Abstract
Acrylamide is a carcinogenic compound which is produced as a result of thermal processing of food materials such as French fries, cereals and meat products. In this study the effects of four different parameters on the level of produced acrylamide in two types of beef burgers during the frying was investigated. Each parameter was used in three levels (temperature at 170, 190, and 210 °C; frying time at 5, 6, and 7 min and meat level at 30, 60, and 85%, and also three types of oil, corn, canola and sunflower). Taguchi’s L9 design was applied to carry out the experiments. While temperature and meat level indicated more effect on the production of acrylamide in the studied samples, type of oil did not show any significant effects at all. Frying time (within the range studied here) showed minor contribution on the acrylamide level produced during the frying.
Keywords: Acrylamide, Beef burger, Taguchi’s design, Meat level, Temperature, Frying time
Introduction
Safety of food products is of great importance. Beef burger is one of the most popular food products that is produced through a frying process, neglecting the frying conditions and other related variables, leads to the formation of a hazardous chemical compound called acrylamide which can cause serious health problems.
(CH2 = CHCONH2) is a carcinogenic compound that is produced during the heating frying, baking, and roasting some specific food at high temperatures and in low humidity (Gokmen et al. 2006; Pedreschi et al. 2006; Gökmen et al. 2009; Yuan et al. 2011). Acrylamide disintegrates in the body and forms glacidamide, which can cause cancer or damage nervous system through affecting DNA and causing mutation. International Agency for Research on cancer (IARC) has introduced acrylamide as a suspicious carcinogenesis compound against human health (Weisshaar 2004; Koh 2006; Ölmez et al. 2008). Studies have demonstrated the carcinogen effect of this compound in mice (Pedreschi et al. 2007; Shin et al. 2010). Acrylamide, as a neurotoxin, is formed in food materials rich in carbohydrates under high temperature conditions over extended heating periods (Parzefall 2008; Tateo et al. 2007). Among food products, the highest level of acrylamide is formed in French fries during the deep-fat frying (Gokmen et al. 2006; Serpen and Gökmen 2009). Effective factors on the acrylamide formation such as cooking period, temperature of frying, humidity, and amount of precursors for Maillard reaction, have been reported previously (Viklund et al. 2010).
Since food products containing meat are also prone to acrylamide production, the objective of the current study was to investigate the effect of four parameters (temperature, frying time, meat level, and oil) on the amount of acrylamide produced during the frying of two types of commercial beef burger. Taguchi experimental design was used to rank the impact of each effect on acrylamide production. Also two types of burger were used in experiment to show that achieved result was more logical.
Materials and methods
Materials
Hexane, potassium hexacyanoferrat (II) trihydrate (Carrez I), zinc sulfate heptahydrate (Carrez II), sodium sulfate, bromic acid, brome, sodium thiosulfate, sodium chloride, calcium chloride, ethylacetate, and acrylamide standard (99%) from Merck chemical company (Darmstadt, Germany) were used. Potassium bromide and valeric acid were supplied from Acros Organic (Paris, France) and Sigma-Aldrich (St. Louis, MO) respectively.
Sample preparation and analysis
Sunflower oil (containing 0.01% TBHQ anti oxidant, 0.01% citric acid, 16% mono unsaturated fatty acid, 72% poly unsaturated fatty acid, and 12% saturated fatty acid), and canola oil (containing 0.01% TBHQ anti oxidant, 0.0095% citric acid, 63% mono unsaturated fatty acid, 30% poly unsaturated fatty acid, and 7% saturated fatty acid) were used in this study. Fried beef burgers were maintained in the environment for 30 min and then they were distorted into powder form by means of mulinex blender. The powder was kept in refrigerator at 4 °C temperature. Then, 30 g of powder was added to 70 ml of hexane for defating. The mixture was stirred by a magnetic stirrer at 700 rpm. The yellow liquid of fat which produced due to stirring was separated manually and then, the sample was dried with nitrogen. Following that, 100 ml of distilled water was added to the sample and the mixture was stirred by a magnetic stirrer for 1 h at 700 rpm. During this stage, acrylamide was extracted into water phase.
Then the sample was centrifuged for 15 min at 4,000 rpm. The upper transparent phase was moved to a balloon and increased to 100 ml by addition of distilled water. For sedimentation of protein, 5 ml Carrez I (150 g potassium hexacyanoferrat (II) trihydrate per liter) and 5 ml Carrez II (300 g zinc sulfate heptahydrate per liter) were added to the solution and stirred at 500 rpm (Ahn et al. 2008; Fiselier et al. 2005; Pittet et al. 2004).
After protein sedimentation, the rest of the solution was centrifuged at 4,000 rmp for 15 min. transparent phase was transferred to a container.
After acrylamide extraction by water, bromination process was carried out. At first 7.5 g of KBr and 0.5 ml of HBr were added to the sample and the mixture was shaken, then 2.5 ml of saturated Br was added and stirred manually. The mixture was maintained in a dark place and 0 °C environment for 90 min. These conditions are essential for bromination reaction. The sample was then transferred out from refrigerator and maintain until the temperature reached to the room temperature (Ahn et al. 2008; Fiselier et al. 2005; Pittet et al. 2004). After bromination, one molar solution of thiosulfate was instilled to the solution for Br neutralization. Instilling was continuing until the solution gets transparent. Finally, 4 g of NaCl was added to the mixture and stirred manually in order to enhance the extraction of ethyl acetate.
The extraction of acrylamide by ethylacetate and concentration
After derivitisation stage, the sample was transferred to a decanter and 20 cc of ethylacetat and 5 cc hexane was added to it and stirred for 10 min. In order to perform overall extraction for acrylamide, two more extraction were carried out on the. Then these three solutions were mixed and 3 g of sodium sulfate was added to the mixture while it was stirring on the magnetic stirrer. Finally, this mixture was centrifuged and transparent phase was separated.
The mixture was evaporated to one ml using nitrogen and then 0.1 ml of valeric acid was added to the mixture. Finally the solution was filtered and the acrylamide was analyzed by a GC-FID system in the presence of valeric acid as internal standard. Features of GC-FID system (pu4410, Scientific Philips) were as following. Capillary separating column of GC (PEG 10, Packed, 2 m length and Ø = 0.46 cm dm), FID detector was containing hydrogen and air at the speed of 33 and 330 ml/min respectively. Vector gas was nitrogen by 99.999% purity at the rate of 30 ml/min. temperatures of injection chamber and detector were both 180°C. The volume of injection was 1 μl. The temperature of the oven was scheduled as following: first at 85°C for one hour at the speed of 9° in minutes, then the temperature increased up to 100°C at the speed of 10° in minutes and finally the temperature enhanced up to 160°C at the speed of 20° in minutes and the sample remained in that temperature for 5 min.
Taguchi experimental design
Taguchi experimental design method is used in this study. This method reduces cost, improves quality, and provides robust design solutions (Taguchi 1986).
Taguchi method utilizes orthogonal arrays (OAs) from experimental design theory to study a large number of variables with a small number of experiments. OAs are subsets of the full factorial experiment which is balanced, i.e., each variable setting occurs the same number of times and none of two experiments are the same (or even mirror images). Using OAs significantly reduces the number of experimental configurations to be studied. However, Taguchi simplified their use by providing tabulated sets of standard OAs and corresponding linear graphs to fit specific projects (Taguchi 1986).
The Taguchi method has developed into an established approach for analyzing interaction effects when ranking and screening various controllable factors. Moreover, this method is applicable to solving a variety of problems involving continuous, discrete and qualitative design variables (Tan and Tang 2001; Sogi et al. 2010). Therefore, the present study adopts the Taguchi method to investigate the main effects on produced acrylamide in fried beef burgers.
Three stages of Taguchi approach to design the experiment are as follows:
Planning a matrix experiment (OA) based on the number control factors (preparation variables) and their alternative levels,
Conducting the matrix experiment,
Analyzing and verifying the results (Tan and Tang 2001).
Four factors each with three levels (low, medium and high) were chosen based on the literature and qualitative experiments (Luftig and Jordan 1998). Three levels were defined for each of the factors as summarized in Table 1. Also each run replicated 3 times (n = 3), and the average of produced acrylamide for each run was reported. The statistical analysis of experiments, carried out through ANOVA method by design expert 8 software.
Table 1.
Design of experiments according to Taguchi method for beef burger A and B
| Run No | Meat value (w/w, %) | Type of oil | Time (min) | Temperature (°C) |
|---|---|---|---|---|
| 1 | 30 | Corn | 5 | 170 |
| 2 | 60 | Canola | 6 | 170 |
| 3 | 85 | Sunflower | 7 | 170 |
| 4 | 85 | Canola | 5 | 190 |
| 5 | 30 | Sunflower | 6 | 190 |
| 6 | 60 | Corn | 7 | 190 |
| 7 | 60 | Sunflower | 5 | 210 |
| 8 | 85 | Corn | 6 | 210 |
| 9 | 30 | Canola | 7 | 210 |
Results and discussion
Verification of the analytical method
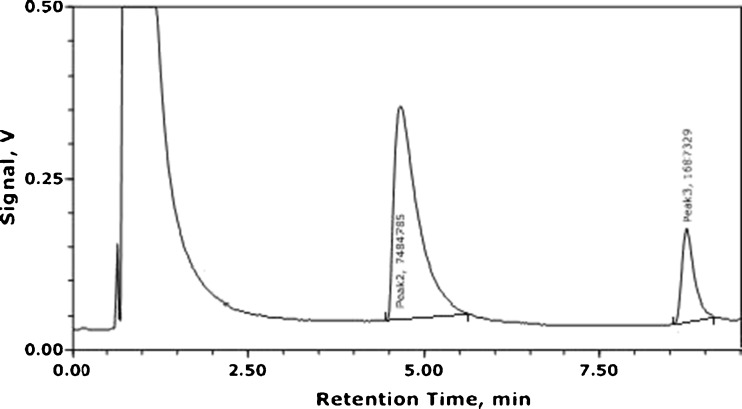
Valeric acid was used as internal standard in order to qualify acrylamide level in each sample. First, four different concentrations of acrylamide (5, 19, 15, and 20 ppm) as internal standard were prepared in three replicates. Then, prior to injection into GC-FID system, 10 μL of valeric acid as internal standard (112.8 g/lit) was added into the standard solutions. Then, mean relative response factor of acrylamide to valeric acid was determined using the chromatograms. A sample chromatogram obtained in this study is shown in Fig. 1. Acrylamide was eluted within 4.5–5.5 min of the chromatogram, and the peak at 8.50 min is for internal standard (valeric acid). Before analyzing the samples for acrylamide 10 μL internal standard was also added to them and a total of 20 μL was injected into the GC. Results related to the analysis of variance (ANOVA) are shown in Tables 2 and 3 for the two types of burger used in this study. Temperature, due to its lowest P-value, was identified as the most effective parameter. Based on Tables 2 and 3, meat proportion is considered the second important parameter on the acrylamide production. ANOVA represents a model, proving the integrity of the analyses, since the level of P-value for both studied burgers are valid and can be used to determine acrylamide levels in the samples. Quantitatively, the estimated effects of a given main effect and its rank relative to other main effects is given via least squares estimation (that is, forming effect estimates that minimize the sum of the squared differences between raw data and the fitted values from such estimates). Having such estimates in hand, one could then construct a list of the main effects ordered by the effect magnitude (Rahmanian et al. 2011). The half-normal probability plot is a graphical tool that uses these ordered estimated effects to help assess which factors are important and which are unimportant (Alam et al. 2010; Afoakwa et al. 2011).
Fig. 1.
Sample of the chromatogram of GC-FID for external standard (15 ppm)
Table 2.
Analysis of variance (ANOVA) for the different parameters of this study for beef burger type A
| Type (A) | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of square | dF | Mean square | F-value | P-value | |
| Prob > F | ||||||
| Model | 84.60 | 6 | 14.10 | 31.20 | 0.0314 | Significant |
| Temperature | 72.70 | 2 | 36.35 | 80.44 | 0.0123 | |
| Time | 1.60 | 2 | 0.80 | 1.77 | 0.3605 | |
| Meat/Other comp. | 10.29 | 2 | 5.15 | 11.39 | 0.0807 | |
| Residual | 0.90 | 2 | 0.45 | – | – | |
R-squared = 0.9894; Adj R-squared = 0.9577
Table 3.
Analysis of variance (ANOVA) for the different parameters of this study for beef burger type B
| Type (B) | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of square | dF | Mean square | F-value | P-value | |
| Prob > F | ||||||
| Model | 82.01 | 6 | 13.67 | 19.90 | 0.0486 | Significant |
| Temperature | 56.72 | 2 | 28.36 | 41.30 | 0.0236 | |
| Time | 1.67 | 2 | 0.84 | 1.22 | 0.4507 | |
| Meat/Other comp. | 10.75 | 2 | 5.37 | 7.83 | 0.1133 | |
| Residual | 1.37 | 2 | 0.69 | – | – | |
R-squared = 0.9835; Adj R-squared = 0.9341
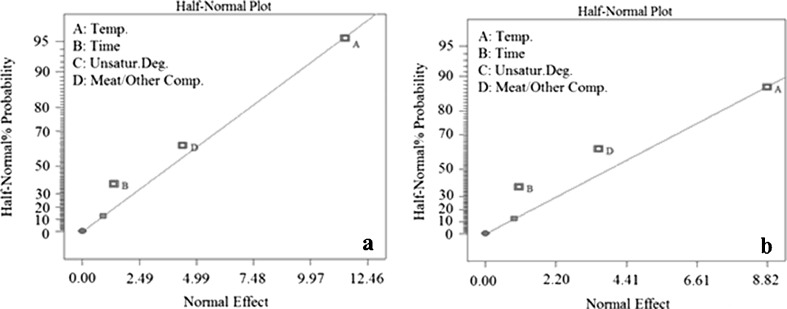
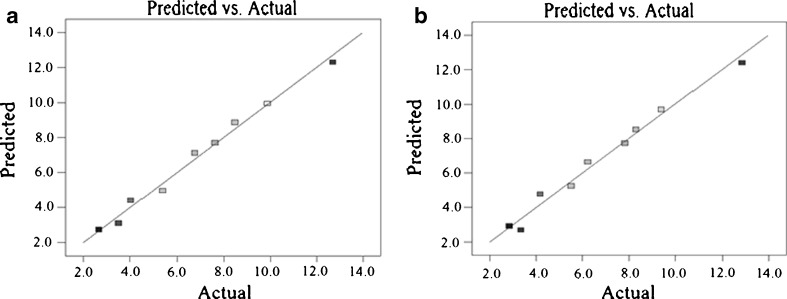
The graphs of half-normal probability plot show three parameters for the ANOVA. Adding next parameter, the oil, makes the model, represented by the ANOVA, insignificant. It means that the oil has no noticeable effect on the level of acrylamide produced. This is demonstrated in Fig. 2 for beef burgers A and B. Such results are consistent with those of Mestdagh et al. (2005). Figure 3 demonstrates the true value of produced arcrylamide in beef burger A and B and corresponding predicted amount of that, represented by ANOVA. As it’s depicted the points are close to the line Y = X. the amounts of R-squared and R-adjusted, represented in Tables 2 and 3, are close to unity, which is another proof on the integrity of the model (Chakraborty et al. 2010).
Fig. 2.
The graph of Half-Normal Plot for beef burgers, a beef burger A, b beef burger B
Fig. 3.
Comparing the predicted and actual levels of acrylamide produced in beef burgers, a beef burger A, b beef burger B
Effect of each main factor on produced acrylamide
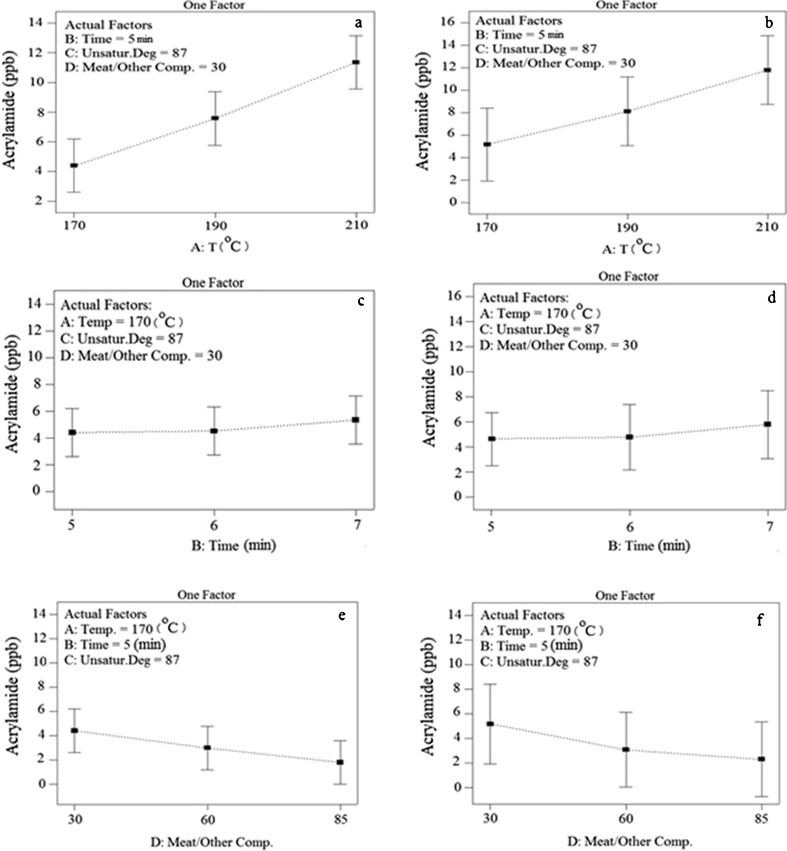
Figure 4 (a and b) shows the effect of the temperature on the level of produced acrylamide. As the temperature increases the level of produced acrylamide in both burgers studied here also increase. There are several mechanisms by which acrylamide production can be explained in food materials. The most important mechanism is through the so-called Maillard reaction. Factors affecting this reaction such as temperature and cooking time can also influence acrylamide formation.
Fig. 4.
Changes in the level of acrylamide produced in considering one parameter, a Effect of temperature in beef burger A, b Effect of temperature in beef burger B, c Effect of time in beef burger A, d Effect of time in beef burger B, d Effect of meat/other compound in beef burger A, d Effect of meat/other compound in beef burger B
Dramatic increase in the acrylamide level was reported by Gokmen et al. (2006) when cooking temperature was changed from 150°C to 220°C. Other researchers have indicated that in temperatures above 175°C acrylamide formation increases. Our results on this aspect agree well with those of some other researchers (Mattus et al. 2004; Fiselier et al. 2005; Gokmen et al. 2006). The effect of frying time on the level of acrylamide produced in both beef burgers (A and B) has been demonstrated in the Fig. 4 (c and d). As it’s shown the effect of the time of frying is less than the temperature itself. In terms of frying time, it should be taken into consideration that there are several parameters which affect acrylamide formation. The most important path toward formation of acrylamide is Maillard reaction; therefore factors which affect this reaction, such as temperature and time, are effective on the rate of acrylamide formation in food materials (Gokmen et al. 2006). Temperature of frying has more effect on arcylamide production compared to the duration of frying (Fiselier et al. 2005). Our results are consistent with this fact. Data related to the correlation between meat level and acrylamide formation in burgers A and B are shown in Fig. 4 (e and f). Unlike two previous parameters, increase in this factor causes reduction in the amount of acrylamide. Hence, in terms of acrylamide formation, the higher the meat level, the lower the amount of the produced acrylamide. Generally, Fig. 4 (a, b, c, d, e, f) show that frying beef burger with higher meat level, at lower temperatures and in shorter time duration causes lower acrylamide formation during frying. Our results show that the higher the level of meat in beef burger, the lower is the amount of acrylamide produced during the frying. The reason of this fact may be due to the more effective role of soya which has higher level in low-meat beef burgers. Soya and cereals can cause more acrylamide compared to meat (Koh 2006), because they have more amounts of glucose and asparagines, which are more prone to produce acrylamide. Food materials with higher amounts of carbohydrates and amino acids such as potato and cereals are more prone to produce acrylamide at high temperatures (Koh 2006). On the other hand, high temperatures in food processing and low access to water in food materials are of great importance in acrylamide formation. In order to form acrylamide at the temperatures above 120°C, the presence of glucose and fructose (reducing sugar) and amino acids such as sitein, metionin, glutamine, and asparagines is necessary. Acrylamide production is affected by some specific features specially the amount of sugar and amino acids (Ooraikal and Moledina 1981). The amount of reducing sugar is a limiting factor in acrylamide forming during frying food materials (Petersson et al. 2006). In meat products, carnocine, which is a dipeptide, acts as a precursor for acrylamid production. Acrylamide changes to another compound called N-methylacrrylamide in a later stage and as a result, the level of acrylamide is decreased (Yaylayan et al. 2004).
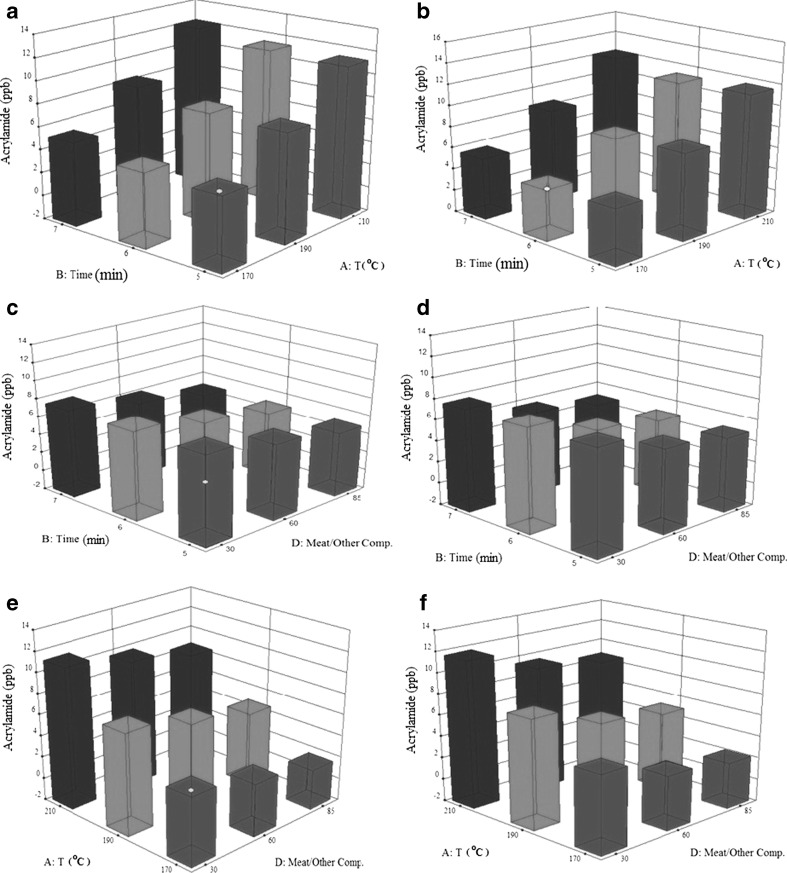
Changes in the level of acrylamide caused by two parameters at the same time
Data related to the combined effects of temperature and frying time on the level of acrylamide produced are shown in Fig. 5 (a and b). The lowest amount of acrylamide was produced at 170°C and in 5 min of frying time. The highest amount of acrylamide was formed at 210°C and with a frying time of 7 min in both burgers (A and B). Correlation between the combined effects of meat level and frying time is shown in Fig. 5 (c and d). As meat level and/or the frying time were decreased, the level of acrylamide produced was also decreased. The lowest amounts of acrylamide 5.37 ng/g for sample A and 5.51 ng/g for sample B were found when a frying time of 5 min and a meat level of 85% were used.
Fig. 5.
Changes in the level of acrylamide considering two parameters (frying time and frying temperature) at a time, a beef burger A, b beef burger B; Changes in the level of acrylamide considering two parameters (frying time and meat/other compound) at a time, c beef burger A, d beef burger B; Changes in the level of acrylamide considering two parameters (frying temperature and meat/other compound) at a time, e beef burger A, f beef burger B
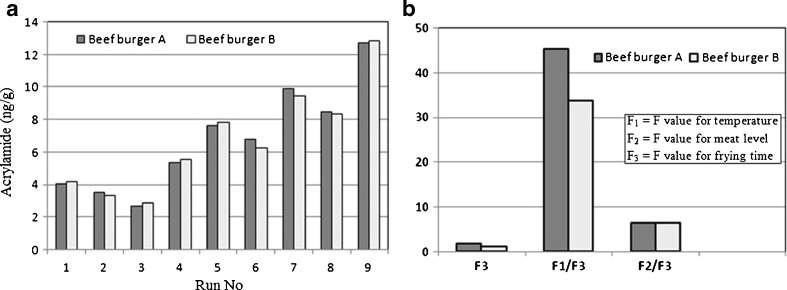
Data related to the amount of the acrylamide with respect to meat level and temperature is shown in Fig. 5 (e and f) for burgers A and B of this study. Lower levels of acrylamide were formed when the level of the meat was the highest (85%) and the temperature was at its lowest (170°C). (2.67 ng/g for sample A and 2.84 ng/g for sample B). Comparing the levels of acrylamide produced in the two commercial burgers used in this study confirms the results of this work in different conditions applied here (Fig. 6a).
Fig. 6.
a Comparing the levels of acrylamide produced in two commercial burgers used in this study (A and B) with similar meat levels, b Relative impacts of the parameters on the amount of acrylamide produced in burgers A and B
Relative effects of the parameters on the level of produced acrylamide
Data about the relative effects of the parameters used in this study on the level of acrylamide produced in the two burgers of the study (A and B) are shown in Fig. 6. Effect of the frying temperature was much higher than those of other parameters. The F-value in ANOVA analysis shows the impact of each parameter on produced acrylamide. So the parameter which has the highest F-value shows the highest impact on acylamide production. Considering the frying time as the least effective parameter (F3 in the Fig. 6b) relative effect of other parameters were determined by dividing their F value to that of frying time. For instance, the F value for the frying temperature in burger A is 45 times higher when compared to that of frying time in the same burger. Also the amount of the impact of the meat proportion is 6 times higher than that of frying time.
Conclusions
Regarding the hazardous effects of acrylamide on human health, the effect of some parameters was considered on the level of acrylamide for two types of beef burger during the frying. According to our results, we can get into this conclusion that temperature, meat value, and time of frying have significant effect on the level of acrylamide, whereas, type of oil had no significant effect on the level of acrylamide produced. Temperature is the most effective parameter following by meat value and time of frying. The most optimum conditions of frying for both beef burgers (A and B), is a result of reduction in frying temperature, reduction in frying time, and increase in meat value of the beef burger. Hence, according to our result the least amount of acrylamide is produced for the third treatment (170 °C, in 7 min frying time, for the beef burgers with meat value of 85% and sunflower oil). Finally, according to the propinquity between two types of beef burgers and with respect to the effect of temperature, time and meat value, we can conclude that in order to obtain the least level of acrylamide, frying meat products should be done in the least possible temperature and frying time and by using high meat burgers.
Contributor Information
Karamatollah Rezaei, Email: krezaei@ut.ac.ir.
Reza Abedini, Email: r.abedini@modares.ac.ir.
References
- Afoakwa EO, Quao J, Takrama J, Budu AS, Saalia FK (2011) Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J Food Sci Technol. doi:10.1007/s13197-011-0446-5 [DOI] [PMC free article] [PubMed]
- Ahn JS, Castte L, Clarke DB, Lioyd AS, Philo MR, Speck DR. Verification of the findings of acrylamide in heated foods. Food Addit Contam. 2008;19:1116–1124. doi: 10.1080/0265203021000048214. [DOI] [PubMed] [Google Scholar]
- Alam MS, Amarjit S, Sawhney BK. Response surface optimization of osmotic dehydration process for aonla slices. J Food Sci Technol. 2010;47:47–54. doi: 10.1007/s13197-010-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty KS, Kumbhar BK, Chakraborty S, Yadav P. Influence of processing parameters on textural characteristics and overall acceptability of millet enriched biscuits using response surface methodology. J Food Sci Technol. 2010;48:167–174. doi: 10.1007/s13197-010-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiselier K, Bazzocco D, Baumgartner FG, Grob K. Influence of the frying temperature on acrylamide formation in French fries. Eur Food Res Technol. 2005;1:1–11. [Google Scholar]
- Gokmen V, Palazoglu TK, Senyuva HZ. Relation between the acrylamide formation and time-temperature history of surface and core regions of French fries. J Food Eng. 2006;77:972–976. doi: 10.1016/j.jfoodeng.2005.08.030. [DOI] [Google Scholar]
- Gökmen V, Morales FJ, Ataç B, Serpen A, Lorenzo GA. Multiple-stage extraction strategy for the determination of acrylamide in foods. J Food Comp Anal. 2009;22:142–147. doi: 10.1016/j.jfca.2008.09.007. [DOI] [Google Scholar]
- Koh BK. Determination of acrylamide content of food products in Korea. J Sci Food Agric. 2006;86:2587–2591. doi: 10.1002/jsfa.2652. [DOI] [Google Scholar]
- Luftig JT, Jordan VS. Design of experiments in quality engineering. New York: McGraw-Hill; 1998. [Google Scholar]
- Mattus B, Hasse NU, Vosmann K. Factors affecting the concentration of acrylamide during deep-fat frying of potatoes. Eur J Lipid Sci Tech. 2004;106:793–801. doi: 10.1002/ejlt.200400992. [DOI] [Google Scholar]
- Mestdagh F, Meulenaer BD, Poucke CV, Detavernier C, Cromphout C, Peteghem CV. Influence of oil type on the amounts of acrylamide generated in a model system and in french fries. J Agric Food Chem. 2005;53:6170–6174. doi: 10.1021/jf0506683. [DOI] [PubMed] [Google Scholar]
- Ölmez H, Tuncay F, Özcan N, Demirel S. A survey of acrylamide levels in foods from the Turkish Market. J Food Comp Anal. 2008;21:564–568. doi: 10.1016/j.jfca.2008.04.011. [DOI] [Google Scholar]
- Ooraikal B, Moledina KH. Physicochemical changes in potato granules during storage. J Food Sci. 1981;46:110–116. doi: 10.1111/j.1365-2621.1981.tb14541.x. [DOI] [Google Scholar]
- Parzefall W. Minireview on the toxicity of dietary acrylamide. Food Chem Toxicol. 2008;46:1360–1364. doi: 10.1016/j.fct.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Pedreschi F, Kaack K, Granby K. Acrylamide content and color development in fried potato Strips. Food Res Int. 2006;39:40–46. doi: 10.1016/j.foodres.2005.06.001. [DOI] [Google Scholar]
- Pedreschi F, Kaack K, Granby K, Troncoso E. Acrylamide reduction under different pre-treatments in French fries. J Food Eng. 2007;79:1287–1294. doi: 10.1016/j.jfoodeng.2006.04.014. [DOI] [Google Scholar]
- Petersson EV, Rosen J, Turner C, Danielsson R, Hellenas KE. Critical factors and pitfalls affecting the extraction of acrylamide from foods: an optimization study. J Anal Chem. 2006;557:287–295. [Google Scholar]
- Pittet A, Perisset A, Oberson JM. Trace level determination of acrylamide in cereal-based foods by gas chromatography-mass spectrometery. J Chromatogr A. 2004;1035:123–130. doi: 10.1016/j.chroma.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Rahmanian B, Pakizeh M, Mansoori SAA, Abedini R. Application of experimental design approach and artificial neural network (ANN) for the determination of potential micellar-enhanced ultrafiltration process. J Hazard Mater. 2011;187:67–74. doi: 10.1016/j.jhazmat.2010.11.135. [DOI] [PubMed] [Google Scholar]
- Serpen A, Gökmen V. Evaluation of the Maillard reaction in potato crisps by acrylamide, antioxidant capacity and color. J Food Comp Anal. 2009;22:589–595. doi: 10.1016/j.jfca.2008.11.003. [DOI] [Google Scholar]
- Shin DC, Kim CT, Lee YC, Choi WJ, Na YJ, Lee KW. Reduction of acrylamide by taurine in aqueous and potato chip model systems. Food Res Int. 2010;43:1356–1360. doi: 10.1016/j.foodres.2010.03.024. [DOI] [Google Scholar]
- Sogi DS, Sharma S, Oberoi DPS, Wani IA. Effect of extraction parameters on curcumin yield from turmeric. J Food Sci Technol. 2010;47:300–304. doi: 10.1007/s13197-010-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi G. Introduction to quality engineering. Tokyo: Asian Productivity Organization; 1986. [Google Scholar]
- Tan KK, Tang KZ. Vehicle dispatching system based on Taguchi-tuned fuzzy rules. Eur J Oper Res. 2001;128:545–557. doi: 10.1016/S0377-2217(99)00373-2. [DOI] [Google Scholar]
- Tateo F, Bononi M, Andreoli G. Acrylamide levels in cooked rice, tomato sauces and some fast food on the Italian market. J Food Comp Anal. 2007;20:232–235. doi: 10.1016/j.jfca.2006.06.006. [DOI] [Google Scholar]
- Viklund G, Olsson KM, Sjöholm IM, Skog KI. Acrylamide in crisps: effect of blanching studied on longterm stored potato clones. J Food Comp Anal. 2010;23:194–198. doi: 10.1016/j.jfca.2009.07.009. [DOI] [Google Scholar]
- Weisshaar R. Acrylamide in heated potato products—analytics and formation routes. Eur J Lipid Sci Technol. 2004;106:786–792. doi: 10.1002/ejlt.200400988. [DOI] [Google Scholar]
- Yaylayan VA, Locas CP, Wnorowski A, O’brien J. The role of creatine in the generation of n-methylacrylamide: a new toxicant in cooked meat. J Agric Food Chem. 2004;52:5559–5565. doi: 10.1021/jf049421g. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shu C, Zhou B, Qi X, Xiang J. Impact of selected additives on acrylamide formation in asparagine/sugar Maillard model systems. Food Res Int. 2011;44:449–455. doi: 10.1016/j.foodres.2010.09.025. [DOI] [Google Scholar]