Abstract
Background
Recent studies have shown that galactose-deficient IgA1 (GdIgA1) has an important role in the pathogenesis of IgA nephropathy (IgAN). Although emerging data suggest that serum GdIgA1 can be a useful non-invasive IgAN biomarker, the localization of nephritogenic GdIgA1-producing B cells remains unclear. Recent clinical and experimental studies indicate that immune activation tonsillar toll-like receptor (TLR) 9 may be involved in the pathogenesis of IgAN. Here we assessed the possibility of GdIgA1 production in the palatine tonsils in IgAN patients.
Methods
We assessed changes in serum GdIgA1 levels in IgAN patients with clinical remission of hematuria and proteinuria following combined tonsillectomy and steroid pulse therapy. Further, the association between clinical outcome and tonsillar TLR9 expression was evaluated.
Results
Patients (n = 37) were divided into two groups according to therapy response. In one group, serum GdIgA1 levels decreased after tonsillectomy (59%) alone, whereas in the other group most levels only decreased after the addition of steroid pulse therapy to tonsillectomy (41%). The former group showed significantly higher tonsillar TLR9 expression and better improvement in hematuria immediately after tonsillectomy than the latter group.
Conclusions
The present study indicates that the palatine tonsils are probably a major sites of GdIgA1-producing cells. However, in some patients these cells may propagate to other lymphoid organs, which may partially explain the different responses observed to tonsillectomy alone. These findings help to clarify some of the clinical observations in the management of IgAN, and may highlight future directions for research.
Introduction
Since the pathogenesis of immunoglobulin A nephropathy (IgAN) remains unclear [1], there is no specific therapy for this disease. Clinical evidence, particularly from kidney transplantation, indicates that the pathogenesis of IgAN is associated with an abnormality of the IgA immune system, rather than an abnormality of renal intrinsic cells [2] [3] [4] [5]. Episodic macroscopic hematuria, coinciding with mucosal infections of the upper respiratory tract [6] or an abnormal response to mucosal vaccination [7] [8] in IgAN patients indicates that dysregulation of the mucosal immune system is important in the pathogenesis of IgAN [9]. However, bone marrow (BM) or BM transplantation (BMT) studies in IgAN patients [10] [11] [12] [13] suggest that mucosal-type polymeric IgA is overproduced in systemic immune sites such as BM. In the 1980s, van Es et al. hypothesized that a “mucosa–BM axis” exists in which there is continuous trafficking of causative cells in the IgA immune system between mucosal sites and the BM [14] [15]. Clinical and experimental studies in the last decade have uncovered a detailed mechanism by which lymphocytes including mucosal B cells, travel between the mucosa and BM or lymphoid tissues [16] [17]. Although these findings support the mucosa-BM axis hypothesis, the causative cell and their origins remain unknown [18], precluding the development of disease-specific therapy for IgAN.
Our recent studies [19] [20] [21] have demonstrated that toll-like receptor (TLR), a key molecule in the innate and mucosal immunity, have pathological roles in IgAN in human and mice models. In particular, TLR9 activation appears to be important for the progression and severity of IgAN. Therefore, it is possible that mucosal cells expressing TLR9, such as tonsillar B cells [20] [21], may be involved in the pathogenesis. In addition, TLR activation also induces IgA switching in mucosal B cells [22]. These findings may explain why tonsillectomy has a favorable effect on the long-term renal survival in some IgAN patients [23] [24]. Other Japanese groups have also recently reported that tonsillectomy followed by steroid pulse therapy is more effective than tonsillectomy alone [25] [26] [27] [28]. It is speculated that steroid therapy may attenuate both inflammation and the number of causative lymphocytes that are capable of migrating into systemic site beyond the tonsil [9] [29]. However, none of these reports has provided data of any specific steroid therapy targets in IgAN.
Mesangial IgA present in the renal deposits is a subclass of IgA1 [30] and displays abnormal O-glycosylation [31] [32]. Furthermore, IgA1 molecules produced by tonsillar lymphocytes undergo aberrant O-glycosylation in IgAN patients [33] [34] [35]. Moldoveanu et al. [36] demonstrated that galactose-deficient IgA1 (GdIgA1) increases in the sera of IgAN patients using a Helix aspersa agglutinin (HAA) lectin assay, whereas recent studies have reported that GdIgA1 is nephritogenic in this disease [37] [38] [39] [40] [41]. We recently reported that serum GdIgA1 levels were clearly associated with disease activity in IgAN [42], indicating that serum GdIgA1 evaluation can be a potential non-invasive diagnostic and activity marker for IgAN [41] [42]. However, further evidence on the extent of GdIgA1-producing cells is necessary.
Our recent experimental findings indicate a role for cluster of differentiation 19-positive (CD19+) B cells: they appear to regulate nephritogenic IgA production, independent of T cells; they are disseminated in multiple lymphoid organs including BM and the spleen; and they are involved in IgAN [43].
The present study aimed to evaluate the changes in serum GdIgA1 levels in IgAN patients with complete remission of urinary abnormalities after tonsillectomy and steroid pulse therapy. We hypothesized that GdIgA1 is produced by the tonsils and should reduce after tonsillectomy.
Subjects and Methods
We designed a cohort study to provide biological evidence of the patient types that show a decrease of serum GdIgA1 after tonsillectomy or additional steroid pulse therapy. The study protocol was approved by the Ethics Review Committee of Juntendo University Faculty of Medicine. Written informed consent was obtained from all patients.
Patients
The inclusion criteria for our cohort were patients with IgAN diagnosed by renal biopsy who received both tonsillectomy and steroid pulse therapy. Patients were required to have clinical remission of hematuria [<5 red blood cells (RBC) per high power field (HPF)] and proteinuria (<0.15 g/g·Cr) after the 3 steroid pulse therapy sessions.
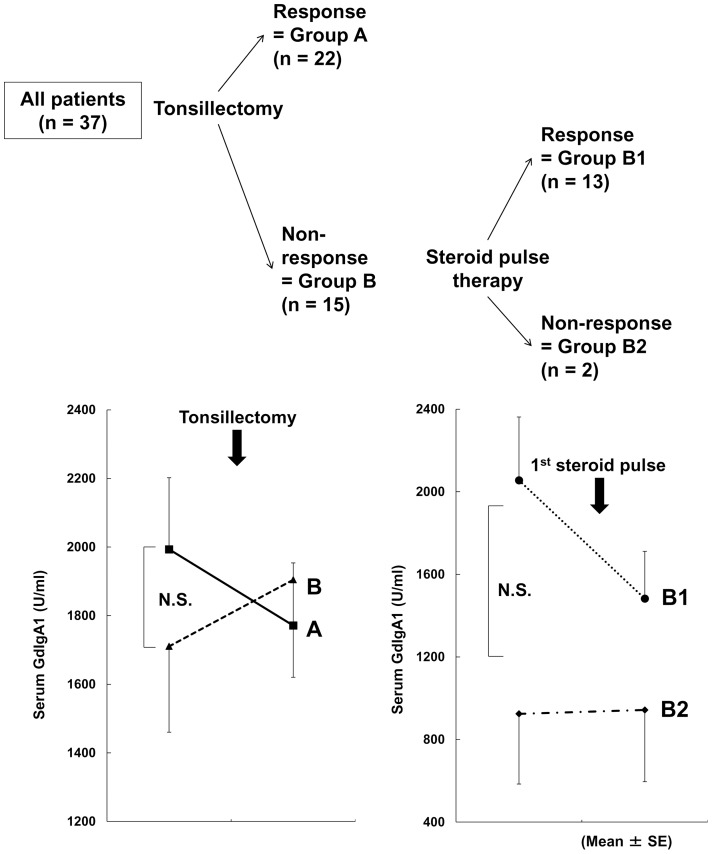
Serum GdIgA1 levels and clinical outcomes were analyzed before and after tonsillectomy, and after the first steroid pulse therapy session. The patients were divided into two groups according to their response to therapy: group A exhibited reduced serum GdIgA1 levels after tonsillectomy alone; group B showed no response to tonsillectomy alone. The patients in group B were further subdivided into those exhibiting reduced GdIgA1 levels after initiation of steroid therapy (group B1) and those not showing a reduction (group B2). The cut-off level for a reduction in GdIgA1 levels was 1 (after/before tonsillectomy or after the first steroid pulse therapy session/after tonsillectomy, <1 indicated a reduction; ≥1 indicated no reduction).
Treatment protocol during the study period
Steroid pulse therapy (500 mg methylprednisolone intravenously [IV] per day for 3 consecutive days, thrice every 2 months) was administered from at least 2 weeks after the tonsillectomy. Between sessions of steroid pulse therapy, patients were prescribed oral prednisolone (0.5 mg/kg body weight) on alternate days.
Analysis of serum and urine
Blood samples were obtained from patients just before tonsillectomy and before the first and second sessions of steroid pulse therapy. Serum GdIgA1 levels were measured by enzyme-linked immunosorbent assay. Costar 96-well U-bottom plates (Corning Inc., Corning, NY, USA) were coated at 4°C overnight with a F(ab')2 fragment of goat anti-human IgA (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at a 3 μg/ml. Plates were blocked at 4°C overnight with 2% bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA) in phosphate-buffered saline containing 0.05% Tween 20 (v/v). Samples diluted in blocking buffer were added to each well and incubated at 4°C overnight. The captured IgA was subsequently desialylated by treatment at 37°C for 3 h with 10 mU/ml neuraminidase from Vibrio cholera (Roche Diagnostics Corp., Indianapolis, IN, USA) in 10 mM sodium acetate buffer, pH = 5 [44]. Samples with and without neuraminidase treatment were analyzed in parallel to assess the proportion of molecules with sialylated and terminal N-acetylgalactosamine (GalNAc) residues. Samples were incubated at 37°C for 3 h with GalNAc-specific biotinylated HAA lectin (Sigma-Aldrich) diluted 1:500 in a blocking buffer [36] [45]. The bound lectin was detected by an avidin-horseradish peroxidase conjugate (ExtrAvidin; Sigma-Aldrich), and the reaction was developed with the peroxidase chromogenic substrate o-phenylenediamine-H2O2 (Sigma-Aldrich). The color reaction was stopped with 1 M sulfuric acid and absorbance at 490 nm was measured using an EL312 BioKinetics microplate reader (BioTek Instruments Inc., Winooski, VT, USA). HAA reactivity of IgA1 was then calculated in each sample as optical density (OD) units per 1 μg of IgA1. Naturally purified GdIgA1 from the plasma of an IgA1 multiple myeloma patient [36] [46] was treated with neuraminidase and used as the standard. The OD units per 1 μg neuraminidase-treated IgA1 were assigned a value of 100%. Total GdIgA1, expressed in U/ml of serum, was calculated as follows: (%× μg IgA/ml)/100 [47].
Urea nitrogen, creatine (Cr) and IgA in sera were measured. Urine samples were obtained at the same time, and urinary protein and RBC levels were assessed. We used the median RBC count per HPF for hematuria classification: 1–5/HPF (3/HPF), 6–10/HPF (8/HPF), 11–15/HPF (13/HPF), 16–20/HPF (18/HPF), 21–25/HPF (23/HPF), 26–30/HPF (28/HPF), and >30/HPF (33/HPF).
Analysis of tonsils
TLR9 transcriptional levels in tonsils were measured by real-time reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted using the RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. Real-time RT-PCR was performed using the 7500 Real-Time RT-PCR System with TaqMan Gene Expression Master Mix and TaqMan Gene Expression Assay (TLR9; Hs00370913_s1, Applied Biosystems, Foster City, CA, USA). The samples were compared with the GAPDH PCR product for quantification (Hs02786624_g1).
Statistical analysis
Associations between different parameters were analyzed by the t-test. Analysis of variance was used to determine differences in the characteristics among the multiple groups. Data are expressed as mean ± SD or median values. P<0.05 was considered significant. All statistical analyses were performed using the Windows version of StatView 5.0 software (Abacus Concepts Inc., Berkeley, CA, USA).
Results
We include 37 patients (16 males) according to the inclusion criteria, and their clinical profiles immediately before tonsillectomy are summarized in Table 1. Group A (responders tonsillectomy alone) comprised 22 patients, whereas group B (non-responders tonsillectomy alone) comprised 15 patients. In group B, GdIgA1 reduced after initiation of steroid therapy in 13 patients (group B1), and remained elevated in 2 patients (group B2; Figure 1). Although group B showed significantly lower serum Cr levels than group A, they were within the normal range. In addition, there were no significant differences in urinary proteinuria between the two groups (Table 1).
Table 1. Profiles of patients with immunoglobulin A nephropathy (IgAN) patients just before tonsillectomy.
| total (n = 37) | A (n = 22) | B (n = 15) | B1 (n = 13) | B2 (n = 2) | |
| Duration from onset to tonsillectomy (year)* | 5 (2–10) | 5.5 (2–8) | 4 (2–12) | 7 (2–12) | 2.5 (2.25–2.75) |
| Age at tonsillectomy (years old) | 31.43±7.72 | 31.55±8.20 | 31.27±8.20 | 31.69±8.36 | 28.50±9.19 |
| Male (%) | 43 (16: 21) | 45 (10: 12) | 40 (6: 9) | 38 (5: 8) | 50 (1: 1) |
| Serum Cr (mg/dl) | 0.83±0.24 | 0.91±0.26 | 0.69±0.16† | 0.69±0.18 | 0.69±0.11 |
| BUN (mg/dl) | 12.86±3.41 | 13.55±3.45 | 11.79±2.49 | 12.08±2.57 | 10.00±0.00 |
| Serum IgA (mg/dl) | 323.3±99.1 | 326.8±88.2 | 317.9±112.8 | 325.3±120.5 | 273.5±29.0 |
| Serum GdIgA1 (U/ml) | 1878.8±971.2 | 1993.4±1024.7 | 1710.6±968.9 | 1834.5±977.6 | 878.8±278.9 |
| Proteinuria / urine Cr (g/g·Cr)* | 0.58 (0.29–0.85) | 0.51 (0.2–0.68) | 0.82 (0.51–1.13) | 0.83 (0.71–1.36) | 0.4 (0.35–0.44) |
| Hematuria (/HPF) | 26.38±10.21 | 28.68±9.62 | 23.00±11.34 | 23.00±11.55 | 23.00±14.14 |
| 1–5/HPF | 1 (2.7%) | 1 (6.7%) | 1 (7.7%) | ||
| 6–10/HPF | 5 (13.5%) | 3 (13.6%) | 2 (13.3%) | 2 (15.4%) | |
| 11–15/HPF | 2 (5.4%) | 2 (13.3%) | 1 (7.7%) | 1 (50.0%) | |
| 16–20/HPF | 1 (2.7%) | 1 (6.7%) | 1 (7.7%) | ||
| 21–25/HPF | 3 (8.1%) | 2 (9.1%) | 1 (6.7%) | 1 (7.7%) | |
| 26–30/HPF | 1 (2.7%) | 1 (6.7%) | 1 (7.7%) | ||
| >30/HPF | 24 (64.9%) | 17 (77.3%) | 7 (46.6%) | 6 (46.1%) | 1 (50.0%) |
Data presented as Mean ± SD or.
*Median (interquartile range).
p<0.05 (compared with group A). BUN, blood urea nitrogen; Cr, creatinine; GdIgA1, galactose-deficient IgA1; HPF, high power field; IgA, immunoglobulin A.
Figure 1. Classification of patients with immunoglobulin A nephropathy (IgAN) based on therapy response.
Group A exhibited reduced serum galactose-deficient IgA1 (GdIgA1) levels after tonsillectomy alone. Group B showed no response to tonsillectomy alone. Group B patients were further subdivided into those exhibiting reduced GdIgA1 levels after initiation of steroid therapy (group B1) and those not showing a reduction (group B2). The cut-off level for a reduction in GdIgA1 levels was 1 (after/before tonsillectomy or after the first steroid pulse therapy session/after tonsillectomy, <1 indicated a reduction; ≥1 indicated no reduction).
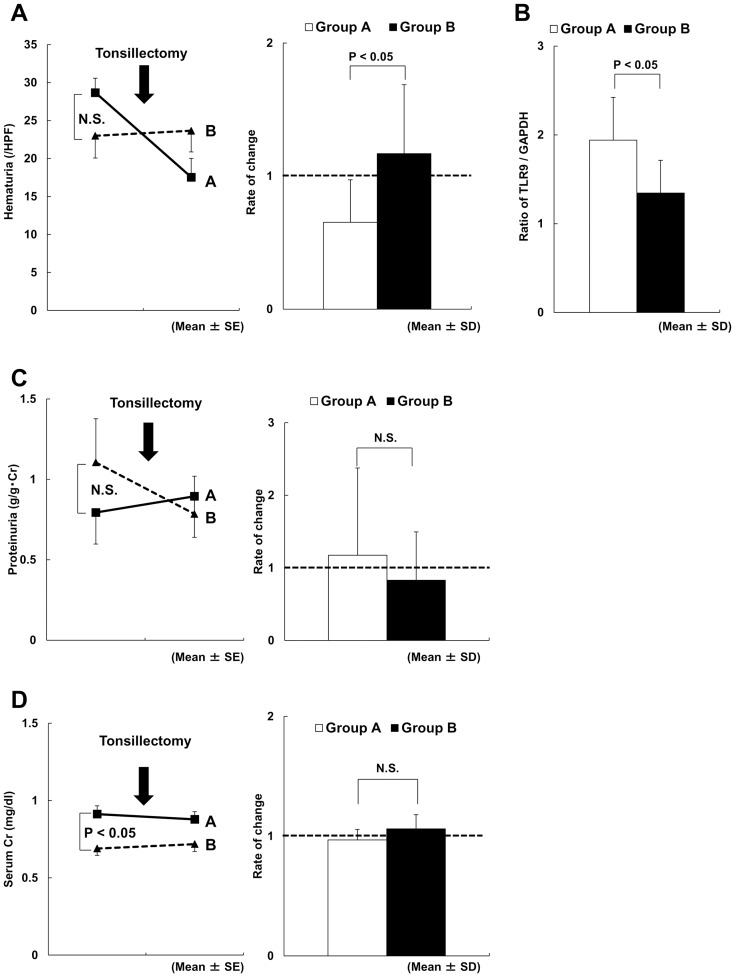
The ratio of changes in hematuria between immediately before and immediately after tonsillectomy in group A was significantly lower than that in group B (Figure 2A). As shown, the mean ratio after/before tonsillectomy in groups A and B were 0.63±0.34 and 1.23±0.66, respectively (P<0.05), suggesting improvement in hematuria after tonsillectomy alone, with a concurrent reduction in serum GdIgA1 levels in group A. Moreover, tonsillar TLR9 expression in group A was significantly higher than that in group B (Figure 2B), indicating that the responding patients comprised those with high tonsillar TLR9 expression. However, the rate of changes in proteinuria and serum Cr, before and after tonsillectomy, were not significantly different between groups A and B (Figures 2C and 2D).
Figure 2. Comparison between groups A and B before and after tonsillectomy.
(A) The rate of change in hematuria was <1 in group A and was significantly lower than that in group B. (B) Tonsillar TLR9 expression in group A was significantly higher than that in group B. (C) The rate of change in proteinuria before and after tonsillectomy was not significantly different between groups A and B. (D) The rate of change in serum Cr before and after tonsillectomy was not significantly different between groups A and B.
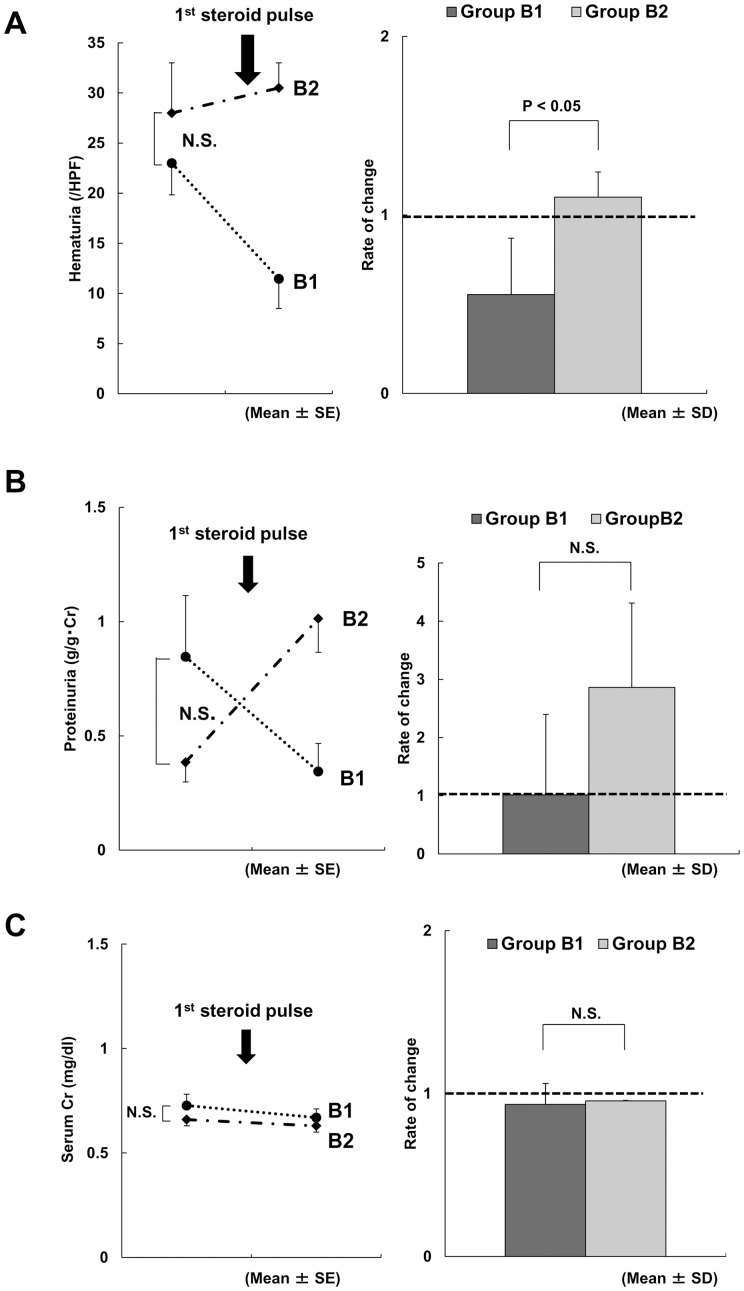
After the first steroid pulse therapy session, group B1 showed improvements in both hematuria and serum GdIgA1 levels, despite the hematuria not improving after tonsillectomy alone (Figure 3A). In contrast, group B2 patients demonstrated no reduction in serum GdIgA1 levels after the first steroid pulse therapy session. No significant differences between groups B1 and B2 were observed in the rate of changes in proteinuria and serum Cr, before and after the first steroid pulse therapy session (Figures 3B and 3C).
Figure 3. Comparison between groups B1 and B2 before and after the first steroid pulse therapy session.
(A) An improvement in hematuria was observed in group B1, but not in group B2. The rate of change in hematuria in group B1 was significantly lower than that in group B2. (B) The rate of change in proteinuria before and after the first steroid pulse therapy session was not significantly different between groups B1 and B2. (C) The rate of change in serum creatinine before and after the first steroid pulse therapy session was not significantly different between groups B1 and B2.
Discussion
Renal biopsy and immunohistochemical analysis of renal tissues remain the gold standard for diagnosing IgAN and evaluating acute lesions. However, new, safe, and reasonably specific non-invasive tests are emerging. Recent clinical studies suggest that elevated circulating GdIgA1 could offer a role in the diagnosis and evaluation of disease activity in IgAN [31] [32] [33] [34] [35] [36] [37] [38] [41] [42], because it appears to be involved in the pathogenesis of IgAN [48]. Other research suggests that β1,3-galactosyltransferase, the key enzyme for galactosylation of hinge-region IgA1 O-glycans, is downregulated in the tonsillar B cells of IgAN patients [49]. It is therefore possible that this alteration of enzyme activity contributes to the production of GdIgA1.
The present data demonstrate that serum GdIgA1 levels and hematuria decreased in 59% (22/37) of patients receiving tonsillectomy alone (group A), suggesting that the palatine tonsils may be a major site contributing to serum GdIgA1 levels. However, some patients (group B) showed improved hematuria and serum GdIgA1 levels following steroid pulse therapy, suggesting that GdIgA1-producing cells may also be localized outside the tonsils.
It has been discussed why mucosal type of polymeric IgA is produced in bone marrow [50]. However, emerging data has revealed that mucosal B cells, even tonsillar B cells, can migrate from inductive mucosal sites to systemic effector sites including BM through guiding adhesion molecules and chemokine/chemokine receptors [16] [17]. In addition, our recent study showed that IgA1 secreted by Epstein–Barr virus (EBV)-immortalized B cells from the peripheral blood of IgAN patients was mostly polymeric with galactose-deficient sialylated O-glycans [39]. These findings support the theory that GdIgA1-producing B cells may travel between the tonsils and systemic lymphoid organs and produce the nephritogenic IgA outside of mucosal sites; this possibly occurs through high endothelial venules (HEV), the lymphatics or the peripheral circulation [16] [17] [18].
The differences observed in the therapeutic responses of hematuria and serum GdIgA1 levels suggest that the number of GdIgA1-producing cells in lymphoid tissues vary among different tissues and among different IgAN patients. The extent of dissemination of GdIgA1-producing cells from mucosal to systemic sites may therefore vary among patients and may represent a determining factor for the efficacy of tonsillectomy with or without steroid pulse therapy.
Group A patients showed a decrease in GdIgA1 after tonsillectomy, but did not show a dramatic improvement in proteinuria. This may be due to short evaluation interval of 2–3 weeks between the tonsillectomy and the first steroid pulse therapy in our treatment protocol. In contrast, hematuria improved in group A despite the short duration. When we consider that the half-life of serum IgA is approximately 5 days, this finding suggests that renal injury and hematuria may relate to the glomerular deposition of GdIgA1 itself. In this regard, one can speculate that serum levels of GdIgA1 may influence renal pathology. Based on this principle, we undertook a preliminary examination of the correlation between these with present serum samples, but failed to find any significant correlation (data not shown). In this study, serum GdIgA1 levels were measured just before tonsillectomy. However, the intervals from renal biopsy to tonsillectomy are different for each individual. Further studies with serum samples collected at renal biopsy are required to assess this issue.
Clinical evidence suggests that acute tonsillitis and an upper respiratory tract infections are associated with IgAN exacerbation, suggesting that mucosal encounters with exogenous antigens may activate the specific tonsillar cells and contribute to disease progression. In this regard, TLR may be involved in the process [19] [20] [21] [51]; we have previously demonstrated that TLR9 expression, particularly in the mucosa, is associated with the progression of IgAN humans and mice [19] [20] [21]. Recently, we also reported that tonsillar TLR9 expression levels are associated with IgAN severity and response to tonsillectomy [20]. Patients with high TLR9 expression showed stronger and earlier remission of hematuria and proteinuria compared with those with a low TLR9 expression. In addition, patients whose serum IgA levels decreased more than average after tonsillectomy (large ΔIgA) showed higher cumulative proteinuria remission rates than patients with a smaller decrease in these levels (small ΔIgA). In this study, tonsillar TLR9 expression was significantly higher in the IgAN patients with reduced hematuria and serum GdIgA1 levels, compared with patients not responding to tonsillectomy alone.
TLR9 is expressed on B cells, and its activation is involved in IgA altering mucosal B cells through BAFF/APRIL (B-cell activating factor/a proliferation-inducing ligand) [22]. Thus, in combination with this study, this data may suggest that TLR9-positive B cells are involved in GdIgA1 production. Humans have two subclasses of IgA, namely IgA1 and IgA2. IgA1 contains O-glycosylation sites, whereas IgA2 and mouse IgA do not. Thus, the mouse model may exclude the aberrant O-glycosylation involved in human IgAN. Furthermore, recent studies suggest that aberrant N-glycan glycosylation may be involved in the pathogenesis of IgAN in mice [52] [53] [54]. We recently reported that aberrant glycosylation of IgA may be involved in the renal inflammatory cascade [55] and prognosis [56] of murine IgAN. These findings suggest that aberrant modifications of serum IgA carbohydrates are involved in the development of IgAN in both humans and mice, with O-glycans and N-glycans, respectively. Therefore, the pathogenesis of IgAN possibly overlaps in humans and mice in terms both of the genetic regulation [9] [56] [57] [58] and the underlying mechanisms, including the causative cell types and TLR9 expression [19] [21].
We recently reported that IgAN-prone mice with genetic and pathological features similar to human IgAN [9] [19] [21] [43] [55] [56] [57] [58] may have pathogenic B cells, but not matured plasma cells, and that they are disseminated to systemic lymphoid tissues such as BM and the spleen [43]. Furthermore, mucosal TLR9 activation appears to be involved in IgAN disease progression through nephritogenic IgA production in mice [21]. These experimental findings further suggest that mucosal/tonsillar TLR9 activation on B cells may be involved in the production of nephritogenic GdIgA1. However, before tonsillectomy the serum GdIgA1 levels were not significantly different between groups A and B, whereas the tonsillar TLR9 expression was significantly different. This finding may indicate that tonsillar TLR9 expression has a week influence on GdIgA1 production at non-tonsillar sites. In addition, because the severity of renal injury was not different between the two groups, the precise origin of GdIgA1 may be not important for its nephritogenicity. However, the mechanisms underlying the regulation of GdIgA1 production at non-tonsillar systemic sites should be evaluated in future studies.
This study was designed only as a preliminary study to test the hypothesis that that GdIgA1 is produced by the tonsils and should reduce after tonsillectomy. However, we can still draw several conclusions; we suggest that GdIgA1-producing cells may be localized to the palatine tonsils, and that the originating mucosal B cells then disseminate to systemic lymphoid organs. Although further research is necessary, these observations may account for the clinical observations that tonsillectomy is sufficient for some patients, whereas others require additional steroid therapy. To be effective in directing treatment, it is necessary to establish an objective method to evaluate the extent of systemic involvement. In the meantime, the current findings provide further clarification on the pathogenesis of IgAN.
Acknowledgments
The authors thank T. Shibata, M. Yamada and all members of the laboratory for technical support and helpful discussions.
Funding Statement
A part of this study was supported by research funds from Grant-in-Aids for Progressive Renal Disease Research, Research on intractable disease, from the Ministry of Health, Labor and Welfare of Japan, a grant from Strategic Japanese (JST)-Swiss (ETHZ) Cooperative Scientific Program and a grant from the Study Group on IgA Nephropathy in Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Berger J, Hinglais N (1968) Les Depots intercapillaries d'IgA-IgG. J Urol Nephrol 74: 694–695. [PubMed] [Google Scholar]
- 2. Floege J, Burg M, Kleiem V (1998) Recurrent of IgA nephropathy after kidney transplantation: not a benign condition. Nephrol Dial Transplant 13: 1933–1935 10.1093/ndt/13.8.1933 [DOI] [PubMed] [Google Scholar]
- 3. Floege J (2004) Recurrent IgA nephropathy after renal transplantation. Semin Nephrol 24: 287–291 10.1016/j.semnephrol.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 4. Cuevas X, Lloveras J, Mir M, Aubia J, Masramon J (1987) Disappearance of mesangial IgA deposits from the kidney of two donors after transplantation. Transplant Proc 19: 2208–2209. [PubMed] [Google Scholar]
- 5. Sanfilippo F, Croker BP, Bollinger RR (1982) Fate of four cadaveric donor renal allografts with mesangial IgA deposits. Transplantation 33: 370–376. [DOI] [PubMed] [Google Scholar]
- 6. Emancipator SN (1990) Immunoregulatory factors in the pathogenesis of IgA nephropathy. Kidney Int 38: 1216–1229 10.1038/ki.1990.337 [DOI] [PubMed] [Google Scholar]
- 7. de Fijter JW, Eijgenraam JW, Braam CA, Holmgren J, Daha MR, et al. (1996) Deficient IgA1 immune response to nasal cholera toxin subunit B in primary IgA nephropathy. Kidney Int 50: 952–961 10.1038/ki.1996.396 [DOI] [PubMed] [Google Scholar]
- 8. Roodnat JI, de Fijter JW, van Kooten C, Daha MR, van Es LA (1999) Decreased IgA1 response after primary oral immunization with live typhoid vaccine in primary IgA nephropathy. Nephrol Dial Transplant 14: 353–359 10.1093/ndt/14.2.353 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki Y, Tomino Y (2008) Potential immunopathogenic role of the mucosa-bone marrow axis in IgA nephropathy: Insights from animal model. Semin Nephrol 28: 66–77 10.1016/j.semnephrol.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 10. Feehally J (1988) Immune mechanisms in glomerular IgA deposition. Nephrol Dial Transplant 3: 361–378. [DOI] [PubMed] [Google Scholar]
- 11. van den Wall Bake AW, Beyer WE, Evers-Schouten JH, Hermans J, Daha MR, et al. (1989) Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest 84: 1070–1075 10.1172/JCI114269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harper SJ, Allen AC, Pringle JH, Feehally J (1996) Increased dimeric IgA producing B cells in the bone marrow in IgA nephropathy determined by in situ hybridisation for J chain mRNA. J Clin Pathol 49: 38–42 10.1136/jcp.49.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imasawa T, Nagasawa R, Utsunomiya Y, Kawamura T, Zhong Y, et al. (1999) Bone marrow transplantation attenuates murine IgA nephropathy: Role of a stem cell disorder. Kidney Int 56: 1809–1817 10.1046/j.1523-1755.1999.00750.x [DOI] [PubMed] [Google Scholar]
- 14. van den Wall Bake AW, Daha MR, van Es LA (1989) Immunopathogenetic aspects of IgA nephropathy. Nephrologie 10: 141–145. [PubMed] [Google Scholar]
- 15. van Es LA, van den Wall Bake AW, Stad RK, van den Dobbelsteen ME, Bogers MJ, et al. (1995) Enigmas in the pathogenesis of IgA nephropathy. Contrib Nephrol 111: 169–176. [DOI] [PubMed] [Google Scholar]
- 16. Brandtzaeg P, Johansen FE (2005) Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev 206: 32–63 10.1111/j.0105-2896.2005.00283.x [DOI] [PubMed] [Google Scholar]
- 17. Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P (2008) The immune geography of IgA induction and function. Mucosal Immunol 1: 11–22 10.1038/mi.2007.6 [DOI] [PubMed] [Google Scholar]
- 18. Kunkel EJ, Butcher EC (2003) Plasma-cell homing. Nat Rev Immunol 3: 822–829 10.1038/nri1203 [DOI] [PubMed] [Google Scholar]
- 19. Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, et al. (2008) Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol 19: 2384–2395 10.1681/ASN.2007121311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sato D, Suzuki Y, Kano T, Suzuki H, Matsuoka J, et al. (2012) Tonsillar TLR9 expression and efficacy of tonsillectomy with steroid pulse therapy in IgA nephropathy patients. Nephrol Dial Transplant 27: 1090–1097 10.1093/ndt/gfr403 [DOI] [PubMed] [Google Scholar]
- 21.Kajiyama T, Suzuki Y, Kihara M, Suzuki H, Horikoshi S, et al. (2011) Different pathological roles of toll-like receptor 9 on mucosal B cells and dendritic cells in murine IgA nephropathy. Clin Dev Immunol: 819646. Available: http://www.hindawi.com/journals/jir/2011/819646/. Accessed 29 January 2014. doi: 10.1155/2011/819646. [DOI] [PMC free article] [PubMed]
- 22. He B, Xu W, Santini PA, Polydorides AD, Chiu A, et al. (2007) Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26: 812–826 10.1016/j.immuni.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 23. Xie Y, Chen X, Nishi S, Narita I, Gejyo F (2004) Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int 65: 1135–1144 10.1111/j.1523-1755.2004.00486.x [DOI] [PubMed] [Google Scholar]
- 24. Sato M, Hotta O, Tomioka S, Horigome I, Chiba S, et al. (2003) Cohort study of advanced IgA nephropathy: Efficacy and limitations of corticosteroids with tonsillectomy. Nephron Clin Pract 93: 137–145 10.1159/000070233 [DOI] [PubMed] [Google Scholar]
- 25. Hotta O, Miyazaki M, Furuta T, Tomioka S, Chiba S, et al. (2001) Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis 38: 736–743. [DOI] [PubMed] [Google Scholar]
- 26. Komatsu H, Fujimoto S, Hara S, Sato Y, Yamada K, et al. (2008) Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: A controlled study. Clin J Am Soc Nephrol 3: 1301–1307 10.2215/CJN.00310108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Chen J, Wang Y, Chen Y, Wang L, et al. (2011) A meta-analysis of the clinical remission rate and long-term efficacy of tonsillectomy in patients with IgA nephropathy. Nephrol Dial Transplant 26: 1923–1931 10.1093/ndt/gfq674 [DOI] [PubMed] [Google Scholar]
- 28. Maeda I, Hayashi T, Sato KK, Shibata MO, Hamada M, et al. (2012) Tonsillectomy has beneficial effects on remission and progression of IgA nephropathy independent of steroid therapy. Nephrol Dial Transplant 27: 2806–2813 10.1093/ndt/gfs053 [DOI] [PubMed] [Google Scholar]
- 29. Coppo R (2010) Can a dysregulated mucosal immune system in IgA nephropathy be controlled by tonsillectomy? Nephrol Dial Transplant 25: 2395–2397 10.1093/ndt/gfq266 [DOI] [PubMed] [Google Scholar]
- 30. Tomino Y, Endoh M, Nomoto Y, Sakai H (1981) Immunoglobulin A1 and IgA nephropathy. N Engl J Med 305: 1159–1160 10.1056/NEJM198111053051922 [DOI] [PubMed] [Google Scholar]
- 31. Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, et al. (2001) Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 59: 1077–1085 10.1046/j.1523-1755.2001.0590031077.x [DOI] [PubMed] [Google Scholar]
- 32. Allen AC, Bailey EM, Brenchley PE, Buck KS, Barratt J, et al. (2001) Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int 60: 969–973 10.1046/j.1523-1755.2001.060003969.x [DOI] [PubMed] [Google Scholar]
- 33. Itoh A, Iwase H, Takatani T, Nakamura I, Hayashi M, et al. (2003) Tonsillar IgA1 as a possible source of hypoglycosylated IgA1 in the serum of IgA nephropathy patients. Nephrol Dial Transplant 18: 1108–1114 10.1093/ndt/gfg108 [DOI] [PubMed] [Google Scholar]
- 34. Horie A, Hiki Y, Odani H, Yasuda Y, Takahashi M, et al. (2003) IgA1 molecules produced by tonsillar lymphocytes are under-O-glycosylated in IgA nephropathy. Am J Kidney Dis 42: 486–496 10.1016/S0272-6386(03)00743-1 [DOI] [PubMed] [Google Scholar]
- 35. Iwanami N, Iwase H, Takahashi N, Kato K, Itoh A, et al. (2008) Similarities between N-glycan glycoform of tonsillar IgA1 and that of aberrant IgA1 abundant in IgA nephropathy patient serum. J Nephrol 21: 118–126. [PubMed] [Google Scholar]
- 36. Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, et al. (2007) Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 1148–1154 10.1038/sj.ki.5002185 [DOI] [PubMed] [Google Scholar]
- 37. Novak J, Julian BA, Tomana M, Mestecky J (2008) IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol 28: 78–87 10.1016/j.semnephrol.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hiki Y (2009) O-linked oligosaccharides of the IgA1 hinge region: roles of its aberrant structure in the occurrence and/or progression of IgA nephropathy. Clin Exp Nephrol13: 415–423 10.1007/s10157-009-0173-7 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, et al. (2008) IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118: 629–639 10.1172/JCI33189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki H, Fan R, Zhang Z, Brown R, Hall S, et al. (2009) Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677 10.1172/JCI33189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berthoux F, Suzuki H, Thibaudin L, Yanagawa H, Maillard N, et al. (2012) Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol 23: 1579–1587 10.1681/ASN.2012010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki Y, Matsuzaki K, Suzuki H, Okazaki K, Yanagawa H, et al.. (2013) Serum levels of galactose deficient IgA1 and related immune complex are associated with disease activity of IgA nephropathy. Clin Exp Nephrol. In press. [DOI] [PMC free article] [PubMed]
- 43. Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, et al. (2013) Experimental evidence of cell dissemination playing a role in pathogenesis of IgA nephropathy in multiple lymphoid organs. Nephrol Dial Transplant 28: 320–326 10.1093/ndt/gfs467 [DOI] [PubMed] [Google Scholar]
- 44. Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, et al. (1997) Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 52: 509–516 10.1038/ki.1997.361 [DOI] [PubMed] [Google Scholar]
- 45. Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, et al. (2007) Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol 44: 2598–2604 10.1016/j.molimm.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, et al. (1999) Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104: 73–81 10.1172/JCI5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu SL, Colvin GA, Rifai A, Suzuki H, Novak J, et al. (2010) Glomerulonephritis after hematopoietic cell transplantation: IgA nephropathy with increased excretion of galactose-deficient IgA1. Nephrol Dial Transplant 25: 1708–1713 10.1093/ndt/gfp693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Glassock RJ (2008) IgA nephropathy: Challenges and opportunities. Cleve Clin J Med 75: 569–576 10.3949/ccjm.75.8.569 [DOI] [PubMed] [Google Scholar]
- 49. Inoue T, Sugiyama H, Kikumoto Y, Fukuoka N, Maeshima Y, et al. (2007) Downregulation of the beta1,3-galactosyltransferase gene in tonsillar B lymphocytes and aberrant lectin bindings to tonsillar IgA as a pathogenesis of IgA nephropathy. Contrib Nephrol 157: 120–124 10.1159/000102315 [DOI] [PubMed] [Google Scholar]
- 50. Barratt J, Eitner F, Feehally J, Floege J (2009) Immune complex formation in IgA nephropathy: a case of the ‘right’ antibodies in the ‘wrong’ place at the ‘wrong’ time? Nephrol Dial Transplant 24: 3620–3623 10.1093/ndt/gfp441 [DOI] [PubMed] [Google Scholar]
- 51. Coppo R, Camilla R, Amore A, Peruzzi L, Daprà V, et al. (2010) Toll-like receptor 4 expression is increased in circulating mononuclear cells of patients with immunoglobulin A nephropathy. Clin Exp Immunol. 159: 73–81 10.1111/j.1365-2249.2009.04045.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nishie T, Miyaishi O, Azuma H, Kameyama A, Naruse C, et al. (2007) Development of immunoglobulin A nephropathy-like disease in β-1,4-galactosyltransferase-I-deficient mice. Am J Pathol 170: 447–456 10.2353/ajpath.2007.060559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miyawaki S, Muso E, Takeuchi E, Matsushima H, Shibata Y, et al. (1997) Selective breeding for high serum IgA levels from noninbred ddY mice: isolation of a strain with an early onset of glomerular IgA deposition. Nephron 76: 201–207 10.1159/000190169 [DOI] [PubMed] [Google Scholar]
- 54. Kobayashi I, Nogaki F, Kusano H, Ono T, Miyawaki S, et al. (2002) Interleukin-12 alters the physicochemical characteristics of serum and glomerular IgA and modifies glycosylation in a ddY mouse strain having high IgA levels. Nephrol Dial Transplant 17: 2108–2116 10.1093/ndt/17.12.2108 [DOI] [PubMed] [Google Scholar]
- 55. Hashimoto A, Suzuki Y, Suzuki H, Ohsawa I, Brown R, et al. (2012) Determination of severity of murine IgA nephropathy by glomerular complement activation by aberrantly glycosylated IgA and immune complexes. Am J Pathol 181: 1338–1347 10.1016/j.ajpath.2012.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okazaki K, Suzuki Y, Otsuji M, Suzuki H, Kihara M, et al. (2012) Development of a model of early-onset IgA nephropathy. J Am Soc Nephrol 23: 1364–1374 10.1681/ASN.2011121160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suzuki H, Suzuki Y, Yamanaka T, Hirose S, Nishimura H, et al. (2005) Genome-wide scan in a novel IgA nephropathy model identifies a susceptibility locus on murine chromosome 10, in a region syntenic to human IgAN1 on chromosome 6q22–23. J Am Soc Nephrol 16: 1289–1299 10.1681/ASN.2004030219 [DOI] [PubMed] [Google Scholar]
- 58.Suzuki Y, Tomino Y (2009) Experimental model of IgA nephropathy. In: Lei KN, editor. Recent Advances in IgA Nephropathy. World Scientific Publishing Co. Pte. Ltd., Singapore: 387–402.