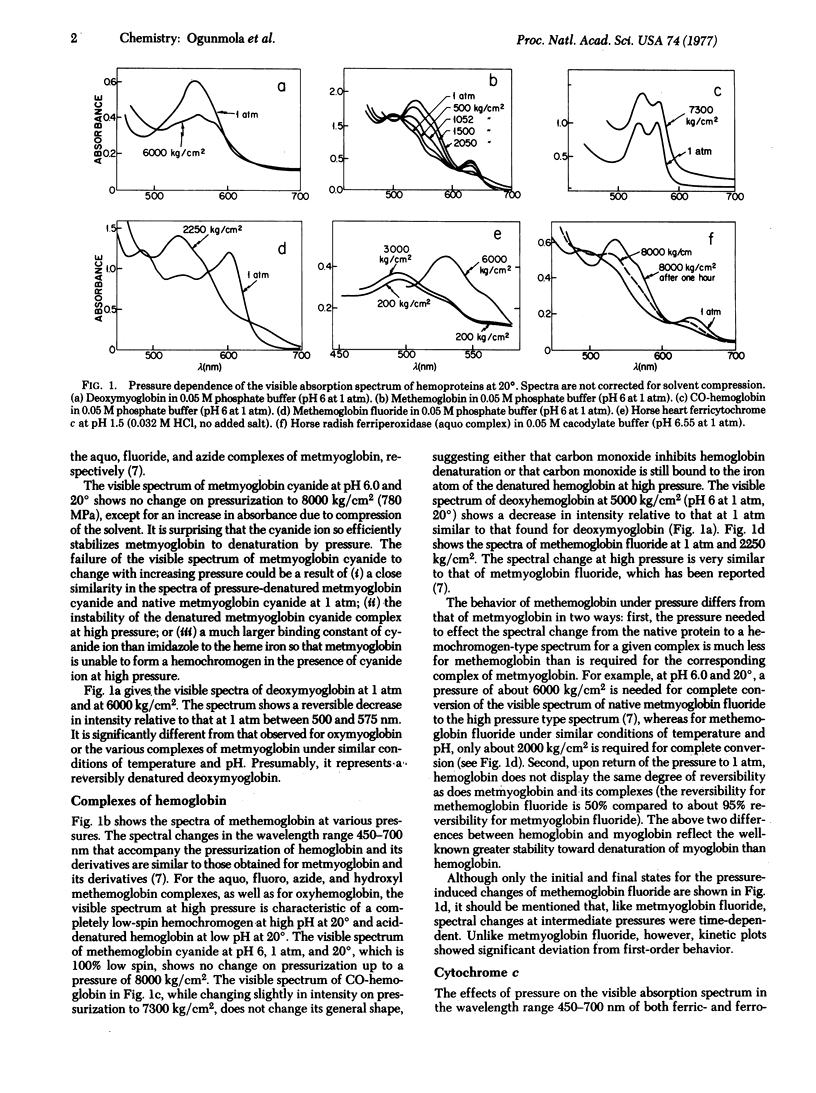
Abstract
The spectra of the ferric form of most heme proteins [metmyoglobin, methemoglobin, horse radish peroxidase (EC 1.11.1.7), and ferricytochrome c at pH 1.5] are converted from high-spin (open crevice) structure to low-spin (closed crevice) form under pressure. Pressures up to 8000 kg/cm2 (780 MPa) have no effect on the spectra of high-spin ferro- and ferricytochrome c, which have a closed crevice structure at pH 7.0. Spectra of deoxy-ferromyoglobin and deoxy-ferrohemoglobin are reduced in intensity, but pressure does not change the positions of the absorption maxima. Cyanide ion prevents pressure-induced spectral changes in metmyoglobin and methemoglobin up to 8000 kg/cm2. Carbon monoxide (with a high affinity for the ferro heme iron) has a similar effect on ferromyoglobin and ferrohemoglobin. The pressure required to cause spectral changes in the heme proteins falls in the order, cytochrome c (pH 7.0) greater than horse radish peroxidase greater than myoglobin greater than hemoglobin. We have calculated a volume change of --50 cm3/mol associated with the configurational change accompanying the reformation of the iron-methionine bond in cytochrome c at low pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEETLESTONE J., GEORGE P. A MAGNETOCHEMICAL STUDY OF EQUILIBRIA BETWEEN HIGH AND LOW SPIN STATES OF METMYOGLOBIN COMPLEXES. Biochemistry. 1964 May;3:707–714. doi: 10.1021/bi00893a019. [DOI] [PubMed] [Google Scholar]
- Fabry T. L., Hunt J. W., Jr Pressure-induced spectral shifts in hemoproteins. Arch Biochem Biophys. 1968 Feb;123(2):428–429. doi: 10.1016/0003-9861(68)90159-8. [DOI] [PubMed] [Google Scholar]
- George P., Lyster R. L. CREVICE STRUCTURES IN HEMOPROTEIN REACTIONS. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1013–1029. doi: 10.1073/pnas.44.10.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Carey F. G. Effect of pressure on the absorption spectrum of some heme compounds. Biochem Biophys Res Commun. 1975 Nov 17;67(2):747–751. doi: 10.1016/0006-291x(75)90876-1. [DOI] [PubMed] [Google Scholar]
- Huestis W. H., Raftery M. A. Observation of cooperative ionizations in hemoglobin. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1887–1891. doi: 10.1073/pnas.69.7.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T., Kotani M. Analysis of thermal equilibrium between high-spin and low-spin states in ferrihemoglobin complexes. Biochim Biophys Acta. 1969 Dec 23;194(2):351–363. doi: 10.1016/0005-2795(69)90096-8. [DOI] [PubMed] [Google Scholar]
- Ogunmola G. B., Kauzmann W., Zipp A. Volume changes in binding of ligands to methemoglobin and metmyoglobin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4271–4273. doi: 10.1073/pnas.73.12.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Fersht A. R., Simon S. R., Roberts G. C. Influence of globin structure on the state of the heme. II. Allosteric transitions in methemoglobin. Biochemistry. 1974 May 7;13(10):2174–2186. doi: 10.1021/bi00707a027. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Blumberg W. E. Studies on the stability of oxyhemoglobin A and its constituent chains and their derivatives. J Biol Chem. 1971 May 25;246(10):3356–3366. [PubMed] [Google Scholar]
- Rachmilewitz E. A., Peisach J., Bradley T. B., Blumberg W. E. Role of haemichromes in the formation of inclusion bodies in haemoglobin H disease. Nature. 1969 Apr 19;222(5190):248–250. doi: 10.1038/222248a0. [DOI] [PubMed] [Google Scholar]
- SIMKO J. P., Jr, KAUZMANN W. The kinetics of the urea denaturation of hemoglobin. I. Beef oxyhemoglobin. Biochemistry. 1962 Nov;1:1005–1017. doi: 10.1021/bi00912a010. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Taniguchi Y., Izui K. Effect of pressure on the combining power of hemoglobin for ethyl isocyanide. J Biochem. 1972 May;71(5):901–904. doi: 10.1093/oxfordjournals.jbchem.a129841. [DOI] [PubMed] [Google Scholar]
- Zipp A., Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973 Oct 9;12(21):4217–4228. doi: 10.1021/bi00745a028. [DOI] [PubMed] [Google Scholar]


