Abstract
Cold pressed and hexane extracted moringa seed oils (CPMSO and HEMSO) were evaluated for their physico-chemical and stability characteristics. The iodine value, saponification value and unsaponifiable matter of CPMSO and HEMSO were found to be 67.8 and 68.5 g I2 / 100 g oil, 190.4 and 191.2 mg KOH / g oil and 0.59 and 0.65%, respectively. The total tocopherols of CPMSO and HEMSO were found to be 95.5 and 90.2 mg/Kg. The fatty acid composition of CPMSO and HEMSO showed oleic acid as the major fatty acid (78–79%). The oxidative, thermal and frying stabilities of the CPMSO were compared with commercial raw and refined groundnut oil (GNO and RGNO). The CPMSO was of adequate thermal stability and better oxidative stability as it showed 79% lesser peroxide formation than GNO. The frying stability of CPMSO was better as it showed lower increase in free fatty acid (28%), peroxide value (10 meq O2/Kg) and color (25%) than RGNO (48%, 22 meq O2/kg and 52%, respectively) after frying.
Keywords: Moringa oleifera seed oil, Quality, Physico-chemical characteristics
Introduction
Vegetable oils constitute an important part of human livelihood all over the world. The widening gap between demand and supply necessitates the need for alternate sources of edible oils to augment global production. Moringa oleifera seeds contain about 42% of a brilliant yellow, high-oleic acid crude oil having a pleasant, peanut-like flavor. The oil consists of 82% unsaturated fatty acids, 70% of which is oleic acid (Tsaknis et al. 1998; Foidl et al. 2001; Farooq et al.2006; Rahman et al. 2009). Apart from the emphasis of modern nutrition on oils that contain high amount of unsaturated fatty acids, high oleic acid oils are known to be healthy alternatives to hydrogenated vegetable oils (Tsaknis et al. 1998; Abdulkarim et al. 2005; Farooq et al. 2006; Rahman et al. 2009). Olive oil, the widest known in this category, is seldom used for frying because of its high cost. There are several reports on the composition and characteristics of Moringa oleifera seed oil varieties from different countries of origin, e.g., India (Lalas and Tsaknis 2002), Kenya (Tsaknis et al. 1999a, b), Malawi (Tsaknis et al. 1998), Malaysia (Abdulkarim et al.2005), Pakistan (Farooq et al. 2006; Manzoor et al. 2007), Bangladesh (Rahman et al. 2009) considering its prospect as an alternative vegetable oil source. Quality characteristics of moringa seed oils from Indian cultivar (PKM-1) and an African cultivar grown in similar agro-climatic conditions of Argentina has also been reported (Ayerza 2011). Moreover, very recent studies on Moringa oleifera seed oil have been conducted to explore the possibility of its use as a biofuel (da Silva et al. 2010; Kibazohi and Sangwan 2011)
Deep-frying is the commonest form in which vegetable oils are consumed in human nutrition. The repeated use of frying oils, usually at very high temperatures in the presence of air and moisture is a common practice in the food service industry; this often changes the physicochemical properties of the frying oil, thus leading to the formation of undesirable constituents that may have serious implication on consumers’ health (Tsaknis et al. 1999a, b; Tsaknis and Lalas 2002; Abdulkarim et al. 2007; Lalas et al. 2006). Oxidative and thermal stabilities are therefore important qualities for frying oils. Frying stability of cold pressed and solvent-extracted Moringa oleifera, variety Mbololo of Kenya, seed oil (Tsaknis et al. 1999a, b), Moringa oleifera seed oil variety “Periyakulam 1” (Tsaknis and Lalas 2002), Moringa stenopetala seed oil (Lalas et al. 2006) as well as Moringa oleifera seed oil (Abdulkarim et al. 2007) has been studied.
The present study provides information on the extraction, quality and stability of Moringa oleifera Jaffna variety seed oil, which has not been reported so far. In the present work, comparative effect of oil extraction procedure on the physico-chemical characteristics of the extracted oils from Moringa oleifera variety Jaffna seeds of Indian origin was studied. Moringa oleifera seed oil was extracted by solvent (hexane) and cold pressing methods and its quality characteristics are reported. Groundnut oil is a common vegetable oil used in India for various purposes like cooking and frying. The oxidative, thermal and frying stabilities of the cold pressed moringa seed oil were also evaluated in comparison with commercial raw and refined groundnut oils and reported in this paper.
Materials and methods
Bulk quantities of dry seeds botanically identified as Moringa oleifera Jaffna variety were procured from Veg-Indian Exports, Erode, Tamil Nadu, India. The seeds were dehulled using an attrition plate mill (Model A-453, Chandra Manufacturing Co., Chennai, India), and the hulls were separated using an aspirator. Foreign matters and immature or defective kernels were removed from the lot, and it was then kept in airtight containers at −20 °C till analyzed. All chemicals and reagents used were of analytical grade. The moisture and crude fat contents of the dehulled kernels were determined according to official methods of AOAC, 2000 (Cunnif 2000). Commercial raw groundnut oil (pressed and filtered)—Diamond brand (GNO) and Commercial refined groundnut oil—Goldwinner brand (RGNO), were procured from a local supermarket in Mysore.
Extraction of Moringa oleifera seed oil
Cold pressing
A hydraulic press (John Mill & Co. Ltd., Max. 90 t, Made in Montgomery, UK) was used for cold pressing. The cylindrical press cage had 1 mm perforations and was 90 mm in diameter and 190 mm high. About 1 kg of kernels (dehulled seeds) was loaded in the press cage per batch. The oil obtained was filtered and the percentage of oil extracted was determined by weight difference. The cold pressed moringa seed oil was named as CPMSO.
Solvent extraction
About 10 kg of moringa kernels were flaked as per the established procedure (Ogunsina et al. 2010; Ogunsina and Radha 2010). Oil extraction from flaked kernels was carried out with hexane (flake to solvent ratio =1:1.5 w/v) by a batch process in a stainless steel percolator - 25 kg capacity (Percolator SS—Central Food Technological Research Institute, Mysore, India). The height of the percolator is 6″. The diameter and mesh size of the perforated plate are 6″ and 0.75 mm respectively. The flaked kernels (10 kg) were loaded into the percolator and washed with 15 L of hexane at room temperature (25–30 °C). There were five repeated washings for each batch with fresh hexane, and all carried out in similar manner. The duration of each wash was 2 h. For each batch, after the fifth washing, the average residual fat content of defatted flakes was determined and found to be 0.87%. This was taken as complete extraction. The miscella was distilled and the extracted moringa seed oil was recovered and desolventized completely using a flash evaporator at 40 °C. The hexane extracted Moringa oleifera seed oil was named as HEMSO.
Determination of physical characteristics
The specific gravity of CPMSO and HEMSO was determined according to ASTM standard test methods ASTM D 445, ASTM D 1298, ASTM D 368, ASTM D 891 (Canning et al. 1991). The density of the CPMSO and HEMSO was determined according to ASTM standard test ASTM D 891, ASTM D 369 (Canning et al. 1991). Viscosity of CPMSO and HEMSO was determined using a viscometer (Model R1:3:M-3, Rheology International Shannon Ltd, Bay K 14A, Shannon Industrial Estate, Shannon, Co Clare, Ireland). About 80 mL of oil sample was filled into the 100 mL sample adaptor and the ASTM spindle size number 3 attached to the viscometer was immersed into the oil to the marked portion. Viscosity was measured at 30 rpm rotor speed under ambient temperature conditions (26 ± 2 °C). Refractive index was determined using Abbe Refactometer (Model NAR-3 T, ATAGO Co., Ltd., Tokyo, Japan) at a temperature range of 30–32 °C. The oil color was determined on triplicate samples by transmission measurement in a 1 in. cell using a Lovibond tintometer (Model—F, The Tintometer Ltd., Salisbury, U.K.) and calculated as 5 × Red units +1× Yellow units (5R + Y value). Values were mean of three readings and expressed as Lovibond units (LU).
Determination of chemical characteristics
Peroxide value (PV), free fatty acids value (FFA), saponification value (SV), Iodine value (IV) and unsaponifiable matter (USM) of CPMSO and HEMSO were determined according to AOCS (1997) Method Nos: Cd 8–53, Ca 5a-40, Cd 3c-91, Cd 1–25 and Ca 6a-40 respectively (Firestone 1997). The total polar matter (TPM) of CPMSO and HEMSO was measured by using Fri-Check instrument (Grote Bean, 375, B2235 Hulshout, Belgium). Oil was filled up to the cylindrical sensor tube and inserted into the Fri-Check unit and the displayed reading on the digital display was taken as the percent total polar matter (TPM) content of the sample. The total tocopherol content of CPMSO and HEMSO was determined by using IUPAC Method No. 2.301, 1987 (Paquot and Havtfenne 1987).
Fatty acid composition
Fatty acid methyl esters (FAME) of CPMSO and HEMSO were prepared by transesterification, using methanolic KOH according to the AOCS Method No: Ce 1–62, 1997 (Firestone 1997). The FAME were analyzed on a gas chromatograph (Model GC-15A, Shimadzu corporation, Kyoto, Japan), equipped with a hydrogen flame ionization detector (FID). Separation was performed using a S.S. column (3 m × 1/8″dia mm i.d.), packed with 15% diethylene glycol succinate (DEGS) on chromosorb W / HP 80–100 mesh as the stationary phase. The column oven temperature was kept at 180 °C. Injector and detector temperatures were 220 and 230 °C respectively and the carrier gas, nitrogen was maintained at a flow rate of 40 mL/min. The fatty acids in the samples were identified based on retention time of reference standard FAME mix C8-C24 (Supelco, Bellefonte, USA) and expressed as relative area percent.
Evaluation of oxidative, thermal and frying stability
Oxidative stability
The oxidative stability of CPMSO was investigated according the established procedure of Bhatnagar et al. (2009). About 40 g × 3 batches of CPMSO were placed in 50 mL beakers and incubated at 37 °C and 55% RH in laboratory incubator. Commercial raw groundnut oil (GNO) as a positive control was also incubated simultaneously under similar conditions. Samples (2 g × 2) were withdrawn at weekly intervals and analyzed for their PV as per AOCS Method No: Cd 8–53, 1997 (Firestone 1997).
Thermal stability
The thermal stability of CPMSO was studied as per the established procedure (Lalas and Tsaknis 2002) with minor modifications. The experimental set-up included a temperature controlled electric stove provided with a digital temperature indicator and a thermocouple immersed in the oil placed over the stove. About 2 L of CPMSO was subjected to continuous heating at 180 ± 5 °C for 36 h. At 0, 6, 12, 18, 24, 30 and 36 h of continuous heating, 50 mL of the oil was drawn in triplicates and analyzed for viscosity, TPM, color, PV and FFA.
Frying stability
The stability of CPMSO during frying was investigated in comparison with commercial refined groundnut oil as per the established procedure (Lalas et al. 2006) with minor modifications. About 5 kg of fresh potatoes purchased from a local supermarket in Mysore, were peeled and sliced into discs of average thickness and diameter of 1.5 mm and 51 mm respectively. Two kilograms each of CPMSO and RGNO were used for frying. Frying protocol involved heating the oil to 180 ± 5 °C and then potato slices (10 batches of 250 g each) were fried in 10 repeated successions spanning a total time of 2 h and there was no replenishment of oil in-between frying. Oil samples were taken in triplicate for analysis immediately before and after frying. The FFA, PV, viscosity and color of oil samples drawn before and after frying were determined.
Statistical analysis
All samples were taken in triplicate and analysis carried out in duplicate making six determinations and mean±standard deviation value reported. The data were analyzed using the statistical program - GraphPad InStat Demo—[DATASET1.ISD] (GraphPad InStat 2010). The two-tailed P value was determined to show the significant changes. A significant change was considered only when p value was ≤0.05.
Results and discussion
Physico-chemical characteristics
This study presents the physico-chemical characteristics of Moringa oleifera Jaffna variety seed oil of Indian origin obtained by cold pressing and hexane extraction. It also reports the stability (oxidative, thermal and frying) of Moringa oleifera seed oil obtained by cold pressing in comparison to commercial groundnut oil. Groundnut oil is very common and popular vegetable oil in India, which is being used in raw and refined forms for many of the culinary applications like cooking and frying in Indian households. Hence the Moringa oleifera Jaffna variety seed oil was compared with commercial raw and refined groundnut oils for stability studies. The moisture content of the dehulled moringa seeds was found to be 2.9% (w/w). The oil obtained by solvent extraction was 39.4% (w/w) while by cold pressing it was 24.2% (w/w). Cold pressing could extract only 61.4% of the total oil present in the dehulled moringa seeds as compared to hexane extraction which extracted almost 100% of the total oil present in the dehulled moringa seeds. The fat content by cold pressing differed significantly (p ≤ 0.05) from fat content by hexane extraction. The values for fat obtained by hexane extraction and cold pressing agreed well with previous findings of 38.3 and 24.5% respectively by Lalas and Tsaknis (2002). The physico-chemical properties of CPMSO and HEMSO are summarized in Table 1. The CPMSO had a golden yellow appearance with slight haziness whereas HEMSO had a clear golden yellow appearance. The Lovibond Tintometer reading in the transmittance mode in 1 in. cell, CPMSO was 30.0 LU whereas HEMSO gave a value of 36.0 LU. The iodine value, saponification value and unsaponifiable matter of CPMSO and HEMSO were found to be 67.8 and 68.5 g I2 / 100 g oil, 190.4 and 191.2 mg KOH / g oil and 0.59 and 0.65% respectively. The total tocopherols content of CPMSO and HEMSO was found to be 95.5 and 90.2 mg/kg respectively; whereas the refractive indices of the two oils showed no significant difference (p > 0.05). The free fatty acid values, peroxide values, saponification values, iodine values, unsaponifiable matter, densities and specific gravities of the two oils were also not significantly different (p > 0.05). Hexane was expected to extract more amounts of tocopherols as compared to cold pressing; however, the case was found to be reverse, which may be due to the higher yield of oil, by hexane extraction than by cold-pressing. The color, FFA, PV values of CPMSO and HEMSO were found to be in the normal limits as far as unrefined oils are concerned. The values for physico-chemical characteristics of CPMSO and HEMSO agreed well with the previous literature reports (Tsaknis et al. 1999a, b; Foidl et al. 2001; Lalas and Tsaknis 2002; Farooq et al. 2006; Rahman et al. 2009). Some variations observed in the results might be due to the varietal difference in seeds and regional agro-climatic variations.
Table 1.
Physico-chemical characteristics of cold pressed moringa seed oil (CPMSO) and hexane extracted moringa seed oil (HEMSO)
| Parameters | CPMSO | HEMSO | Moringa oleifera Periyakulam-1 seed oil (Indian origin) (Lalas and Tsaknis 2002) | Moringa concanensis seed oil (Pakistan origin) (Latif and Anwar 2008) | Moringa oleifera Lam. seed oil (Bangladesh origin) (Rahman et al. 2009) |
|---|---|---|---|---|---|
| Color – 1 in. cell (Lovibond units, Y+5R) | 30.0a ± 1.0 (25 Y & 1 R) | 36.0b ± 1.2 (30 Y & 1.2 R) | 39.0 | 29.5 | 37.8 |
| FFA (as oleic acid), % | 3.5a ± 0.12 | 4.0b ± 0.05 | 1.1 | 0.32 | 0.7 |
| Peroxide value, meq O2 / kg | 1.0a ± 0.01 | 1.02a ± 0.01 | NR | NR | 1.5 |
| Iodine value, g I2 / 100 g oil | 67.8a ± 0.42 | 68.5a ± 0.36 | 65.6 | 66.7 | 68.9 |
| Saponification value, mg KOH / g oil | 190.4a ± 0.57 | 191.2a ± 0.52 | 188.0 | 180 | 180.0 |
| Unsaponifiable matter, % | 0.59a ± 0.05 | 0.65a ± 0.03 | NR | 0.76 | 0.77 |
| Total tocopherols, mg/kg | 95.5a ± 1.0 | 90.2a ± 1.7 | 35.4 | 120.4 | 251.5 |
| Total polar materials, % | 3.1a ± 0.05 | 0.0b | NR | NR | NR |
| Refractive index | 1.47a ± 0.001 | 1.47a ± 0.001 | 1.46 | 1.46 | 1.46 |
| Density at 25 °C, g/mL | 0.90a ± 0.01 | 0.92a ± 0.01 | 0.91 | 0.87 | 0.90 |
| Specific gravity | 0.93a ± 0.01 | 0.90a ± 0.01 | NR | NR | |
| Viscosity, mPa.s | 43.8a ± 0.14 | 43.6a ± 0.25 | 45.1 | NR | 56.5 |
NR not reported
Values on the same row followed by different superscripts differ significantly at p ≤ 0.05
Samples were taken in triplicate and analysis carried out in duplicate making six determinations (n = 6) and mean ± standard deviation value reported
The fatty acid profiles of CPMSO and HEMSO are presented in Table 2. Oleic acid was the major unsaturated fatty acid; palmitic, stearic, arachidic and behenic acids were the saturated fatty acids while palmitoleic and linolenic acids were present in small amounts. For the two oils, significant difference was observed only in myristic and stearic fatty acids (p ≤ 0.05). With about 79% oleic acid in its fatty acid composition CPMSO and HEMSO are high-oleic oils which are of great importance because of their superior stability compared to PUFA rich oils (Manzoor et al. 2007). The CPMSO and HEMSO had 79.5 and 78.7% of monounsaturated oleic acid and 80.7 and 79.9% of unsaturated fatty acids respectively. Only stearic acid which increased from 3.9% in CPMSO to 4.6% in HEMSO and behenic acid which reduced from 5.1% in CPMSO to 4.5% in HEMSO showed significant difference (p ≤ 0.05). No significant difference was found in rest of the fatty acids of CPMSO and HEMSO. The fatty acid composition of CPMSO and HEMSO agreed well with the previous literature reports (Tsaknis et al. 1999a, b; Foidl et al. 2001; Lalas and Tsaknis 2002; Farooq et al. 2006; Rahman et al. 2009). The fatty acid composition of GNO suggested that oleic acid was the major fatty acid (~44%) followed by linoleic acid (~30%) and palmitic acid (~14%). Stearic acid and arachidic acid were present in almost equal amounts (~4% each) while linolenic acid (~1%) was present in minor amount. The fatty acid composition of GNO agreed well with the previous literature report on commercial raw groundnut oil (Bhatnagar et al. 2009).
Table 2.
Fatty acid composition of cold pressed moringa seed oil (CPMSO), hexane extracted moringa seed oil (HEMSO) and commercial raw groundnut oil (GNO)
| Fatty acid Composition (relative area %) | CPMSO | HEMSO | Moringa oleifera Periyakulam-1 seed oil (Indian origin) (Lalas and Tsaknis 2002) | Moringa concanensis seed oil (Pakistan origin) (Latif and Anwar 2008) | Moringa oleifera Lam. seed oil (Bangladesh origin) (Rahman et al. 2009) | *GNO |
|---|---|---|---|---|---|---|
| Myristic (C14:0) | 0.24a ± 0.05 | 0.72b ± 0.13 | 0.1 | NR | 0.1 | ND |
| Palmitic (C16:0) | 5.8a ± 0.11 | 6.1a ± 0.24 | 6.5 | 11.9 | 6.2 | 14.5 ± 1.07 |
| Palmitoleic(C16:1) | 1.2a ± 0.35 | 1.2a ± 0.0 | 1.5 | 2.4 | 1.1 | ND |
| Stearic (C18:0) | 3.9a ± 0.54 | 4.6b ± 0.22 | 5.9 | 3.6 | 4.8 | 4.7 ± 0.31 |
| Oleic (C18:1) | 79.5a ± 1.11 | 78.7a ± 1.21 | 71.2 | 67.3 | 74.4 | 44.7 ± 1.21 |
| Linoleic (C18:2) | ND | ND | 0.7 | 1.8 | 1.2 | 30.5 ± 1.75 |
| Linolenic (C18:3) | 2.2a ± 0.62 | 1.8a ± 0.34 | 0.2 | NR | 0.3 | 1.2 ± 0.22 |
| Arachidic (C20:0) | 2.2a ± 0.43 | 2.3a ± 0.05 | 3.6 | 3.7 | 3.5 | 4.4 ± 0.67 |
| Gadoleic (C20:1) | ND | ND | 2.2 | 1.8 | 1.6 | ND |
| Behenic (C22:0) | 5.1a ± 0.47 | 4.5a ± 1.53 | 6.4 | 7.6 | 6.2 | ND |
| % SFA | 17.2a | 18.3a | 22.5 | 26.8 | 20.8 | 23.9 |
| % MUFA | 80.7a | 79.9a | 74.9 | 71.5 | 77.1 | 44.4 |
| % PUFA | 2.2a | 1.8a | 0.9 | 1.8 | 1.5 | 31.7 |
NR not reported
ND not detected
*Fatty acid composition of GNO and RGNO was similar
Values on the same row followed by different superscripts differ significantly at p ≤ 0.05
Samples were taken in triplicate and analysis carried out in duplicate making six determinations (n = 6) and mean ± standard deviation value reported
Stability of CPMSO
Oxidative stability
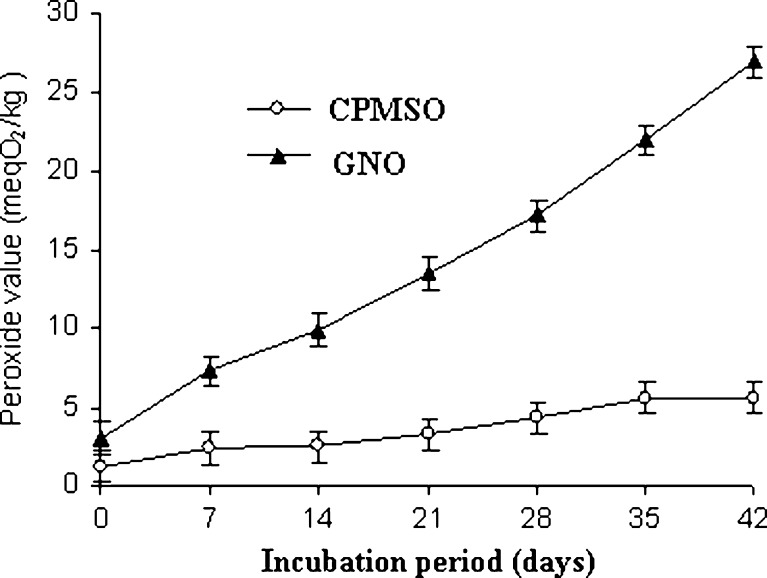
Changes in PV of CPMSO and GNO during incubation at 37 °C and 55% RH are presented in Fig. 1. The results indicated greater inhibitory effect of CPMSO against formation of peroxides than GNO. The PV of GNO increased steadily from 3.0 meq O2/kg on day zero to 26.93 meq O2/kg on the 42nd day, whereas for CPMSO, it increased from 1.2 meq O2/kg on day zero to 5.6 meq O2/kg on the 35th and 42nd days showing a relatively better stability against oxidation. The fatty acid composition of CPMSO and GNO suggested that they contained 31.7 and 2.2% of polyunsaturated fatty acids (PUFA). The steady rise in the peroxides in GNO can be attributed to the presence of more amounts of PUFA than in CPMSO since the oxidative stability of oil is inversely proportional to its PUFA content (Bhatnagar et al. 2009).
Fig. 1.
Oxidative stability of cold pressed moringa seed oil (CPMSO) and commercial raw groundnut oil (GNO). Samples were taken in triplicate and analysis carried out in duplicate making six determinations (n = 6) and mean ± standard deviation value reported
Thermal stability
The changes in FFA, PV, TPM and kinematic viscosity of CPMSO in response to heat (180 ± 5 °C) spanning 0–36 h at 6 h interval are shown in Table 3. As the duration of heating increased, corresponding increase was observed in PV whereas, FFA decreased from 3.5% to 2.2% with the heating period. This might have happened due to the volatile nature of free fatty acids getting evaporated and lost with high intensity of progressive heating. TPM increased gradually from 3% at 0 h to 66% at 36 h as expected. After 24 h of heating, TPM rose to >24% which is considered as the limit beyond which any particular frying oil should be discarded (Abdulkarim et al. 2007). The TPM is a good quality indicator for oils under heating conditions; it indicates the total amount of newly formed compounds having higher polarity than that of triacylglycerols (Abdulkarim et al. 2007). Earlier reports suggested that a change in TPM is the most reliable measure of the extent of deterioration in heat-abused oils especially due to repeated use as frying medium under severe heating conditions (Abdulkarim et al. 2007). The sensor tube in the Fricheck unit monitor changes in the viscosity of such oils. This explains why changes in viscosity in both cases are similar. The formation of high molecular weight polymers often lead to increase in viscosity. The changes in color intensity of CPMSO in response to the thermal treatment (180 ± 5 °C) spanning 36 h is also given in Table 3. The color of CPMSO increased from 30 to 80 LU after 36 h of heating (at 175 ± 0.1 °C) showing more than 2.5 fold increase in color intensity. Most volatile fatty acids are lost when heating of vegetable oils is prolonged causing an accumulation of non-volatile decomposed products (oxidized triacylglycerols and FFA) and changing color intensity.
Table 3.
Thermal stability and frying stability of cold pressed moringa seed oil (CPMSO) and commercial refined groundnut oil (RGNO)
| Duration of thermal treatment (h) | Oil color (Lovibond units) | Free Fatty Acids (%) | Peroxide value (meqO2 / Kg) | Viscosity (mPa.s) | Total polar materials (%) |
|---|---|---|---|---|---|
| 0 | 30.0 ± 1.0 a (25.0Y & 1.0R) | 3.5 ± 0.05 a | 1.5 ± 0.05 a | 43.8 ± 0.55 a | 3.1 ± 0.14 a |
| 6 | 50.0 ± 1.5 b (35.0Y & 3.0R) | 3.4 ± 0.05 b | 2.1 ± 0.05 b | 107.3 ± 1.53 b | 6.8 ± 0.19 b |
| 12 | 65.0 ± 1.5 c (40.0Y & 5.0R) | 3.0 ± 0.03 c | 2.5 ± 0.05 c | 115.0 ± 1.22 c | 12.2 ± 0.37 c |
| 18 | 75.0 ± 2.0 d (43.0Y & 6.4R) | 2.7 ± 0.03 d | 3.1 ± 0.05 d | 197.0 ± 1.81 d | 16.0 ± 0.39 d |
| 24 | 79.0 ± 1.0 d (47.0Y & 6.4R) | 2.4 ± 0.03 e | 4.1 ± 0.03 e | 234.7 ± 2.14 e | 27.5 ± 0.56 e |
| 30 | 80.0 ± 1.0 d (48.0Y & 6.4R) | 2.3 ± 0.01 f | 4.9 ± 0.05 f | 241.4 ± 1.46 f | 46.5 ± 0.58 f |
| 36 | 80.0 ± 1.0 d (48.0Y & 6.4R) | 2.2 ± 0.01 g | 5.6 ± 0.14 g | 337.5 ± 1.97 g | 65.8 ± 0.97 g |
| CPMSO | |||||
| Before frying | 30.0 ± 1.0 a (20Y + 2R) | 3.5 ± 0.01 a | 1.00 ± 0.01 a | 43.8 ± 2.56 a | 3.1 ± 0.16a |
| After frying | 40.0 ± 1.5 b (25Y + 3R) | 4.9 ± 0.01 b | 10.2 ± 0.24 b | 65.1 ± 3.07b | 90.8 ± 0.98 b |
| RGNO | |||||
| Before frying | 12.0 ± 0.5 c (7Y + 1R) | 0.72 ± 0.01 c | 1.5 ± 0.01 c | 45.3 ± 2.06 c | 1.1 ± 0.17 c |
| After frying | 25 ± 1.0 d (15Y + 2R) | 1.4 ± 0.02 d | 21.9 ± 0.10 d | 50.2 ± 2.04 d | 95.2 ± 1.45 d |
Values on the same column followed by different superscripts differ significantly at p ≤ 0.05
Samples were taken in triplicate and analysis carried out in duplicate making six determinations (n = 6) and mean ± standard deviation value reported
Frying stability
The data of frying study is also given in Table 3. CPMSO showed a FFA increase of 28.6% while RGNO showed a FFA increase of 48.6% after frying. The food to be fried does contain some water. Abdulkarim et al. (2007) suggests that in the presence of moisture, a chemical reaction is initiated in the frying oil which causes a progressive increase in its FFA. Water, a weak nucleophile, attacks the ester linkage of triacylglycerols and produces di- and mono-acylglycerols, glycerol, and free fatty acids (Choe and Min 2007). The deteriorative effect of oxidation and polymerization was lower in CPMSO than in RGNO indicating CPMSO to be stable oil for frying. In most deep fat frying operations, the amount of FFA produced by hydrolysis is too small to affect the quality of the food; adverse effects are usually due to the oxidation of unsaturated fatty acids (Abdulkarim et al. 2007). An increase was also observed in the PV of CPMSO from 1.0 to 10.22 meq O2/kg before and after frying respectively; whereas corresponding change in RGNO was from 1.5 to 21.9 meq O2/kg. Increase in PV was higher for RGNO than for CPMSO. Peroxide formation under frying conditions was evident in both the oils but the rate was more in RGNO than in CPMSO. An increase was observed in the viscosity of the oils after frying. This may be due to the formation of high molecular weight polymers. The viscosity of CPMSO increased more than that of RGNO after frying. This might be due to the refined nature of groundnut oil since refining process removes the phospholipids, waxes and free fatty acids which are also responsible for increase in viscosity of frying oils. CPMSO showed a 25% increase in color intensity while RGNO showed a 52% increase in color intensity after frying. Checking oil quality and knowing when to change used frying oil are critical to the quality of fried food. When a frying oil should be discarded depends on good judgment (Abdulkarim et al. 2005), the type of frying oil, frying temperature, number of repeated frying and analytical measurement of frying oil quality indicators (Choe and Min 2007). CPMSO may therefore be more suitable for repeated frying than RGNO. The results clearly indicate that the deteriorative effect of oxidation and polymerization on CPMSO is lower than that on RGNO indicating CPMSO to be superior frying oil.
Conclusions
The present study provides information on the extraction, quality and stability of Moringa oleifera Jaffna variety seed oil which has not been reported so far. The quality characteristics of the cold pressed and solvent extracted Moringa oleifera variety Jaffna seed oil such as peroxide value, saponification value, unsaponifiable matter, refractive index, density and specific gravity were found to be almost similar. Moringa oleifera Jaffna variety seed oil, based on its high oleic acid content can become another valuable source of high-oleic acid oil. The high oleic acid content also provides good stability to Moringa oleifera seed oil. The oil had good thermal stability. The oxidative stability and frying stability of cold pressed Moringa oleifera Jaffna variety seed oil was better than commercial raw and refined groundnut oils respectively. Based on the present findings, Moringa oleifera Jaffna variety seed oil has shown enough promise to be considered as more stable and healthy substitute for commercial groundnut oil as a cooking and frying medium.
Acknowledgements
This research work was funded by the United Nations University and Central Food Technological Research Institute (CFTRI), Mysore, India. Authors acknowledge Dr V. Prakash, Director, CFTRI, for providing infrastructural facilities. Thanks are due to Dr A. G. Appu Rao, Head of Protein Chemistry and Technology and Dr B.R. Lokesh, Head of Lipid Science and Traditional Foods for their valuable suggestions.
References
- Abdulkarim SM, Long K, Lai OM, Muhammad SKS, Ghazali HM. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005;93:253–263. doi: 10.1016/j.foodchem.2004.09.023. [DOI] [Google Scholar]
- Abdulkarim SM, Long K, Lai OM, Muhammad SKS, Ghazali HM. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007;105:1382–1389. doi: 10.1016/j.foodchem.2007.05.013. [DOI] [Google Scholar]
- Ayerza R. Seed yield components, oil content, and fatty acid composition of two cultivars of moringa (Moringa oleifera Lam.) growing in the Arid Chaco of Argentina. Ind Crops Prod. 2011;33:389–394. doi: 10.1016/j.indcrop.2010.11.003. [DOI] [Google Scholar]
- Bhatnagar AS, Prasanthkumar PK, Hemavathy J, Gopalakrishna AG. Fatty acid composition, oxidative stability and radical scavenging activity of vegetable oil blends with coconut oil. J Am Oil Chem Soc. 2009;86:991–999. doi: 10.1007/s11746-009-1435-y. [DOI] [Google Scholar]
- Canning SP, Fazio PC, Fisher D, Gutman EL, Hsia CT, Kauffman SL, Kramer J, Lane M, Leinweber CM, Mcgee PA (1991) Methods of the American Society for Testing and Materials, ASTM D 445, ASTM D 1298, ASTM D 368, ASTM D 891, ASTM D 369. pp 168–173.
- Choe E, Min DB. Chemistry of deep-fat frying oils. J Food Sci. 2007;72(5):R77–86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Cunnif P (2000) Official methods of association of official analytical chemists. International Association of Official Analytical Chemists (AOAC). Vol. II, 17th Ed. (pp 1–37). Arlington VA USA.
- da Silva JPV, Serra TM, Gossmann M, Wolf CR, Meneghetti MR, Meneghetti SMP. Moringa oleifera oil: Studies of characterization and biodiesel production. Biomass Bioenergy. 2010;34:1527–1530. doi: 10.1016/j.biombioe.2010.04.002. [DOI] [Google Scholar]
- Farooq A, Syeda NZ, Umer R. Characterization of Moringa oleifera seed oil from drought and irrigated regions of Punjab, Pakistan. Grasas Y Aceites. 2006;57(2):160–168. [Google Scholar]
- Firestone D (1997) Official methods and recommended practices of the American Oil Chemists Society, AOCS method no. ca 5a-40, AOCS method no. ca 6a-40, AOCS method no. cd 8–53, AOCS method no. cd 3c-91, AOCS method no. ce 1–62. 5th Ed. AOCS press, 1608, Broadmoore drive Champaign, Illinois-61826 USA.
- Foidl N, Makkar HPS, Becker K (2001) The potential of Moringa oleifera for agricultural and industrial uses. Moringa review proceedings in Dar Es Salaam, Tanzania, Oct. 20th – Nov. 2nd 2001.
- Graphpad instat demo-[dataset1.isd]. Graphpad Software Inc. 11452, EI Camino Real, #215, San Digeo, 92130 USA. www.graphpad.com (Accessed on Jan. 9, 2010).
- Kibazohi O, Sangwan RS. Vegetable oil production potential from Jatropha curcas, Croton megalocarpus, Aleurites moluccana, Moringa oleifera and Pachira glabra: assessment of renewable energy resources for bio-energy production in Africa. Biomass Bioenergy. 2011;35:1352–1356. doi: 10.1016/j.biombioe.2010.12.048. [DOI] [Google Scholar]
- Lalas S, Gortzi O, Tsaknis J. Frying stability of Moringa stenopetala seed oil. Plant Foods Hum Nutr. 2006;61:93–102. doi: 10.1007/s11130-006-0022-8. [DOI] [PubMed] [Google Scholar]
- Lalas S, Tsaknis J. Characterization of Moringa oleifera seed oil variety Periyakulam-1. J Food Comp Ana. 2002;15(1):65–77. doi: 10.1006/jfca.2001.1042. [DOI] [Google Scholar]
- Latif S, Anwar F. Quality assessment of Moringa concanensis seed oil extracted through solvent and aqueous-enzymatic techniques. Grasas Y Aceites. 2008;59:69–75. [Google Scholar]
- Manzoor M, Anwar F, Iqbal T, Bhanger MI. Physico-chemical characterization of Moringa concanensis seeds and seed oil. J Amer Oil Chem Soc. 2007;84:413–419. doi: 10.1007/s11746-007-1055-3. [DOI] [Google Scholar]
- Ogunsina BS, Radha C, GovardhanSingh RS. Physicochemical and functional properties of full-fat and defatted Moringa oleifera kernel flour. Int J Food Sci Technol. 2010;45:2433–2439. doi: 10.1111/j.1365-2621.2010.02423.x. [DOI] [Google Scholar]
- Ogunsina BS, Radha C. Comparative study of the functional and physico-chemical properties of debittered Moringa oleifera seeds and soybeans flours. Ife J Technol. 2010;19(1):85–92. [Google Scholar]
- Paquot C, Havtfenne A (1987) IUPAC method no. 2.301. Standard methods for analysis of oils, fats and derivatives. International Union of Pure and Applied Chemists, 7th Ed. Blackwell, Oxford, pp 174–182.
- Rahman IMM, Barua S, Nazimuddin M, Begum ZA, Rahman MA, Hasegawa H. Physicochemical properties of Moringa oleifera Lam. seed oil of the indigenous-cultivar of Bangladesh. J Food Lipids. 2009;16:540–553. doi: 10.1111/j.1745-4522.2009.01165.x. [DOI] [Google Scholar]
- Tsaknis J, Lalas S, Gergis V, Spiliotis V. A total characterisation of Moringa oleifera Malawi seed oil. La Rivista Italiana Delle Sostanze Grasse. 1998;75:21–27. [Google Scholar]
- Tsaknis J, Lalas S, Gergis V, Dourtoglou V, Spilitois V. Characterization of Moringa oleifera seed oil (mbololo variety of Kenya) J Agric Food Chem. 1999;47:4495–4499. doi: 10.1021/jf9904214. [DOI] [PubMed] [Google Scholar]
- Tsaknis J, Spiliotis V, Lalas S, Gergis V, Dourtoglou V. Quality changes of Moringa oleifera, variety Mbololo of Kenya, seed oil during frying. Grasas Y Aceites. 1999;50:37–48. doi: 10.3989/gya.1999.v50.i1.634. [DOI] [Google Scholar]
- Tsaknis J, Lalas S. Stability during frying of Moringa oleifera seed oil variety “Periyakulam 1”. J Food Comp and Anal. 2002;15:79–101. doi: 10.1006/jfca.2001.1043. [DOI] [Google Scholar]