin, putative | −/++ | no SP | possibly | 0.8299 |
| NUCLEUS or CYTOPLASM a | ||||||
| PBANKA_011240 | PF3D7_0614200 | conserved Plasmodium protein, unknown function | −/− | no SP | non-mito | 0.0141 |
| PBANKA_083490 | PF3D7_0934100 | XPD/ERCC2, DNA excision-repair helicase, putative | −/− | no SP | non-mito | 0.0106 |
| PBANKA_091970 | PF3D7_1128500 | conserved protein, unknown function | −/− | non-mito | 0.1301 | |
| PBANKA_101520 | PF3D7_1429500 | diphthamide synthesis protein, putative | −/− | no SP | non-mito | 0.2517 |
| PBANKA_102890 | PF3D7_1413800 | diphthamide synthesis protein, putative | −/− | no SP | possibly | 0.0176 |
| PBANKA_103410 | PF3D7_1408400 | DNA-repair helicase, putative | −/− | no SP | possibly | 0.0139 |
| PBANKA_103530 | PF3D7_1406900 | radical SAM protein, putative | −/+ | no SP | possibly | 0.1722 |
| PBANKA_133970 | PF3D7_1324500 | DEAD box helicase, putative | −/− | no SP | non-mito | 0.4691 |
| SUBCELLULAR LOCALIZATION NOT PREDICTED a | ||||||
| PBANKA_011230 | PF3D7_0614100 | conserved Plasmodium protein, unknown function | −/++ | no SP | possibly | 0.2399 |
| PBANKA_070600 | PF3D7_0824600 | anamorsin related protein, putative | −/− | no SP | non-mito | 0.1138 |
| PBANKA_081200 | PF3D7_0910900 | DNA primase large subunit, putative | −/++ | no SP | possibly | 0.0509 |
| PBANKA_090570 | PF3D7_1143300 | DNA-directed RNA polymerase I, putative | −/− | no SP | non-mito | 0.0677 |
| PBANKA_100950 | PF3D7_1435300 | NAD(P)H-dependent glutamate synthase, putative | −/− | no SP | non-mito | 0.0131 |
| PBANKA_114410 | PF3D7_1368200 | RNAse L inhibitor protein, putative | −/− | no SP | possibly | 0.0535 |
| PBANKA_123970 | PF3D7_0524900 | tRNA-YW synthesizing protein, putative | −/0 | no SP | non-mito | 0.0527 |
| PBANKA_144250 | PF3D7_1227800 | histone S-adenosyl methyltransferase, putative | −/− | no SP | non-mito | 0.0660 |
Gene IDs of the P. berghei and P. falciparum orthologs and the predicted localizations of the proteins were retrieved from PlasmoDB (http://PlasmoDB.org) or identified by similarity searches using A. thaliana [Fe-S] cluster proteins as query sequences [41].
Putative targeting of the P. falciparum [Fe-S] cluster-containing proteins to the apicoplast or mitochondrion was predicted using four different algorithms. PlasmoAP [51] indicates the likelihood of the presence of the required signal peptide followed by the likelihood of an apicoplast localization (“−” = unlikely, “0″ = undecided, “+” = likely, “++” = very likely). ApicoAP [52] is a different algorithm that can identify apicoplast proteins in multiple Apicomplexa (“No SP” = no signal peptide, “No ATP” = signal peptide but no transit peptide, “ATP” = apicoplast targeted protein). PlasMit [53] predicts the likelihood of a mitochondrial localization for P. falciparum proteins (“non-mito” (99%), “possibly” (91%), and “mito” (99%)). MitoProtII [54] gives a probability score for the likelihood of mitochondrial localization but is not optimized for Plasmodium sequences.
We previously reported that a Plasmodium-specific component of the apicoplast [Fe-S] cluster biosynthesis pathway, nitrogen fixation factor U (NifU)-like domain containing protein (NFUapi), can be deleted in the murine malaria model parasite P. berghei and plays an auxiliary role in liver stage merozoite formation [32]. Here, we present a systematic experimental genetics analysis of the P. berghei apicoplast SUF system. We show that four of five nuclear-encoded P. berghei SUF genes are refractory to gene deletion and, hence, can be considered likely essential for blood stage proliferation. Endogenous tagging confirmed accessibility of the loci to targeted gene modification and the resulting fluorescent fusion proteins showed co-localizations with an apicoplast resident protein.
Results
The Principal Components of the Plasmodium berghei SUF Pathway are Refractory to Targeted Deletion in vivo
We first wanted to investigate whether the SUF genes of the Plasmodium apicoplast [Fe-S] cluster biosynthesis pathway were susceptible or refractory to targeted gene deletion. For this systematic genetic characterization, we employed the murine malaria model system P. berghei that permits in vivo selection of recombinant parasites. We attempted to generate loss-of-function mutants of the five nuclear-encoded components (Fig. 1). SUFB (PBANKA_API0012) is encoded on the apicoplast circular genome and cannot be targeted by the available transfection technologies.
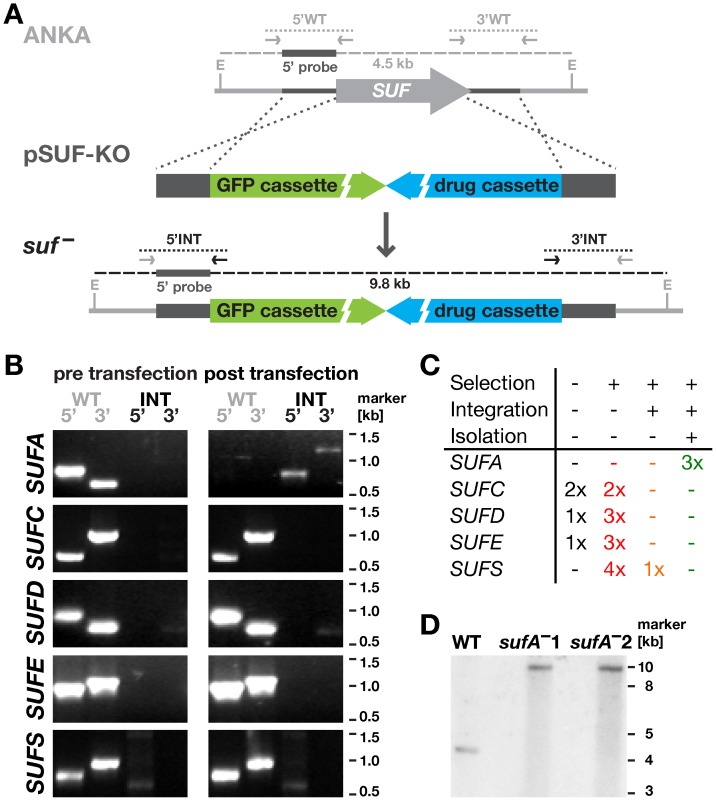
Figure 1. Systematic gene targeting of Plasmodium berghei SUF genes.
(A) Replacement strategy to delete the five nuclear-encoded PbSUF genes. The respective ANKA strain wild type (WT) SUF loci were targeted with replacement plasmids (pKO) containing upstream 5′ and downstream 3′ regions (dark gray bars) flanking the open reading frames (light gray arrow), a high-expressing GFP cassette (green), and the hDHFR-yFcu drug-selectable cassette (blue). Integration-specific (5′INT and 3′INT) and wild type-specific (5′WT and 3′WT) primer combinations (Table S1) are indicated by arrows; expected PCR fragments by dotted lines. The probe used for Southern blot analysis of the two isogenic sufA – parasites lines corresponds to the 5′ integration sequence and hybridized to EcoRI (E) restriction-digested gDNA; expected fragments and their sizes are indicated by gray dashed lines. (B) Representative diagnostic PCR results of the SUF loci of WT ANKA (pre transfection) and drug-selected (post transfection) parasites are shown. For SUFA, diagnostic PCR of isogenic gene deletion parasites confirms successful integration and absence of WT parasite contamination. (C) Overview of all transfection experiments summarizing the number of times no pyrimethamine-resistant parasites were selected (black), selection of pyrimethamine-resistant parasites was achieved (red), integration-specific PCR demonstrated targeted deletion of the SUF gene (orange), and isolation of WT-free, isogenic recombinant parasites (green). (D) Southern blot analysis of two isogenic sufA – parasite lines reveals the expected size shifts.
We employed currently available experimental genetics techniques [33], [34] to delete the open reading frames of all five target genes (Fig. 1A). Upon successful double homologous/ends out recombination events, recombinant parasites are predicted to contain high-expressing GFP- and drug-selectable cassettes in place of the respective genes. After positive selection with the antimalarial drug pyrimethamine, potential recombinant parasites were isolated by flow cytometry [35] and subjected to genotyping by diagnostic PCR (Fig. 1B and Table S1).
In three independent transfection attempts, we were able to select sufA – parasites (Fig. 1B and C). In marked contrast, we were unable to generate recombinant gene deletion parasites for SUFC (PBANKA_102920), SUFD (PBANKA_094350), SUFE (PBANKA_030380), or SUFS (PBANKA_061430) in four independent transfection experiments. In a single transfection experiment targeting SUFS, we observed integration-positive PCR products. Repeated attempts to isolate gene deletion mutants via flow cytometry or limiting dilution cloning always resulted in a wild type (WT)-containing population, as tested by a WT-specific PCR reaction that only amplifies the WT locus, suggesting a requirement for the presence of SUFS as shown previously for other essential genes, e.g. the chloroquine resistance transporter [36]. Refractoriness to targeted gene deletion strongly suggests that the four SUF genes are crucial for propagating a successful blood infection, the phase of the Plasmodium life cycle where transfection is performed.
Collectively, our reverse genetics findings indicate that four out of five Plasmodium SUF proteins, namely SUFC, SUFD, SUFE, and SUFS, perform critical functions during blood infection of the malarial parasite.
SUFA is Dispensable for Plasmodium life Cycle Progression
Repeated successful deletion of SUFA (PBANKA_123740) during our transfection experiments already indicated non-vital roles of the target gene for erythrocytic parasite propagation. In order to determine potential in vivo roles during the parasite life cycle, we selected two isogenic sufA – populations. Genotyping, including Southern blot analysis (Fig. 1D), confirmed the homogenous presence of sufA – parasites only.
To mimic a natural infection, we propagated the two selected sufA – populations through the mosquito vector, female Anopheles stephensi, and isolated sporozoites from infected salivary glands. Intravenous injection of 10,000 wild type (WT) or sufA – sporozoites were performed to infect and monitor transmission to C57BL/6 mice (Fig. 2). This analysis showed the typical pre-patent period, which is the time until first detection of blood stage parasites in peripheral blood following infection with sporozoites and which includes the liver stage development, of three days in all animals tested. Moreover, during the following days, sufA – and WT-infected animals displayed similar development of parasitemias. All parasites first replicated exponentially before entering a plateau phase, once the parasitemia was close to 1%. The exposure of three naïve C57BL/6 mice to bites of five infected mosquitoes also resulted in successful natural transmission in two mice. In conclusion, this analysis demonstrated that SUFA does not play important roles in establishment and propagation of an erythrocytic infection and, hence, is not a valid target for rational drug design.
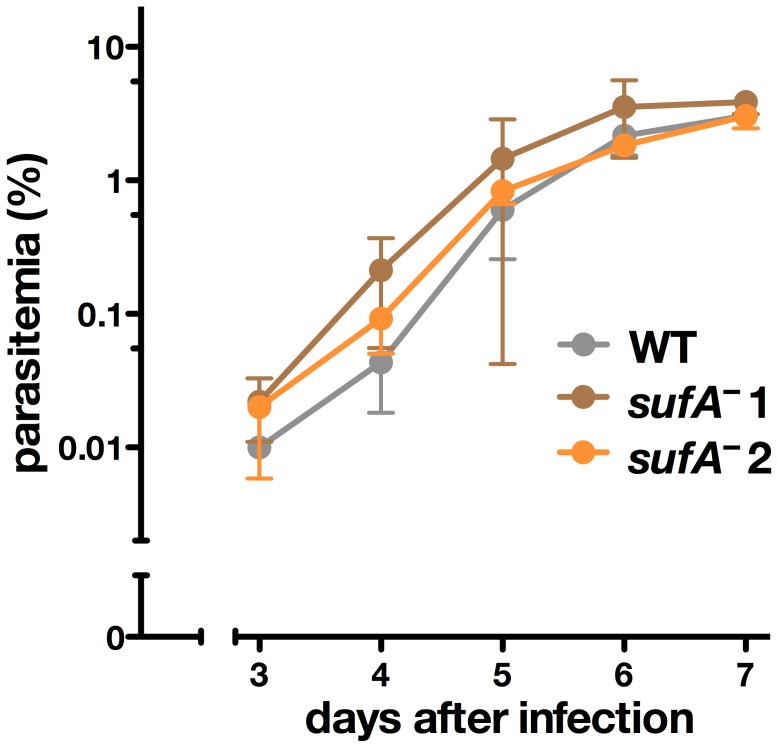
Figure 2. SUFA is dispensable for blood infection in vivo.
Parasitemias of sufA –-infected animals in comparison to mice infected with wild type (WT) parasites. Female C57BL/6 mice (WT, n = 3; sufA –1 and 2, n = 5 each) were injected intravenously with 10,000 freshly dissected salivary gland sporozoites and infection was monitored by microscopic examination of Giemsa-stained blood films. The two isogenic sufA – parasite lines (brown and orange lines) and WT parasites (gray line) showed equal pre-patent periods (three days) and similar exponential parasite growth (P>0.05; two-way ANOVA followed by Bonferroni posttests).
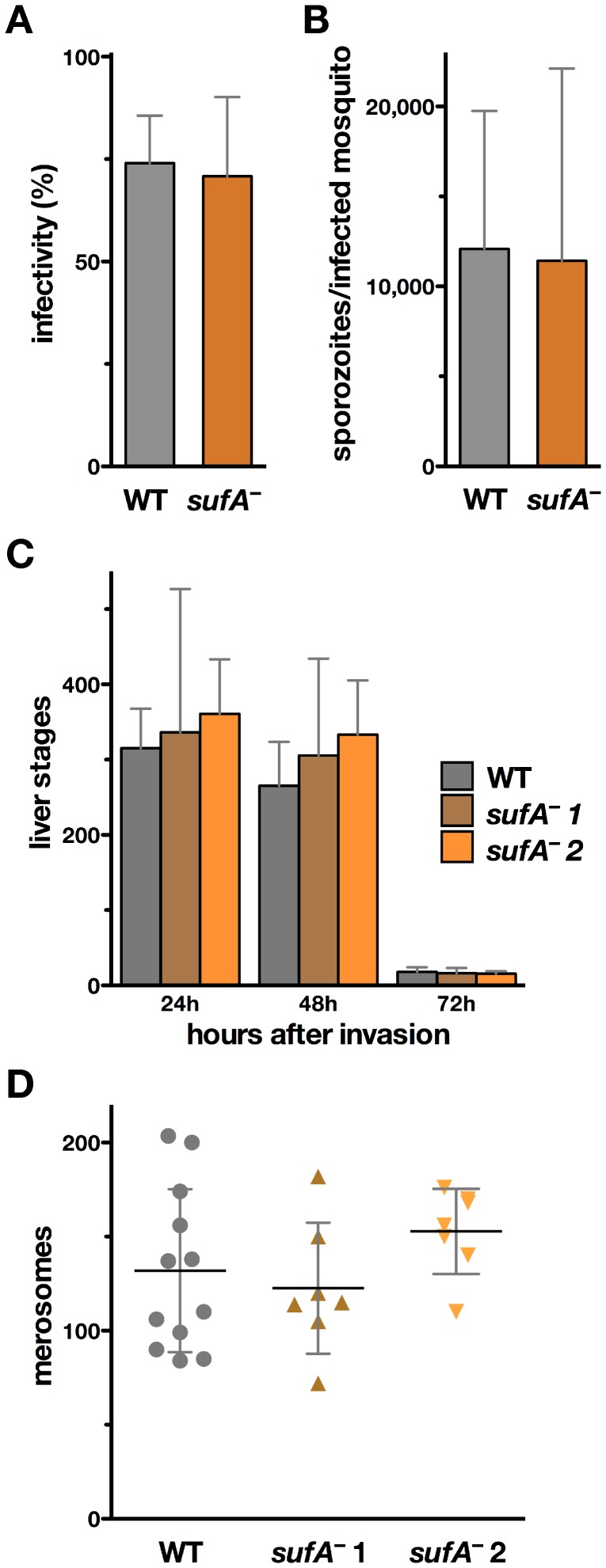
Successful sufA – sporozoite isolation and infection of mice were already indicative of unaltered life cycle progression in the absence of SUFA. To exclude modest defects, we systematically assessed parasite development in the mosquito vector and during pre-erythrocytic growth (Fig. 3). Transmission to mosquitoes and sporogony were indistinguishable from WT parasites, as exemplified by similar infectivity to mosquitoes (Fig. 3A) and normal sporozoite numbers in salivary glands, the final target organ in the mosquito vector (Fig. 3B). Also when we quantified intrahepatic parasite stages in cultured hepatoma cells (Fig. 3C) and merosomes from culture supernatants, representing emerging merozoites (Fig. 3D), we could not distinguish sufA– from WT parasites. In both parasite lines, merosomes are released into the supernatant between 48 and 72 h after infection, leading to a remarkable drop in parasite numbers in hepatoma cells seen at 72 h after infection.
Figure 3. Normal development of sufA – parasites in the mosquito vector and during liver cell infection.
(A) Percentage of A. stephensi mosquitoes infected with WT (gray, n = 4) and sufA – (brown, n = 6) parasites. Shown is the mean percentage (± S.D.) from independent mosquito feeding experiments. For sufA – infections, both isogenic strains, sufA –1 (n = 4) and sufA –2 (n = 2), were transmitted to mosquitoes and data combined. (B) Mean sporozoite number (± S.D.) in salivary glands (days 17–21 after infection) from the same independent mosquito feedings as shown in panel A (WT, n = 4; pooled sufA –, n = 6). (C) Liver stages development of WT and sufA – parasites in cultured hepatoma cells. Shown are mean numbers (± S.D.) of intracellular parasites at the time points indicated from two experiments done in quadruplicate each. (D) Merosome formation at 72 h after infection. Shown are mean values (± S.D.). None of the sufA – data points were significantly different from the corresponding WT values (P>0.05; non-parametric, two-tailed Mann-Whitney’s test).
Together, our analysis demonstrates that absence of SUFA is compatible with effective host switch, parasite stage conversion, and population expansion throughout the entire Plasmodium life cycle.
Apicoplast Localization of Plasmodium SUF Proteins
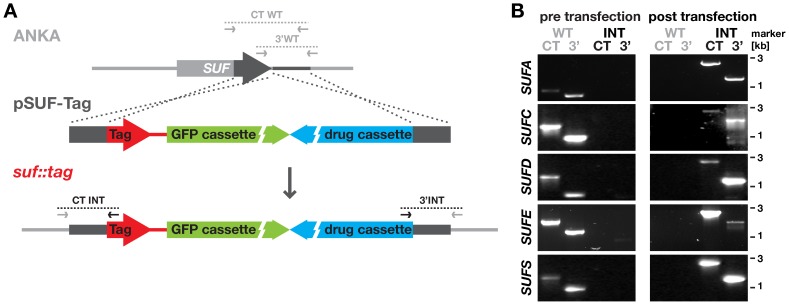
In order to gain independent confirmation that the four essential SUF loci are susceptible to genetic manipulation, we targeted SUFC, D, E, and S along with SUFA by double homologous/ends-out recombination introducing a carboxy-terminal in-frame fusion with the combined mCherry-3xMyc tag (Fig. 4A), using the same strategy we previously employed to tag NFUapi [32]. Upon successful recombination, selection of recombinant parasites by the antifolate pyrimethamine, and WT-free isolation by flow cytometry, we readily obtained all five desired recombinant parasites, i.e. sufA::tag, sufC::tag, sufD::tag, sufE::tag, and sufS::tag, after the first transfection attempt (Fig. 4B). In addition to demonstrating accessibility of the loci to genetic modification, the parasite populations provided templates for the verification of the respective diagnostic PCRs of the 3′ integration of the unsuccessful gene deletion attempts (Fig. 1B), which use the same homologous sequence for integration.
Figure 4. Control transfections of Plasmodium berghei SUF genes and generation of fluorescently tagged SUF parasite lines.
(A) Replacement strategy to generate stable parasite lines that express the endogenous SUF proteins fused to an mCherry-3xMyc tag (red). The respective ANKA strain wild type (WT) SUF loci were targeted with replacement plasmids (pSUF-Tag) containing carboxy terminal (CT) and downstream 3′ regions (dark gray bars), a high-expressing GFP cassette (green), and the hDHFR-yFcu drug-selectable cassette (blue). Integration-specific (CT INT and 3′INT) and wild type-specific (CT WT and 3′WT) primer combinations (Table S1) and expected fragments are indicated by arrows and dotted lines, respectively. (B) PCR-based genotyping of the suf::tag parasites to verify successful fusion of the respective SUF genes with the mCherry-3xMyc tag and WT-free isolation of the recombinant suf::tag parasites. Note that the 3′ WT- and integration-specific PCRs are identical to those designed for targeted gene deletion (Figure 1).
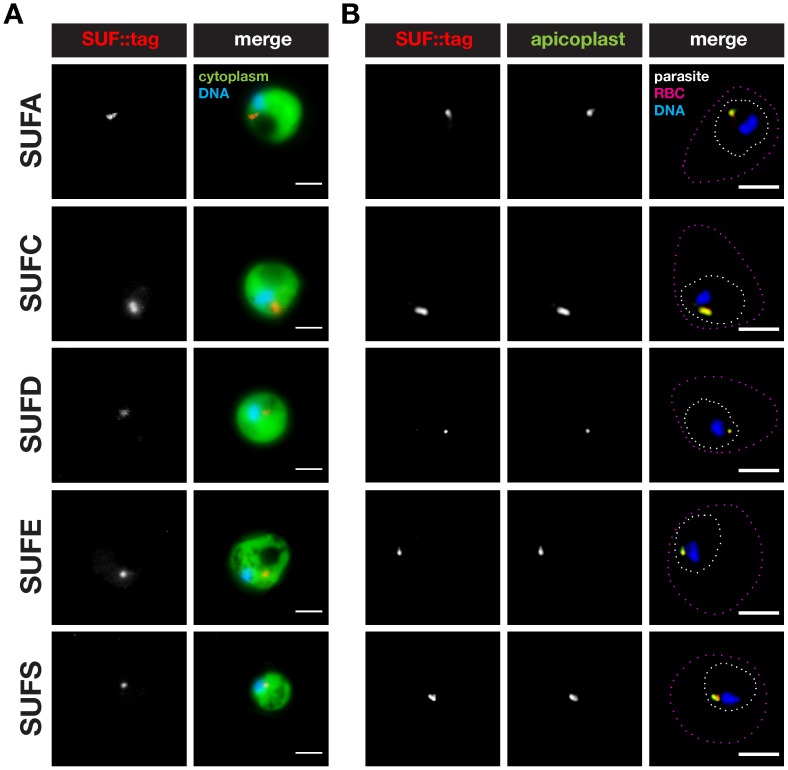
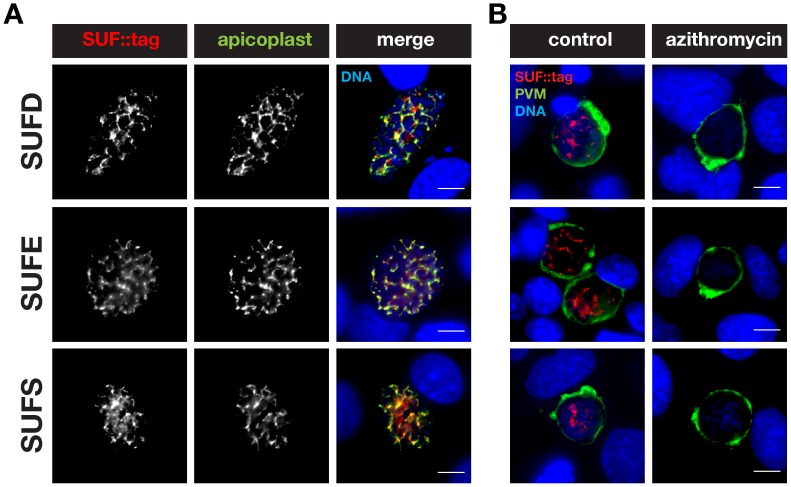
These parasites also provided an opportunity to verify the predicted apicoplast targeting by fluorescence microscopy (Fig. 5 and 6). As expected, all five fusion proteins displayed a punctate staining in live P. berghei blood stage trophozoites, reminiscent of the apicoplast (Fig. 5A). The localization of PbSUFC::tag fully supports the previous finding of a punctate pattern in P. falciparum-infected erythrocytes observed with an anti-SUFC serum [27]. We confirmed the apparent apicoplast localization in fixed blood stage parasites by staining these with anti-mCherry antibodies and an anti-serum recognizing acyl carrier protein (ACP), an apicoplast signature protein (Fig. 5B). Using the same antibodies, we obtained supporting evidence for an apicoplast localization of three SUF fusion proteins, SUFD::tag, SUFE::tag, and SUFS::tag, in developing liver stage parasites (Fig. 6A). As expected in the case of an apicoplast localization, the characteristic, SUFD, E, and S-positive branched structures were destroyed following treatment with azithromycin (Fig. 6B).
Figure 5. Localization of Plasmodium berghei SUFs to the apicoplast in blood stages.
(A) Epifluorescent micrographs of live suf::tag parasite-infected mouse erythrocytes. Shown are representative micrographs of trophozoite stage parasites. The parasite cytoplasm is labeled by GFP and parasite nuclei by the DNA-dye Hoechst. Bars, 2 µm. (B) Co-staining of fixed trophozoite stage suf::tag parasites using anti-mCherry antibodies and anti-sera against acyl carrier protein (ACP), a signature protein of the apicoplast. Substantial overlap can be observed between the SUF::tag proteins and the signature apicoplast protein in a small structure, i.e. the apicoplast. The outlines of the parasite and the infected red blood cell (RBC) are indicated by white and magenta dotted lines, respectively. Nuclei were stained with the DNA-dye Hoechst. Bars, 2 µm.
Figure 6. Localization of Plasmodium berghei SUFD, E, and S to the apicoplast in liver stages.
(A) Co-staining of fixed, sufD::tag, sufE::tag, or sufS::tag parasite-infected hepatoma cells 48 h after sporozoite infection using anti-mCherry antibodies and anti-sera against acyl carrier protein (ACP). Note substantial overlap between the SUF::tag proteins and the signature apicoplast protein. (B) Drug treatment of suf::tag-infected hepatoma cells to corroborate apicoplast localization of the SUF::tag proteins. During liver stage development suf::tag-infected cells were left untreated (control) or treated with 1 µM azithromycin. Liver stages were stained with anti-mCherry antibodies and anti-sera against upregulated in infective sporozoite protein 4 (UIS4), a signature protein of the parasitophorous vacuolar membrane (PVM). Nuclei were stained with the DNA-dye Hoechst. Bars, 10 µm.
We note that all tagged SUF proteins displayed similar localization, yet differed in intensity in the live blood stage parasites, with SUFC::tag yielding the most prominent signal, while SUFA::tag and SUFD::tag were only detectable after prolonged exposure times. Transcription data available online (http://PlasmoDB.org) confirm generally low transcript levels, particularly for SUFA and SUFD, with SUFE being most abundantly expressed [37]. Though we could not confirm this in live blood stage parasite, SUFE::tag signals were the most prominent during liver stage development. This initial observation indicates that a detailed biochemical study to investigate the stoichiometry and order of the apicoplast [Fe-S] cluster biogenesis pathway is warranted.
Discussion
Our data provide the first genetics evidence that four of five SUF proteins are refractory to targeted gene deletion and, hence, most likely vital for blood stage development. We provide additional experimental support for this notion by successful fluorescent tagging through a complementation strategy. Based on the important role(s) of SUFC for parasite growth, we hypothesize that, by analogy, its partner protein SUFB, encoded in the apicoplast genome and inaccessible to experimental genetics, likely exerts essential functions as well.
Two aspects provide further confidence that the central components of the Plasmodium apicoplast SUF system, namely SUFC, SUFD, SUFE, and SUFS, are critical for survival during blood stage growth. First, we used the most recent and efficient techniques available for experimental genetics in P. berghei [34], [35] that have previously enabled the challenging generation of a slow growing recombinant line lacking a putative protein export regulator [38]. Second, we successfully deleted only two components of the apicoplast [Fe-S] cluster biogenesis pathway, namely SUFA, described herein, and NFUapi [32], both of which are estimated to have carrier rather than assembly functions in E. coli [19], [39]. This finding combined with the absence of a plastid-like SUF system in humans provides a rationale for further studies towards the development of antimalarial drugs targeting the Plasmodium SUF pathway.
[Fe-S] cluster biogenesis has been well studied in bacteria and yeast, and to a lesser extent in other eukaryotes [16], [24], [40]–[42]. However, very little functional data are yet available for apicomplexan parasites. The dispensable function of PbSUFA could be compatible with a role as a transfer protein as suggested by studies in E. coli [19] rather than that of a scaffold protein. Similarly, we discussed a potential function of PbNFUapi as a transfer protein [32]. Together, our data suggest that the putative transfer proteins in the apicoplast [Fe-S] biosynthesis pathway are not essential for parasite survival. Alternatively, both proteins might perform at least partially redundant functions, a possibility that might be further tested through the generation of recombinant parasite lines lacking both NFUapi and SUFA.
The corresponding SUF [Fe-S] cluster biogenesis pathway in the related apicomplexan parasite, Toxoplasma gondii, which causes toxoplasmosis, is likely also localized to the apicoplast, despite the uniform absence of a signal peptide and apicoplast-targeting sequences in the SUF proteins [24]. It remains elusive how this alternative targeting to the apicoplast might have evolved and if there are specific reasons why all of the SUF components in T. gondii are targeted through this alternative pathway. Apparently, other apicomplexan parasites, such as Babesia bovis and Theileria annulata, target these components through a classical signal peptide and apicoplast-targeting sequence-dependent import pathway, although these parasites appear to encode a reduced set of proteins, namely SUFE and SUFS and, in the case of T. annulata, also NFUapi [25], [32].
In E. coli, deficiencies in the SUF pathway do not result in phenotypes under normal growth conditions. However, when cultured in low iron or increased oxidative stress conditions, bacteria demonstrate marked growth problems [21], [43], [44]. As all our analyses were performed under optimized in vivo and cell culture conditions, we cannot exclude a phenotype of sufA – under suboptimal growth conditions, e.g. in malnourished mice.
Plasmodium is an obligate intracellular eukaryotic pathogen and apparently cannot compensate for the loss of the apicoplast SUF pathway. Recent data suggest that a functional non-mevalonate isoprenoid biosynthesis (DOXP) pathway is the major vital role of the apicoplast in P. falciparum parasites in vitro [31]. Presence of [Fe-S] clusters in the penultimate and ultimate enzymes, ISPG and ISPH, provide a plausible explanation for the observed refractoriness of the SUF assembly machinery to targeted gene deletion. To test if the DOXP pathway of isoprenoid biosynthesis is the sole reason for the essentiality of [Fe-S] cluster biogenesis in the apicoplast, one could attempt to delete P. falciparum SUF pathway components under isopentenyl pyrophosphate supplementation [31].
In conclusion, our study identified the four key components of the Plasmodium apicoplast [Fe-S] biosynthetic pathway and revealed that SUFA is dispensable for efficient progression through the Plasmodium life cycle.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the German ‘Tierschutzgesetz in der Fassung vom 22. Juli 2009’ and the Directive 2010/63/EU of the European Parliament and Council ‘On the protection of animals used for scientific purposes’. The protocol was approved by the ethics committee of the Berlin state authority (‘Landesamt für Gesundheit und Soziales Berlin’, permit number G0469/09).
Experimental Animals, Parasites, and Cell Lines
Female NMRI and C57BL/6 mice were purchased from Charles River Laboratories (Sulzfeld, Germany). C57BL/6 mice were used for sporozoite infections. All other parasite infections were conducted with NMRI mice. Experimental genetics were all performed in P. berghei strain ANKA (WT), as control lines GFPcon [33] or Berred [38] parasites were used. In vitro liver stage parasite development was analyzed using cultured HuH7 hepatoma cells.
Generation of SUF Targeting and Tagging Plasmids
For targeted gene deletion of the P. berghei SUF genes, fragments of the upstream 5′ and downstream 3′ flanking regions (FR) were amplified from gDNA using gene-specific primers. PCR fragments were cloned into the P. berghei adaptable transfection vector (pBAT-SIL6) [34], which contains drug-selectable and high-expressing GFP cassettes. First, the 3′FR homologous sequences were cloned following restriction digestion of vector and insert with HindIII and KpnI. Then, the 5′FR homologous sequences digested with SacII and EcoRV were cloned into SacII and PvuII linearized vector, thus removing the mCherry-3xMyc tag from the original vector. The resulting plasmids were linearized with SalI. To provide transfection controls and confirm the apicoplast localization of SUF proteins, mCherry-3xMyc tagged parasite lines were generated. For this purpose, the carboxy-terminal parts of the SUF genes were PCR amplified using gene-specific primers. After restriction digestion, the respective fragments were cloned into the SacII and HpaI digested pBAT-SIL6 vector already containing the 3′FR sequence of the respective SUF genes, thus fusing the SUF carboxy-terminal sequence in frame with the mCherry-3xMyc tag sequence. The resulting plasmids were linearized with SalI. All primers are listed in Table S1.
Parasite Transfection, Selection and Genotyping of Recombinant Parasites
For targeted gene deletions and carboxy-terminal tagging, 106 to 107 purified P. berghei schizonts were transfected with digested plasmids using the Amaxa Nucleofector system as described [33]. Transfected parasites were subsequently injected into naïve NMRI mice selected by oral pyrimethamine (70 µg/ml) in the drinking water. Genotyping of drug-resistant parasites was performed by diagnostic PCR using gDNA as template and integration-specific primers. Two isogenic sufA – parasite lines from two independent transfection experiments were generated by flow cytometry-assisted isolation as described [35]. The genotype of the two selected sufA– parasite populations was confirmed by Southern blot analysis using the PCR DIG Probe Synthesis kit and the DIG Luminescent Detection kit (Roche), according to the manufacturer’s protocol. For amplification of the hybridization probe, gene-specific primers TV-5’SUFA-F and TV-5’SUFA-R were used. The hybridization probe was annealed to EcoRI restriction-digested gDNA, resulting in bands of 4.5 kb (WT) and 9.8 kb (sufA –). All primers are listed in Table S1.
Plasmodium Life Cycle Progression
Gametocyte differentiation and exflagellation of microgametes were examined prior to mosquito feeding. Anopheles stephensi mosquitoes were raised under a 14 h light/10 h dark cycle, 75% humidity and at 28°C (non-infected) or 20°C (infected), respectively. Sporozoite populations were isolated and analyzed as described previously [45]. Mosquito infectivity, i.e. the percentage of mosquitoes with midgut oocysts, was assessed through dissection of mosquito midguts at day 10 after feeding. All parasite strains that were used express GFP in all life cycle stages, allowing the determination of the number of midguts containing GFP-positive oocysts using a fluorescence binocular. Salivary gland-associated sporozoites were quantified at days 17–21. To determine sporozoite infectivity, sporozoites were liberated from salivary glands and injected intravenously into young, naïve C57BL/6 mice (10,000 sporozoites/inoculation). Patency was determined by daily examination of Giemsa-stained thin blood smears.
P. berghei in vitro liver stages were cultured and analyzed using standard techniques [46]. In brief, 30,000 hepatoma cells were seeded per well in 8-well chamber slides (Nalge Nunc International) and inoculated with freshly-dissected 10,000 sporozoites 24 h later. Thereafter, standard procedures for culturing infected hepatoma cells were followed [47]. Merosomes were harvested from the cell culture supernatant and counted in a Neubauer chamber 72 h after inoculation.
Fluorescence Microscopy
For confirmation of expression and determination of the subcellular localization of tagged SUF proteins, live and fixed suf::tag blood stage parasites were imaged using Leica DMR epifluorescence microscope. Infected erythrocytes were fixed using a previously published protocol with minor modifications [48]. 5 µl of tail blood from an infected mouse was mixed with 125 µl of RPMI1640 and 15 µl of cell suspension was allowed to settle 5 min onto poly-L-lysine coated cover slips. Cover slips were transferred to a 24-well plate containing 500 µl of 4% EM-grade paraformaldehyde and 0.0075% EM-grade glutaraldehyde in microtubule stabilizing buffer (MTSB, 10 mM MES, 150 mM NaCl, 5 mM EGTA, 5 mM glucose, 5 mM MgCl2 pH 6.9), fixed for 20 min and washed with PBS. Cells were permeabilized for 10 min with 0.1% Triton X-100 in PBS and blocked 3 h with 10% foetal calf serum (FCS) in PBS. The samples were incubated with rat anti-mCherry antibodies (1∶1,000 dilution, Chromotek) and rabbit anti-P. berghei ACP peptide antiserum (1∶300 dilution; [49]) in 10% FCS in PBS overnight at 4°C. Bound antibodies were detected using goat anti-rat IgG Alexa Fluor 546 and goat anti-rabbit IgG Alexa Fluor 633 conjugated antibodies (1∶3,000 dilution, Invitrogen). Nuclei were visualized with the DNA-dye Hoechst 33342 (Invitrogen; 1∶1,000 dilution). Coverslips were mounted with Fluoromount-G (Southern Biotech).
Liver stages were fixed with ice-cold methanol for immunofluorescence assays at the indicated time points. For quantification of sufA – liver stage parasite numbers, these were visualized using monoclonal mouse anti-P. berghei heat shock protein 70 (HSP70) antibodies (1∶300 dilution) [50]. For confirmation of expression and determination of the subcellular localization of the SUF::tag proteins 48 h after infection, fixed suf::tag liver stage parasites were incubated with rat anti-mCherry antibodies (1∶1,000 dilution, Chromotek) and rabbit anti-P. berghei ACP peptide antiserum (1∶300 dilution; [49]). To confirm the apicoplast localization at 48 h after infection, we treated sporozoite-infected hepatoma cells with 1 µM azithromycin (Pfizer), as described previously [46]. Parasites were identified by staining with rabbit anti-upregulated in infective sporozoites protein 4 (UIS4) peptide antiserum (1∶2,000 dilution; kindly provided by G. Montagna, MPI-IB, Berlin). Branched anti-mCherry-positive structures extending into the area delineated by the anti-UIS4 antiserum were defined as apicoplasts. Bound antibodies were detected using donkey/goat anti-rabbit/rat/mouse IgG Alexa Fluor 488/546 conjugated antibodies (1∶3,000 dilution, Invitrogen). Nuclei were visualized with DNA-dyes Hoechst 33342 (Invitrogen) and DRAQ5 (Axxora; both 1∶1,000 dilution). Coverslips were mounted with Fluoromount-G (Southern Biotech). Total numbers of parasites were counted using a Leica DM2500 epifluorescence microscope. Images were recorded using a Zeiss AxioObserver Z1 epifluorescence microscope.
All images were processed minimally with ImageJ (http://rsb.info.nih.gov/ij/). Following subtraction of background fluorescence levels of non-infected cells within the same recording, minimum and maximum intensities of the specific signals were optimized to use the full dynamic range of the look-up-tables. No gamma adjustments were applied.
Supporting Information
Primer sequences.
(DOCX)
Acknowledgments
We would like to acknowledge the assistance of the FCCF at the Deutsches Rheuma-Forschungszentrum (Berlin).
Funding Statement
This work was supported by the Max Planck Society and partly by the EVIMalaR network (#34). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bych K, Kerscher S, Netz DJA, Pierik AJ, Zwicker K, et al. (2008) The iron-sulphur protein Ind1 is required for effective complex I assembly. EMBO J 27: 1736–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakurai H, San Pietro A (1985) Association of Fe-S center(s) with the large subunit(s) of photosystem I particles. J Biochem 98: 69–76. [DOI] [PubMed] [Google Scholar]
- 3. Klinge S, Hirst J, Maman JD, Krude T, Pellegrini L (2007) An iron-sulfur domain of the eukaryotic primase is essential for RNA primer synthesis. Nat Struct Mol Biol 14: 875–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porello SL, Cannon MJ, David SS (1998) A substrate recognition role for the [4Fe-4S]2+ cluster of the DNA repair glycosylase MutY. Biochemistry 37: 6465–6475. [DOI] [PubMed] [Google Scholar]
- 5. Kispal G, Sipos K, Lange H, Fekete Z, Bedekovics T, et al. (2005) Biogenesis of cytosolic ribosomes requires the essential iron-sulphur protein Rli1p and mitochondria. EMBO J 24: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haile DJ, Rouault TA, Tang CK, Chin J, Harford JB, et al. (1992) Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci USA 89: 7536–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Malkin R, Rabinowitz JC (1966) The reconstitution of clostridial ferredoxin. Biochem Biophys Res Commun 23: 822–827. [DOI] [PubMed] [Google Scholar]
- 8. Outten FW, Djaman O, Storz G (2004) A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli . Mol Microbiol 52: 861–872. [DOI] [PubMed] [Google Scholar]
- 9. Lill R (2009) Function and biogenesis of iron-sulphur proteins. Nature 460: 831–838. [DOI] [PubMed] [Google Scholar]
- 10. Tokumoto U, Takahashi Y (2001) Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron-sulfur proteins. J Biochem 130: 63–71. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi Y, Tokumoto U (2002) A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem 277: 28380–28383. [DOI] [PubMed] [Google Scholar]
- 12. Mühlenhoff U, Lill R (2000) Biogenesis of iron-sulfur proteins in eukaryotes: a novel task of mitochondria that is inherited from bacteria. Biochim Biophys Acta 1459: 370–382. [DOI] [PubMed] [Google Scholar]
- 13. Kispal G, Csere P, Prohl C, Lill R (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18: 3981–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma AK, Pallesen LJ, Spang RJ, Walden WE (2010) Cytosolic iron-sulfur cluster assembly (CIA) system: factors, mechanism, and relevance to cellular iron regulation. J Biol Chem 285: 26745–26751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hidese R, Mihara H, Esaki N (2011) Bacterial cysteine desulfurases: versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl Microbiol Biotechnol 91: 47–61. [DOI] [PubMed] [Google Scholar]
- 16. Johnson DC, Dean DR, Smith AD, Johnson MK (2005) Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem 74: 247–281. [DOI] [PubMed] [Google Scholar]
- 17. Loiseau L, Ollagnier-de-Choudens S, Nachin L, Fontecave M, Barras F (2003) Biogenesis of Fe-S cluster by the bacterial Suf system: SufS and SufE form a new type of cysteine desulfurase. J Biol Chem 278: 38352–38359. [DOI] [PubMed] [Google Scholar]
- 18. Outten FW, Wood MJ, Munoz FM, Storz G (2003) The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in Escherichia coli . J Biol Chem 278: 45713–45719. [DOI] [PubMed] [Google Scholar]
- 19. Chahal HK, Dai Y, Saini A, Ayala-Castro C, Outten FW (2009) The SufBCD Fe-S scaffold complex interacts with SufA for Fe-S cluster transfer. Biochemistry 48: 10644–10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wollers S, Layer G, Garcia-Serres R, Signor L, Clemancey M, et al. (2010) Iron-sulfur (Fe-S) cluster assembly: the SufBCD complex is a new type of Fe-S scaffold with a flavin redox cofactor. J Biol Chem 285: 23331–23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nachin L, Loiseau L, Expert D, Barras F (2003) SufC: an unorthodox cytoplasmic ABC/ATPase required for [Fe-S] biogenesis under oxidative stress. EMBO J 22: 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitaoka S, Wada K, Hasegawa Y, Minami Y, Fukuyama K, et al. (2006) Crystal structure of Escherichia coli SufC, an ABC-type ATPase component of the SUF iron-sulfur cluster assembly machinery. FEBS Lett 580: 137–143. [DOI] [PubMed] [Google Scholar]
- 23. Ellis KE, Clough B, Saldanha JW, Wilson RJ (2001) Nifs and Sufs in malaria. Mol Microbiol 41: 973–981. [DOI] [PubMed] [Google Scholar]
- 24. Seeber F (2002) Biogenesis of iron-sulphur clusters in amitochondriate and apicomplexan protists. Int J Parasitol 32: 1207–1217. [DOI] [PubMed] [Google Scholar]
- 25. Seeber F, Soldati-Favre D (2010) Metabolic pathways in the apicoplast of Apicomplexa . Int Rev Cell Mol Biol 281: 161–228. [DOI] [PubMed] [Google Scholar]
- 26. Wilson RJM, Denny PW, Preiser PR, Rangachari K, Roberts K, et al. (1996) Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum . J Mol Biol 261: 155–172. [DOI] [PubMed] [Google Scholar]
- 27. Kumar B, Chaubey S, Shah P, Tanveer A, Charan M, et al. (2011) Interaction between sulphur mobilisation proteins SufB and SufC: Evidence for an iron-sulphur cluster biogenesis pathway in the apicoplast of Plasmodium falciparum . Int J Parasitol 41: 991–999. [DOI] [PubMed] [Google Scholar]
- 28. Rangachari K, Davis CT, Eccleston JF, Hirst EMA, Saldanha JW, et al. (2002) SufC hydrolyzes ATP and interacts with SufB from Thermotoga maritima . FEBS Lett 514: 225–228. [DOI] [PubMed] [Google Scholar]
- 29. Xu XM, Adams S, Chua N-H, Møller SG (2005) AtNAP1 represents an atypical SufB protein in Arabidopsis plastids. J Biol Chem 280: 6648–6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gisselberg JE, Dellibovi-Ragheb TA, Matthews KA, Bosch G, Prigge ST (2013) The suf iron-sulfur cluster synthesis pathway is required for apicoplast maintenance in malaria parasites. PLoS Pathog 9: e1003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeh E, DeRisi JL (2011) Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum . PLoS Biol 9: e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haussig JM, Matuschewski K, Kooij TWA (2013) Experimental genetics of Plasmodium berghei NFU in the apicoplast iron-sulfur cluster biogenesis pathway. PLoS ONE 8: e67269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janse CJ, Franke-Fayard BMD, Mair GR, Ramesar J, Thiel C, et al. (2006) High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145: 60–70. [DOI] [PubMed] [Google Scholar]
- 34. Kooij TWA, Rauch MM, Matuschewski K (2012) Expansion of experimental genetics approaches for Plasmodium berghei with versatile transfection vectors. Mol Biochem Parasitol 185: 19–26. [DOI] [PubMed] [Google Scholar]
- 35. Kenthirapalan S, Waters AP, Matuschewski K, Kooij TWA (2012) Flow cytometry-assisted rapid isolation of recombinant Plasmodium berghei parasites exemplified by functional analysis of aquaglyceroporin. Int J Parasitol 42: 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ecker A, Lakshmanan V, Sinnis P, Coppens I, Fidock DA (2011) Evidence that mutant PfCRT facilitates the transmission to mosquitoes of chloroquine-treated Plasmodium gametocytes. J Infect Dis 203: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, et al. (2003) Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301: 1503–1508. [DOI] [PubMed] [Google Scholar]
- 38. Matz JM, Matuschewski K, Kooij TWA (2013) Two putative protein export regulators promote Plasmodium blood stage development in vivo . Mol Biochem Parasitol 191: 44–52. [DOI] [PubMed] [Google Scholar]
- 39. Py B, Gerez C, Angelini S, Planel R, Vinella D, et al. (2012) Molecular organization, biochemical function, cellular role and evolution of NfuA, an atypical Fe-S carrier. Mol Microbiol 86: 155–171. [DOI] [PubMed] [Google Scholar]
- 40. Lill R, Mühlenhoff U (2006) Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol 22: 457–486. [DOI] [PubMed] [Google Scholar]
- 41. Balk J, Pilon M (2011) Ancient and essential: the assembly of iron-sulfur clusters in plants. Trends Plant Sci 16: 218–226. [DOI] [PubMed] [Google Scholar]
- 42. Xu XM, Møller SG (2011) Iron-sulfur clusters: biogenesis, molecular mechanisms, and their functional significance. Antioxid Redox Signal 15: 271–307. [DOI] [PubMed] [Google Scholar]
- 43. Nachin L, El Hassouni M, Loiseau L, Expert D, Barras F (2001) SoxR-dependent response to oxidative stress and virulence of Erwinia chrysanthemi: the key role of SufC, an orphan ABC ATPase. Mol Microbiol 39: 960–972. [DOI] [PubMed] [Google Scholar]
- 44. Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, et al. (2001) DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183: 4562–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vanderberg J (1975) Development of infectivity by the Plasmodium berghei sporozoite. J Parasitol 61: 43–50. [PubMed] [Google Scholar]
- 46. Haussig JM, Matuschewski K, Kooij TWA (2011) Inactivation of a Plasmodium apicoplast protein attenuates formation of liver merozoites. Mol Microbiol 81: 1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Silvie O, Goetz K, Matuschewski K (2008) A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog 4: e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deligianni E, Morgan RN, Bertuccini L, Kooij TWA, Laforge A, et al. (2011) Critical role for a stage-specific actin in male exflagellation of the malaria parasite. Cell Microbiol 13: 1714–1730. [DOI] [PubMed] [Google Scholar]
- 49. Friesen J, Silvie O, Putrianti ED, Hafalla JCR, Matuschewski K, et al. (2010) Natural immunization against malaria: causal prophylaxis with antibiotics. Sci Transl Med 2: 40ra49. [DOI] [PubMed] [Google Scholar]
- 50. Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F (1994) Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res 80: 16–21. [DOI] [PubMed] [Google Scholar]
- 51. Foth BJ, Ralph SA, Tonkin CJ, Struck NS, Fraunholz MJ, et al. (2003) Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum . Science 299: 705–708. [DOI] [PubMed] [Google Scholar]
- 52. Cilingir G, Broschat SL, Lau AOT (2012) ApicoAP: the first computational model for identifying apicoplast-targeted proteins in multiple species of Apicomplexa . PLoS ONE 7: e36598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bender A, van Dooren GG, Ralph SA, McFadden GI, Schneider G (2003) Properties and prediction of mitochondrial transit peptides from Plasmodium falciparum . Mol Biochem Parasitol 132: 59–66. [DOI] [PubMed] [Google Scholar]
- 54. Claros MG (1995) MitoProt, a Macintosh application for studying mitochondrial proteins. Comput Appl Biosci 11: 441–447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences.
(DOCX)