Abstract
Alcoholic-alkaline treatment of starch results in granular cold water-soluble starch. The nature of structural alterations occurred in starch due to the treatment is however relatively vague. Potato starch was treated at various alcoholic-alkali conditions and subjected to light microscopy, Fourier transform infrared (FTIR) spectroscopy and X-ray diffractometry. Alcoholic-alkaline treatment increased the solubility of starch in a temperature-dependant manner. The treated starch suspension was also more turbid than that of native counterpart at all concentrations, due probably to either the presence of higher number of water-soluble granules or leaching of amylose during alkalization. Alcoholic–alkali treating of starch did not disintegrate the granular assembly; albeit, decreased the crystalinity. Both native and treated starches showed the B-type pattern in X–ray diffractometry. FTIR spectroscopy revealed that characteristic peaks of hydroxyl groups were of low transmittance in spectrum of treated starch compared with that of native counterpart implying in participation of hydroxyl groups in interactions with modifying agents.
Keywords: Alcoholic-alkaline treatment, Starch, Fourier transform infrared spectroscopy, X-ray diffraction
Introduction
The renewable, inexpensive and biodegradable polymer, starch, is biosynthesized as semi-crystalline granules composed of two glucosidic macromolecular components: amylose and amylopectin. Although there are various applications for native starch, its industrial usage is limited due to the some unfavorable characteristics such as poor solubility in cold water, tendency to retrograde and high viscosity once it is gelatinized (Shi et al. 2011). Therefore, considerable effort has been focused on production of starch derivatives or modified products in order to broaden the application range of this biopolymer. Various chemical methods including etherification, esterification, cross-linking, grafting and hydrolysis are applied in starch modification. As well, physical procedures, alone or in combination with chemical reactions, have been applied to alter the starch structure (Jane 1992). Alcoholic-alkaline treatment of starches from various sources such as corn, waxy corn and high amylose corn results in cold water-soluble starch that still possess the granular assembly (Chen and Jane 1994a). Singh and Singh (2003) observed that alcoholic-alkaline treatment brought substantial changes in physico-chemical, morphological, thermal and rheological properties of corn and potato starches. Recently, it was hypothesized based on intrinsic viscosity measurements that some degradation takes place in the alcoholic-alkaline treated starches accompanied by changes in X-ray diffraction (XRD) patterns (Kaur et al. 2011). The nature and fate of molecular alterations, transitions and interactions occurred in starch structure because of alcoholic-alkaline treatment however remain still relatively vague. The objective of the present study was therefore to prepare the granular cold water-soluble potato starch by alcoholic-alkaline treatment and follow up the changes and modifications in starch structure by means of various techniques including Fourier transform infrared (FTIR) spectroscopy and XRD.
Materials and methods
Materials
Native potato starch, hydrochloric acid 37 %, ethanol and sodium hydroxide were purchased from Merck (Darmstadt, Germany). All other chemicals were of analytical grade and used without further purification. Double distilled water was used throughout the work.
Preparation of cold water-soluble starch
The granular cold water-soluble starch was prepared following the method of Chen and Jane (1994a) with slight modifications. Ten g starch was suspended in 40 g ethanol (40 %) at two different temperatures (25 and 35 °C) and stirred mechanically for 10 min. This was followed by adding 12 g NaOH (3 M on the solvent basis) at rate of 4 g/min. The suspension was gently stirred for 15 min; afterwards an additional 40 g ethanol (40 %) was added slowly and stirred for another 10 min. The slurry was left at room temperature (25 °C) for 30 min in order to give sufficient time for the treated starch to settle down. The settled granules were washed with fresh ethanol solution (40 %), neutralized with 3 M HCl in absolute ethanol, and then washed with 60 % and 95 % ethanol solutions. The obtained starch was dehydrated with absolute ethanol, and finally oven-dried at 80 °C for 3 h.
Cold water-solubility
Cold water-solubility was measured following the method of Eastman and Moore ((1984) with slight modification. Aqueous suspensions (1 %) of native and alcoholic-alkaline modified starches were prepared and shaken at 1,000 rpm on a rotary shaker (S 150, Stuart®, Bibby Scientific Ltd., UK) for 45 min at 25 or 35 °C. The suspensions were then centrifuged (D- 78532, Tettich, Germany) at 1,200 g for 15 min and supernatant was dried at 105 °C for 6 h. The cold water-solubility of samples was calculated as:
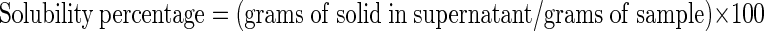 |
Turbidity
The change in turbidity of starch aqueous suspension due to alcoholic-alkaline modification was measured as an index of solubility following the method of Perera and Hoover (1999). Both native and modified starches were suspended in double distilled water with pH = 7.0 at several concentrations (1, 2 and 3 %) and stirred (250 rpm) for 120 h at 35 °C. To evaluate the effect of acidification, three concentrations (1, 2 and 3 %) of native starch suspension was prepared at pH = 4.0 and their turbidity was measured over 120 h. Starch suspensions were read at 640 nm against water blank using an ultraviolet–visible spectrophotometer (Cecil instrument, CE 2502, Cambridge, UK).
Light microscopy
Granular morphology of native and alcoholic-alkaline modified starches was observed with a light microscope (Olympus BX 41, Tokyo, Japan) equipped with a digital color video camera (Sony, Tokyo, Japan).
XRD pattern
The XRD patterns for native and modified starches were obtained using an X-ray powder diffractometer (XPert MPD, Philips, Netherlands) with Cu K radiation at 40 kV and 25 mA at a diffraction angle ranging from 6 to 50°. The step-scan was set at an angle of 0.02° and count time was 2 s per step. Samples crystallinity was determined by plotting the peaks baseline on the diffractogram and calculating the area using the spectrum viewer software (Version 2.6). The area above and under the curve corresponded to crystalline domains and amorphous regions, respectively. The ratio of upper area to total area was taken as the degree of crystallinity in the form of following equation.
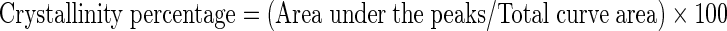 |
FTIR spectroscopy
Samples were grinded together with potassium bromide and pressed into a pellet for scanning with a FTIR spectrometer (Perkin Elmer Spectrum one, MA, USA). The spectra were recorded for total 16 scans between 4,000 and 400 cm−1 with resolution of 4 cm−1.
Results and discussion
Cold water-solubility
Cold water-solubility measured mainly the soluble molecules and highly swollen granules, which did not sediment by moderate centrifugation (Rajagopalan and Seib 1992). The native starch solubility was negligible (~4 %) in cold water at 25 °C; however, it increased significantly (~23 %) when starch suspension was prepared at 35 °C. Alcoholic-alkaline treatment increased the solubility of potato starch to 51 % at 25 °C and 74 % at 35 °C indicating the efficiency of the modification method to solubilize the starch. It has been suggested that alkali condition opens up the starch granule structure, resulting in breaking the intermolecular hydrogen bonds thereby enhancing the water solubility (Han and Lim 2004). The presence of alcohol not only restricts the swelling of granules by decreasing the effective water concentration but also acts as a complexing agent to stabilize the dissociated starch molecules and conserve the granules integrity (Chen and Jane 1994a).
Turbidity
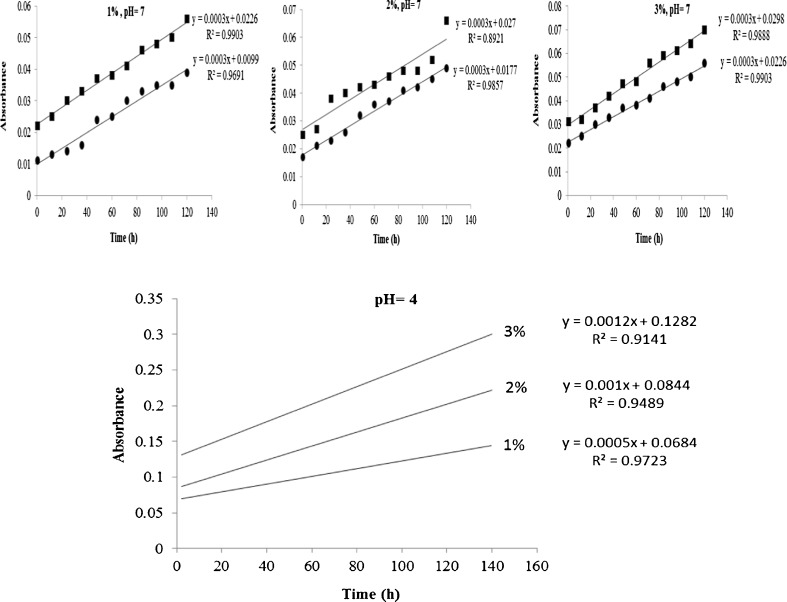
The absorbance values of native and modified starch suspensions are shown in Fig. 1. Alcoholic-alkali treated starch suspension was more turbid than its native counterpart at all concentrations because probably of the presence of higher number of water-soluble granules. It is also worthy to note that leaching of amylose during treatment might contribute to the increased turbidity (Singh and Singh 2003). Turbidity of all samples increased progressively with time most probably due to the penetration of water into granules and their swelling, as well as, leaching of linear and branched starch molecules out (Jacobson et al. 1997). The interaction between leached amylose and amylopectin chains could form function zones, which might reflect or scatter light (Perera and Hoover 1999). Fig. 1 shows the absorbance of native starch suspensions with different concentrations at pH 4.0 (lower panel). Absorbance increased progressively with increase in turbidity. It is clear that acidic condition increased significantly the turbidity in comparison to pH 7.0 (Fig. 1, upper panel). This is attributed to the enhanced hydrophilic character and partial hydrolysis of starch at lower pH values resluting in disintegration of granules (Alam and Hasnain 2009).
Fig. 1.
Upper panel: absorbance at 640 nm of native (filled circle) and alcoholic-alkaline modified (filled square) starches suspensions at different concentrations (1, 2 and 3 %) at their rudiment pH 7.0; lower panel: absorbance at 640 nm of native starch suspensions at different concentrations; 1 %, 2 % and 3 % in acidic condition (pH = 4)
Light microscopy
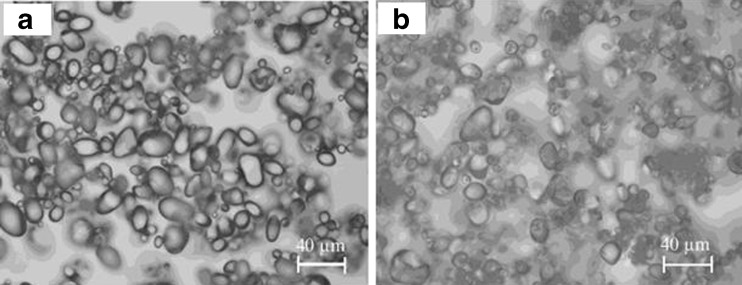
The light micrographs of native and modified starches are shown in Fig. 2. It is argued that starch leaching out during modification took place though the integrity of granules preserved. The difference in morphology of native and treated starch granules was however negligible indicating that ethanol was efficient enough to prevent from destruction of granules by alkali and dissociation of starch double-helical conformation (Chen and Jane 1994b; Kaur et al. 2011).
Fig. 2.
Light micrographs of native (a), and alcoholic-alkaline modified (b) granular potato starch at 20× magnification
XRD pattern
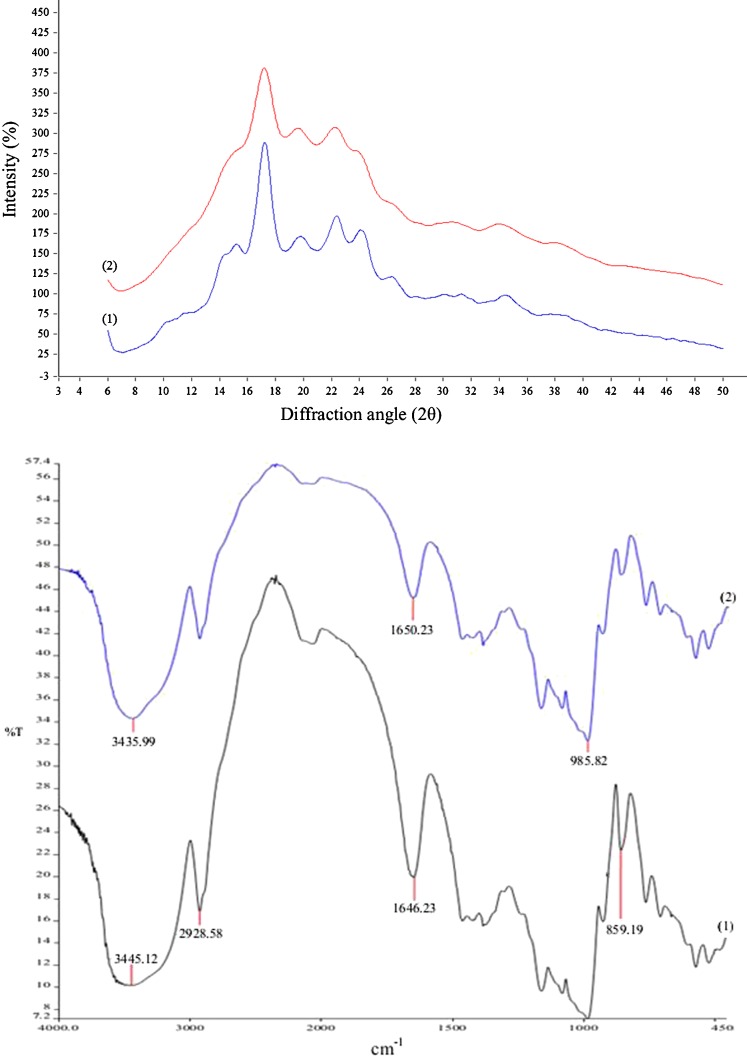
B-type scattering pattern (Gernat 1993) was observed for both native and alcoholic-alkaline treated samples with clear diffraction peaks, a strong peak at 17.2°, a weak peak at 19.9° and a double peak at 22.4° and 24.2° (Fig. 3). The crystallinity degree of native and modified starches was 35 and 28 %, respectively showing a decrease in ordered arrangement of macromolecular components in granular assembly of starch due to alcoholic-alkaline treatment. The similarity in scattering patterns of modified starch with that of native counterpart however emphasizes in preserved organization of starch granules. In agreement to our result, Kaur et al. (2011) found that alcoholic-alkaline treatment did not displace the scattering pattern of Sago starch when the amount of sodium hydroxide was less than 90 % of starch dry weight.
Fig. 3.
Upper panel: X-ray diffraction pattern of potato native starch (1), and alcoholic- alkaline modified starch (2); lower panel: FTIR spectra of potato native starch (1), and alcoholic- alkaline modified starch (2)
FTIR spectra
The FTIR spectra of both native and alcoholic-alkaline treated starches (Fig. 3) showed a peak at 985 cm−1 due to the CO bond stretching. A characteristic peak for native starch at 1,646 cm−1 is due to the presence of bound water in starch (Fang et al. 2004). Alcoholic-alkaline treatment shifted this peak to 1,650 cm−1 and decreased its transmittance. The treatment also increased the intensity of band at 2,928 cm−1 which is characteristic of C–H stretching associated with the methine (methylidyne or methyne) ring’s hydrogen atoms. A broad band due to hydrogen bonded hydroxyl group (O–H) appeared at 3,445 and 3,435 cm−1, respectively in native and modified starches. This is attributed to the complex vibrational stretching associated with free, inter and intra molecular bound hydroxyl groups (Fang et al. 2002). This peak was low transmittance in treated starch compared to native starch, indicating that most of hydroxyl groups contributed in reaction during modification of starch. In general, by comparing the FTIR spectrum of native and alcoholic-alkaline modified starches, it can be said that during the modification most of starch hydroxyl groups participated in interactions with ethanol and/or sodium hydroxide.
Conclusions
Alcoholic-alkali treatment of starch is an efficient method to increase the solubility of starch while conserving its granular assembly. The crystalinity degree of starch is however reduced due to treatment although the overall diffraction pattern of X–ray by starch is not altered. Hydroxyl groups of starch interact with ethanol and/or sodium hydroxide during modification resulting in low transmittance.
Acknowledgment
Authors would like to thank the Iranian Research Organization for Science and Technology (IROST) for supporting to experiments of this research.
References
- Alam F, Hasnain A. Studies on swelling and solubility of modified starch from Taro (Colocasia esculenta): effect of pH and temperature. Agric Conspec Sci. 2009;74:45–50. [Google Scholar]
- Chen J, Jane J. Preparation of granular cold-water-soluble starches by alcoholic-alkaline treatment. Cereal Chem. 1994;71:618–622. [Google Scholar]
- Chen J, Jane J. Preparation of granular cold-water-soluble starches by alcoholic-alkaline treatment. Cereal Chem. 1994;71:623–626. [Google Scholar]
- Eastman JE, Moore CO. Cold-water-soluble granular starch for gelled food compositions. US Patent. 1984;4:465–702. [Google Scholar]
- Fang JM, Fowler PA, Tomkinson J, Hill CAS. The preparation and characterization of a series of chemically modified potato starches. Carbohydr Polym. 2002;47:245–252. doi: 10.1016/S0144-8617(01)00187-4. [DOI] [Google Scholar]
- Fang JM, Fowler PA, Sayers C, Williams PA. The chemical modification of a range of starches under aqueous reaction condition. Carbohydr Polym. 2004;55:283–289. doi: 10.1016/j.carbpol.2003.10.003. [DOI] [Google Scholar]
- Gernat C. Crystalline parts of three different conformations detected in native and enzymatically degraded starches. Starch-Starke. 1993;45:309–314. doi: 10.1002/star.19930450905. [DOI] [Google Scholar]
- Han JA, Lim ST. Structural changes in corn starches during alkaline dissolution by vortexing. Carbohydr Polym. 2004;55:193–199. doi: 10.1016/j.carbpol.2003.09.006. [DOI] [Google Scholar]
- Jacobson MR, Obanni M, BeMiller JN. Retrogradation of starches from different botanical sources. Cereal Chem. 1997;74:571–578. doi: 10.1094/CCHEM.1997.74.5.511. [DOI] [Google Scholar]
- Jane J. Preparation and food applications of physically modified starches. Trends Food Sci Tech. 1992;3:145–148. doi: 10.1016/0924-2244(92)90169-W. [DOI] [Google Scholar]
- Kaur B, Fazila A, Karim AA. Alcoholic-alkaline treatment of sago starch and its effect on physicochemical properties. Food Bioprod Process. 2011;89:463–471. doi: 10.1016/j.fbp.2010.09.003. [DOI] [Google Scholar]
- Perera C, Hoover R. Influence of hydroxypropylation on retrogradation properties of native, defatted and heat-moisture treated potato starches. Food Chem. 1999;64:361–375. doi: 10.1016/S0308-8146(98)00130-7. [DOI] [Google Scholar]
- Rajagopalan S, Seib PA. Granular cold-water-soluble starches prepared at atmospheric pressure. J Cereal Sci. 1992;16:13–28. doi: 10.1016/S0733-5210(09)80076-3. [DOI] [Google Scholar]
- Shi AM, Li D, Wang LJ, Li BZ, Adhikari B. Preparation of starch-based nanoparticles through high-pressure homogenization and miniemulsion cross-linking: Influence of various process parameters on particle size and stability. Carbohyd Polym. 2011;83:1604–1610. doi: 10.1016/j.carbpol.2010.10.011. [DOI] [Google Scholar]
- Singh J, Singh N. Studies on the morphological and rheological properties of granular cold water soluble corn and potato starches. Food Hydrocolloid. 2003;17:63–72. doi: 10.1016/S0268-005X(02)00036-X. [DOI] [Google Scholar]