Abstract
Protein hydrolysates prepared by hydrolysis of shrimp waste (Penaeus monodon and Penaeus indicus) for 90 min. using Alcalase enzyme following pH-stat method. Antioxidative activities of SWPH were assessed determining FRAP, ABTS and DPPH radical scavenging activities, which increased linearly with increasing concentration of protein hydrolysate upto 5 mg/ml maintaining good correlation. SWPH showed high stability over wide ranges of pH (2–11) and temperature (up to 100 °C for 150 min), in which the activity of more than 80% was retained. Protein hydrolysate solution with a concentration of 5 mg/ml significantly lowered TBA values of Croaker fish fillet and maintained yellowishness of skin colour compared to untreated control sample during 10 days of refrigerated storage at 4 °C. SWPH also restricted the increase of PV and FFA values in Croaker fish fillet within acceptable limit.
Keywords: Shrimp waste, Protein hydrolysate, Antioxidative activity
Introduction
Food quality is directly affected by oxidation, and is commonly associated with changes of flavour and texture. Therefore, prevention of lipid oxidation is a great concern in the food industry. The use of synthetic antioxidants is an old practice and their safety could be questioned by the consumer. The alternative natural compounds with efficient antioxidative activity have been paid increasing attention. Byproducts from shrimp waste have attracted considerable attention due to their different food application as inferred from different research works in the recent past. Additionally, shrimp waste comprising head and shell is enriched in amino acids, peptides, proteins and other useful nutrients. Penaeid shrimps have become the economically important species in India and are widely cultured in ponds. Black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus indicus) are commonly cultured and exported with a catch volume over 1000 t per year. Waste after processing of shrimp may account for up to 35–40% of the original weight of raw material (Shahidi et al. 1992). Those by-products consist of 71.4% head and 28.6% shell (Meyers 1986) and may pose a disposal problem due to the ease of spoilage causing environmental pollution (Quaglia and Orban 1987; Gildberg 1993). Effective utilization of this waste would be a promising approach to reduce such a problem as well as earn revenue for its application in food industry. Protein hydrolysates from different fish species such as mackerel (Wu et al. 2003), herring (Sathivel et al. 2003), tuna cooking juice (Jao and Ko 2002) and black soy bean (Shih et al. 2002) have been found to show antioxidative activity against peroxidation of lipids or fatty acids. Different initiative for recovery of protein and amino acids (Mandeville et al. 1992), colourants (Chen and Meyers 1982), flavourants (Pan 1990) and chitin, chitosan (Coward-kelly et al. 2006) from shrimp head and shell has been undertaken of which enzymatic hydrolysis approach was effective (Ruttanapornvareesakul et al. 2006). Very few research has been undertaken regarding antioxidative effect of protein hydrolysate or peptides from fish and shellfish, as well as their by-products (He et al. 2006). However, little information regarding antioxidants of a protein concentrate from shrimp waste and its application has been reported. Therefore, the objective of this investigation was to characterise and determine antioxidative activities of shrimp waste protein hydrolysate and study the use of it as a natural antioxidant in comparison to traditional chemical antioxidant used in food industry.
Materials and methods
Industrial shrimp waste from a processing plant at Kolkata, India mainly comprising of cephalothorax, shell and tail of Penaeus monodon and Penaeus indicus were collected. The waste was washed under running water, freeze dried, ground, homogenized, vacum packed and kept in refrigerated storage at 4 °C and used within a month. Prior to the hydrolysis process, a portion of this waste was thawed for analysis of proximate composition and physical characteristics of raw material.
Chemicals
Alcalase 2.4 L was obtained from Novo Nordisk (Bagsvaerd, Denmark). Ethanol and methanol were obtained from Merck (Darmstadt, Germany). 2,2-azino-bis(3-ethylbenzothiazoline- 6-sulfonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and 2,2-diphenyl-1-picryl hydrazyl (DPPH) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 2,4,6-Tripyridyls-triazine (TPTZ), ferric chloride hexahydrate and potassium persulfate were procured from Fluka Chemical Co. (Buchs, Swizerland).
Enzymatic hydrolysis
Shrimp waste protein hydrolysate prepared according to method of Holanda and Netto (2002) with slight modification. Freeze-dried raw waste thawed and suspended (1:1, w/v) in distilled water in Sorvall bottles and the mixture was heated at a temperature of 90 °C for 30 min to inactivate the endogenous hydrolyzing enzyme. The mixture was then homogenized and pH was adjusted to 8.5 with 1N NaOH at 60 °C for enzymatic hydrolysis using Alcalase. The Sorvall bottles were then preheated in a water bath to optimum temperature of 5 °C. Reactions were done in duplicates in 1 L polyethylene jacketed glass vessel in a thermostatically controlled water bath with an automatic temperature compensator (ATC) probe, a pH electrode, a mixer shaft for addition of alkali to maintain the desired pH. The hydrolytic reaction was started by addition of enzymes at levels of 0.05% (w/w) based on protein concentration and the reaction conducted at pH 8.5 and temperature 60 °C using pH stat method as described by Adler-Nissen (1986) for 90 min. The reaction was terminated by heating at 90 °C for 15 min in a water bath with occational stirring. Samples were cooled and then centrifuged at 16000 g for 15 min at 4 °C using REMI centrifuge. The supernatants were collected, concentrated and freeze dried using a freeze drier (Vertis Freeze Mobile, 6ES, USA). Protein hydrolysate powder prepared by Alcalase was kept in polyethylene bag under vaccum at room temperature in dessicator until use.
Proximate analysis and determination of physical properties
Moisture, protein, fat, ash and salt contents of raw waste and protein hydrolysate powder were determined according to the method of AOAC (1999). Chitin was determined according to the method of Spinelli and Mahnken (1978). Colour was measured by Hunter lab and reported in CIE system. L*, a* and b* parameters indicate lightness, redness/greenness and yellowness/blueness, respectively. Water activity was measured using water activity analyzer (Thermoconstanter, Novasina, Swizerland). pH was determined by pH meter CG 842 (Schott, Germany) as described by Benjakul et al. (1997).
Estimation of DPPH and ABTS radical scavenging activity, FRAP (Ferric reducing antioxidant power)
The DPPH and ABTS radical scavenging activity was determined using the method of Yen and Wu (1999) and Arnao et al. (2001) respectively with some modification. Sample (1.5 ml) was added with 1.5 ml of 0.15 mM 2,2-diphenyl-1-picryl hydrazyl (DPPH) in 95% ethanol and working solution mixing stock solution of 7.4 mM ABTS and 2.6 mM potassium per sulphate respectively. The mixture in both the cases was mixed vigorously and allowed to stand at room temperature in the dark for 30 min. The absorbance of the resulting solution was measured at 517 nm using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan). The blank was prepared in the same manner, except that distilled water was used instead of the sample. A standard curve was prepared using Trolox in the range of 10–60 lM. The activity was expressed as μmol Trolox equivalents (TE)/g shrimp protein hydrolysate. A standard curve of Trolox ranging from 50 to 600 μM was prepared.
FRAP (Ferric reducing antioxidant power)
FRAP was assayed according to Benzie and Strain (1996). Stock solutions included 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, and 20 mM FeCl3. 6H2O solution. A working solution was prepared freshly by mixing 25 ml of acetate buffer, 2.5 ml of TPTZ solution and 2.5 ml of FeCl3. 6H2O solution. The mixed solution was incubated at 37 °C for 30 min and was referred to as FRAP solution. A sample (150 μl) was mixed with 2850 μl of FRAP solution and kept for 30 min in dark. The ferrous tripyridyltriazine complex (coloured product) was measured by reading the absorbance at 593 nm. The standard curve was prepared using Trolox ranging from 50 to 600 μM. The activity was expressed as μmol Trolox equivalents (TE)/g shrimp protein hydrolysate.
Effect of concentration of protein hydrolysate on antioxidative activity
Protein hydrolysate with 15% DH, which exhibited the highest antioxidative activity, compared with those with DHs of 5 and 25% (Klompong et al. 2007), were dissolved in distilled water to obtain the various concentration of 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5.0 mg ⁄mL. Antioxidative activity of protein hydrolysates at different concentrations was measured by monitoring the DPPH, FRAP and ABTS assays.
Effect of pH on antioxidative activity
Protein hydrolysate with 15% DH at a concentration of 3 mg ⁄mL were prepared using distilled water as a medium. The 5 mL of sample solutions were adjusted to pHs 2,3,4,5,6,7,8,9,10 and 11 with 1 or 6 m HCl and 1 or 6 m NaOH and incubated at room temperature for 30 min. The pHs of sample solutions were then adjusted to 7.0 with 1 m phosphate buffer. The final volume of all solutions was brought up to 20 mL using the distilled water. Residual antioxidative activity of protein hydrolysates was determined by DPPH, FRAP and ABTS assays.
Effect of temperatures on antioxidative activity
Protein hydrolysate with 15% DH were dissolved in distilled water to obtain the concentration of 3 mg ⁄ mL at pH 7.0. The sample solutions were incubated at 30, 40, 50, 60, 70, 80, 90 and 100 °C placed in a temperature controlled water bath (Memmert, Schwabach, Germany) for 30 min. The sample without incubation (25 °C) was used as the control. The residual antioxidative activities were determined by DPPH, ABTS and FRAP assays. The effect of heating times at 100 °C on antioxidative activity was also investigated by heating for 30, 60, 90, 120 and 150 min. The treated samples were immediately cooled in iced water after the designated time. The residual antioxidative activities were measured by DPPH, ABTS, and FRAP assays.
Application of antioxidative activity
Samples of whole Croaker fish (Johnius gangeticus) with skin on were individually dipped into the following solutions as different treatments for 5 min: control containing only distilled water, 0.1%, 0.2%, and 0.5% (w/v) crude antioxidant solution, or 0.05% (w/v) sodium erythorbate (C6H7NaO6) solution. For each treatment, three whole fish were used as replicates. After treatment, whole fish samples were put into individual Fisher brand polyethylene bags and stored in the refrigerator at 4 °C for 10 days. One half fillet of a whole fish on the skin side was used to measure color while the other half on the cut exposed flesh side was used to determine TBA, PV and FFA values.
Color measurement
The skin color of Croaker fish was measured as described earlier before and during refrigerated storage. Tristimulus L*, a*, b* values were obtained using a white color standard. The measuring head of chroma meter CR 300 had an 8 mm diameter measuring area and used diffuse illumination. Each fillet was measured in three locations average was taken. Averages and standard deviations of L*, a*, b* values were calculated.
TBA analysis
2-Thiobarbituric acid value (TBA) was determined for Croaker fish (Johnius gangeticus) fillets during the refrigerated storage as described by Yu and Sinnhuber (1975). The fillets were ground, mixed, and subjected to analysis. TBA number was expressed as mg malonaldehyde/kg sample.
Peroxide value (PV) and Free fatty acid (FFA)
The PV of the lipid was determined from lipid extract using iodometric method as described by Jacobs (1958). The FFA content in sample was determined by the method recommended by Takagi et al. (1984).
Statistical analysis
Data were taken triplicate and subjected to Analysis of Variance (ANOVA) and the differences between means were evaluated by Duncan’s Multiple Range Test (Steel and Torrie 1980). SPSS statistic program (Version 10.0) (SPSS, 1.2) was used for data analysis.
Results and discussion
Proximate compositions of raw shrimp waste and protein hydrolysate are shown in Table 1. SWPH consisted of 8.05% moisture, 42.24% protein, 16.11% ash and 5.84% fat. It was found that protein and carbohydrate were the major components in it. SWPH is produced by hydrolyzing with enzyme and then the supernatant collected was freeze dried where the extract was concentrated, water was mostly removed and the protein became concentrated, as indicated by the high protein content in the finished product. The high content of carbohydrate (27.65%) most likely resulted from the addition of sugar to improve the flavour and taste. SWPH contained 3.58% salt. This might contribute to high ash content found in the sample. Chitin constituted in the sample at very low content (0.29%). Since chitin was not soluble in water, it could be removed during the filtration process of the extract before evaporation. SWPH was dark brown in colour as evidenced by low L*-value (9.42). The brown colour of SWPH might be developed via the reaction between sugar and amino acid known as the Maillard reaction, which takes place in thermally processed food (Carabasa-Giribet and Ibarz-Ribas 2000). SWPH had a* value of 8.64 and b*-value of 12.34. Water activity (Aw) of SWPH was 0.78. Normally, the browning rate is usually maximum in intermediate moisture foods in which Aw is about 0.7 (Fennema 1996). SWPH had neutral pH (7.21).
Table 1.
Chemical composition and some physical properties of SWPH prepared by alkaline hydrolysis
| Properties | Shrimp waste protein hydrolysate |
|---|---|
| Moisture | 8.0 ± 0.07 |
| Protein | 42.2 ± 0.49 |
| Lipid | 5.9 ± 0.85 |
| Ash | 16.1 ± 0.02 |
| Carbohydrate | 27.6 ± 0.64 |
| Salt | 3.6 ± 0.10 |
| Chitin | 0.2 ± 0.01 |
| Aw | 0.7 ± 0.00 |
| pH | 7.2 ± 0.05 |
| Color | |
| L* | 9.4 ± 0.22 |
| a* | 8.6 ± 0.25 |
| b* | 12.3 ± 0.34 |
Means ± SD from triplicate determinations
Alcalase is endopeptidase capable of hydrolysing proteins with broad specificity for peptide bonds and prefer for a large uncharged residue. Hydrolysed short chain peptides containing water soluble hydrophilic peptides have been reported to possess antioxidative properties (Je et al. 2005). Additionally, peptides also exhibited their antioxidative activity via radical scavenging activity, as well as reducing power (Benjakul et al. 2005). The effect of antioxidants on DPPH radical scavenging was thought to be due to their hydrogen donating ability (Siddhuraju and Becker 2007). ABTS assay is an excellent tool for determining the antioxidant activity of hydrogen-donating antioxidants (scavengers of aqueous phase radicals) and of chain breaking antioxidants (scavenger of lipid peroxyl radicals) (Leong and Shui 2002). Therefore, the water soluble protein hydrolysate was able to scavenge free radicals, thereby preventing lipid oxidation via a chain breaking reaction. The ferric reducing antioxidant power, generally measures the antioxidant effect of any substance in the reaction medium as its reducing ability. Effect of different factors on the antioxidative effect has been studied in the following section.
Effect of the concentration of SWPH on antioxidative activity
The antioxidant property of protein hydrolysate determined by DPPH, ABTS radical scavenging activities and FRAP assays, measured as Trolox equivalent antioxidant capacity (TEAC) generally increased (p < 0.05) with the concentration up to 5 mg/ml as depicted in Fig. 1 (I), (II) and (III). Despite of same DH (35%) the antioxidant activities varied with the concentration of protein in the hydrolysate solution. These results were in agreement with Suetsuna (2000) who reported that prawn protein hydrolysate showed varying antioxidative activity with different concentration applied. The results suggested that antioxidative compounds in the fraction tested were capable of radical scavenging with reducing power to a greater extent when higher concentrations were used. This result was in accordance with Jao and Ko (2002), who reported that the DPPH radical scavenging activity of protein hydrolysates from tuna cooking juice, increased when the concentration increased from 17% to 75%. The greater reducing power indicated that hydrolysates could donate electron to free radical, leading to the prevention or retardation of propagation. In the propagation step, the free radical reacts with oxygen to form peroxy radical, which reacts with more unsaturated lipids to form hydroperoxide. However, the continuous increase in metal chelating activity (Fig. 1 (III)) was found with increasing concentrations up to 5 mg/mL. Metal ion is an effective prooxidant, which can accelerate the initiation process (Gordon 2001). As a consequence, the ability of hydrolysate in chelating those metal ions could lead to the prevention of lipid oxidation. From the result, it can be recommended that SWPH could function as both primary and secondary antioxidant via scavenging the free radical and chelating the metal ions.
Fig. 1.
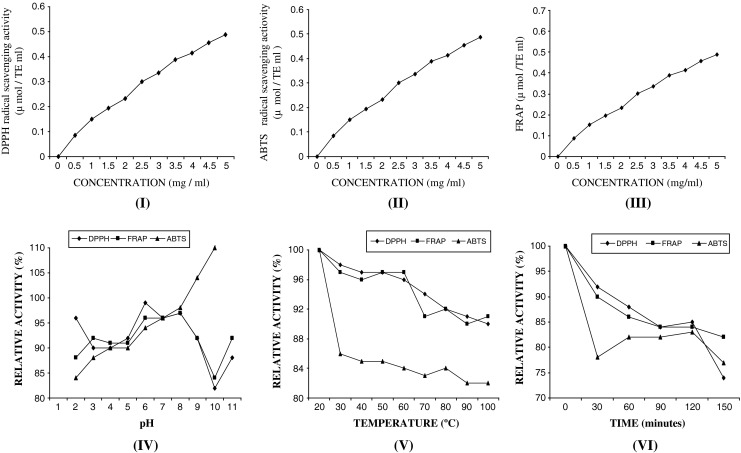
Antioxidative activities [DPPH (I), ABTS (II) radical scavenging activity and FRAP (III)] and variation of relative activity (combination of three antioxidative activities) with pH (IV), temperature (V) and time (VI) depicting pH and thermal stability of shrimp waste protein hydrolysate prepared by alcalase hydrolysis at different concentration. (n = 3, Mean data represented in the graph)
Effect of pH on antioxidative activity of protein hydrolysate
The DPPH, ABTS radical scavenging activity and FRAP of hydrolysate were stable over a wide pH range (Fig. 1, IV). However, the DPPH radical scavenging activity and FRAP tended to decrease in high alkalinity i.e. above eight. Peptides with the short chain peptides and amino acids in protein hydrolysate are not much affected by charge modification governed by pH changes. Basically, protein hydrolysate is soluble over a wide pH range, showing low influence by pH, whereas native proteins with tertiary and quarternary structure are affected considerably by pH (Gbogouri et al. 2004). Nevertheless, metal chelating activity of hydrolysate decreased in high alkaline and acidic pH ranges (p < 0.05). Conversely, the increase in ABTS radical scavenging activity was noticeable. At alkaline pH, antioxidative compounds exhibiting ABTS radical scavenging activity might be activated, while those with DPPH radical scavenging activity and FRAP lost their activity to some extent. Thus, alkaline pHs mostly affected antioxidative activities of the protein hydrolysates. Binsan et al. (2008) who reported that ABTS radical-scavenging activity of Mungoong was activated at alkaline pH.
Effect of temperature and time on antioxidative activity of protein hydrolysate
Thermal stability of hydrolysate as monitored by DPPH, ABTS radical scavenging activities and FRAP is shown in Fig. 1 (V) as relative activity (%). An increase in FRAP was noticeable when subjected to higher temperature, especially upto 60 °C with increasing heating time (45–60 min), where activities of more than 80% remained which got reduced with further increase in temperature and heating time. Some losses in ABTS radical scavenging activity was also found after the water soluble fraction was incubated at 30 °C for 30 min. Thereafter, no further changes in activity were observed with increasing temperature up to 100 °C. The result indicated that antioxidant activity of peptides with low molecular weight were most likely stable at very low temperature and time. In general, proteins were vulnerable to heat treatment, leading to the aggregation of protein and exposure of hydrophobic domain (Sikorski and Naczk 1981). Low MW fish protein hydrolysates were influenced by heat treatment minutely, whereas proteins with second, tertiary and quarternary structure were affected greatly by heat (Mutilangi et al. 1996). The antioxidant activity slightly decreased after being heated for 30 min but the activity remained after heating for up to 120 min, in which 80% activity was still retained. Accumulated energy or enthalpy might be sufficient for antioxidative compounds to undergo denaturation and loss of their activities (Pokorny and Schmidt 2001). This result reconfirmed thermal stability of protein hydrolysate is a important character of a good antioxidant. Binsan et al. (2008) reported that antioxidative activities of Mungoong slightly decreased with prolonged heating time.
Table 2 shows that all the three parameters are well correlated in respect of increase in concentration of SWPH and the correlation values are (ABTS and DPPH: r = 0.978 ABTS and FRAP: r = 0.989, FRAP and DPPH: r = 0.952) significant at 1% level. Significant correlation between the parameters was also noticed in respect temperature and heating time though for pH parameters were negatively correlated except DPPH and FRAP (r = 0.757) which was significant at 5% level. Antioxidant capacity of Mungoong (an extracted paste from shrimp waste), determined by DPPH and ABTS radical-scavenging assays and FRAP showed similar co relationship as described by Binsan et al. (2008) (Table 3).
Table 2.
Correlation coefficient (r) between average values of antioxidant activities of shrimp waste protein hydrolysate varied with concentration, temperature, time and pH
| pH | Temperature | Time | Concentration of SWPH | |
|---|---|---|---|---|
| ABTS and DPPH activity | −0.51 | 0.71a | 0.75 | 0.97b |
| DPPH and FRAP activity | 0.758a | 0.93 | 0.91a | 0.95b |
| ABTS and FRAP activity | −0.11 | 0.72a | 0.85a | 0.98b |
aCorrelation is significant at the 0.05 level (2-tailed)
bCorrelation is significant at the 0.01 level (2-tailed)
Table 3.
Correlation coefficient (r) between average values of auto oxidation parameters of Croaker fish fillet varied with different antioxidant treatment and refrigerated storage days
| Between treatment | Between storage days | |
|---|---|---|
| PV and FFA | 0.75a | 0.87a |
| PV and TBA | 0.68 | 0.76a |
| PV and Color | 0.89a | 0.99b |
| FFA and TBA | 0.64 | 0.71 |
| FFA and Color | 0.99b | −0.92b |
| TBA and Color | 0.52 | −0.81 |
aCorrelation is significant at the 0.05 level (2-tailed)
bCorrelation is significant at the 0.01 level (2-tailed)
Antioxidative effect of SWPH during refrigerated storage of Croaker fish fillet
Changes in colour
Results in Fig. 2 (I) indicated that during refrigerated storage, b* values (yellowness) for the control decreased more rapidly than samples dipped in protein hydrolysate solution and sodium erythorbate. After 4 days refrigerated storage, it could be visually observed that the yellowish color at the skin side of control sample, without antioxidant, got faded, while the fillet treated with antioxidants maintained the yellowish skin color. At the end of storage, the b* value of the control was lower (p ≤ 0.05) compared to antioxidant treatments and decreased from 18.8 to 8.8 during 10 days of refrigerated storage, while no significant difference was found in respect of L* (lightness) and a* (redness) among treatments or storage time . Protein hydrolysate at a higher concentration (5% w/v) equally prevented color degradation in Croaker fish fillet though the effectiveness was lower (p ≤ 0.05) at later stage of storage compared to sodium erythorbate (0.05% w/v). Hsieh and Kinsella (1989) illustrated that lipid hydroperoxides interacted with pigments and other macromolecules in fish causing discoloration and off-flavor. Antioxidant extracted from shrimp shell waste in the form of protein hydrolysate could decrease the carotenoid degradation by inhibiting autoxidation and/or lipoxygenase activity.
Fig. 2.
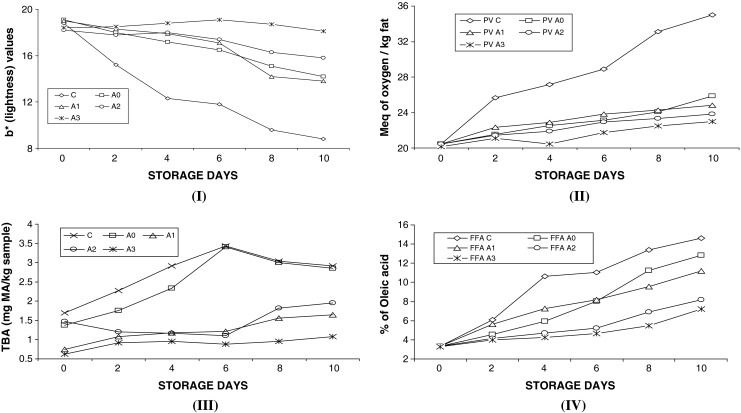
Physico chemical changes in terms of b* (lightness) value for skin side of Croaker fillet (I), per oxide (PV) value (II), thio barbituric acid (TBA) content (III), free fatty acid (FFA) content (IV) of Croaker fish fillet with four different antioxidant treatments during 10 days of refrigerated storage at 4 °C. (n = 3, Mean data represented in the graph). C = Control (no additive), A 0 = with 0.2% of 5 mg/ml concentration SWPH solution, A 2 = with 1.0% of 5 mg/ml concentration SWPH solution, A 1 = with 0.5% of 5 mg/ml concentration SWPH solution, A 3 = with 0.05% w/v sodium erythroborate (C6H7NaO6) solution
Changes in TBA value
Thio barbiuteric acid (TBA) values of all samples tended to increase throughout the storage time specially 0 to 6 days of refrigerated storage after which no significant increment was noticed in Fig. 2 (III). The increase in TBA indicated formation of secondary lipid oxidation products (Kolakowska 2002). TBA value has been used to measure the concentration of relatively polar secondary reaction products, especially aldehydes (Nawar 1996). The highest TBA values of Croaker muscle was found in control and 4% SWPH solution sample throughout the storage period (p < 0.05). The marked increase in TBA at the end of storage was found in control (pre-soaked with distilled water) which most likely due to higher rate of lipid oxidation in the sample. Furthermore, the released free iron and other prooxidants from the muscle during storage time increased the oxidation of lipid in control fillet. The presence of sodium erythroborate and 5% SWPH solution could prevent the oxidation initiated by metal ions probably by several mechanisms including free radical-scavenging action, metal ion-chelating property and/or reducing activity. Results suggested that both color degradation and TBA values were significantly different with effects of treatments and soaking in protein hydrolysate solution effectively arrested oxidation of fat in Croaker muscle.
Changes in PV and FFA
The lipid oxidation as measured by the primary lipid oxidation products, per oxide value occurred in Croaker fillet for control rapidly increased continuously during first 10 days of refrigerated storage, probably due to the absence of antioxidants added [Fig (II)]. However PV value was comparatively lower (p ≤ 0.05) in the presence of sodium erythroborate and SWPH solution (5%). The sample treated with sodium erythroborate solution showed the lowest PV upto 10 days of storage when compared to treatment of SWPH solution and distilled water, respectively. PV values increased (p < 0.05) with storage days for all the samples. However, no significant differences in PV values between antioxidant treatments were observed at the end of storage period. Lajolina et al. (1983) opined that a PV value upto 30 Meq of O2/Kg of fat should be acceptable for fishery products suggesting fillets were consumable even after 10 days of refrigerated storage. Reddy and Srikar (1991) observed PV values for products prepared from Pink perch and Croaker as 11.35 and 10.23 Meq of O2/Kg of fat respectively during 98 days of frozen storage. Free fatty acids are the first product of lipid hydrolysis due to the action of lipolytic enzyme. The release of non-heme iron with absence of antioxidants in croaker muscle during extended refrigerated storage might enhance the oxidation process in the muscle (Chaijan et al. 2005). Changes in FFA values of Croaker fillet pre-soaked with sodium erythroborate and SWPH occurred increased (p < 0.05) with storage days though it was compoaratively lower than that of control sample (p ≤ 0.05). Significant differences (p < 0.05) in FFA values were also observed between treatment, where SWPH showed almost similar effectivity though both were lower than that of control [Fig (IV)]. Reddy and Srikar (1991) observed FFA value in increasing trend through out the storage period of fish finger prepared from Pink perch and Croaker.
References
- Adler-Nissen J. Enzymatic hydrolysis of food proteins. London: Elsevier Applied Science; 1986. p. 427. [Google Scholar]
- Official method of analytical chemists. 15. Arlington: The Association of Official Analytical Chemists, Inc; 1999. [Google Scholar]
- Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- Benjakul S, Seymour TS, Morrissey MT, An H. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Benjakul S, Visessanguan W, Thongkaew C, Tanaka M. Effect of frozenstorage on chemical and gel-forming properties of fish commonly used for surimi production in Thailand. Food Hydrocolloids. 2005;19(2):197–207. doi: 10.1016/j.foodhyd.2004.05.004. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- Carabasa-Giribet M, Ibarz-Ribas A. Kinetics of colour development in aqueous glucose systems at high temperatures. J Food Eng. 2000;44:181–189. doi: 10.1016/S0260-8774(00)00027-3. [DOI] [Google Scholar]
- Chaijan M, Benjakul S, Visessanguan W, Faustman C. Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005;93:607–617. doi: 10.1016/j.foodchem.2004.10.035. [DOI] [Google Scholar]
- Chen HM, Meyers SP. Extraction of astaxanthin pigment from crawfish waste using a soy oil process. J Food Sci. 1982;47:892–896. doi: 10.1111/j.1365-2621.1982.tb12739.x. [DOI] [Google Scholar]
- Coward-Kelly G, Agbogbo FK, Holtzapple MT. Lime treatment of shrimp head waste for the generation of highly digestible animal feed. Carbohydr Polym. 2006;97:1515–1520. doi: 10.1016/j.biortech.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Fennema OR (1996). Amino acids, peptides, and proteins. In: Food chemistry. Marcel Dekker, New York, pp 321–420
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates. J Food Sci. 2004;69:615–622. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- Gildberg A. Enzymatic processing of marine raw materials. Process Biochem. 1993;28:1–15. doi: 10.1016/0032-9592(94)80030-8. [DOI] [Google Scholar]
- Gordon M. Antioxidants and food stability. In: Pokorny J, Yanishlieva N, Gordon M, editors. Antioxidant in food. New York: CRC; 2001. pp. 7–21. [Google Scholar]
- He H, Chen X, Sun C, Zhang Y, Gao P. Preparation and functional evaluation of oligopeptide-enriched hydrolysate from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Bioresour Technol. 2006;97:385–390. doi: 10.1016/j.biortech.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Holanda HD, Netto FM (2002) Optimization of the conditions for the enzyme hydrolysis of shrimp residue, using response surface methodology (RSM). In book of abstracts, 2002 IFT Annual Meeting, Anaheim, Calif., USA. p 194
- Hsieh RJ, Kinsella JE. Oxidation of polyunsaturated fatty acids: mechanisms, products, and inhibition with emphasis on fish. In: Kinsella JE, editor. Advances in food and nutrition research. San Diego: Academic; 1989. pp. 233–341. [DOI] [PubMed] [Google Scholar]
- Jacobs MB. The chemical analysis of foods and food products. New York: Krieger; 1958. pp. 393–394. [Google Scholar]
- Jao CL, Ko WC. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolysates from tuna cooking juice. Fish Sci. 2002;68:430–435. doi: 10.1046/j.1444-2906.2002.00442.x. [DOI] [Google Scholar]
- Je JY, Park PJ, Kim SK. Antioxidant activity of peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res Int. 2005;38:45–50. doi: 10.1016/j.foodres.2004.07.005. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kolakowska A. Lipid oxidation in food systems. In: Sikorski ZE, Kolakowska A, editors. Chemical and functional properties of food lipids. New York: CRC; 2002. pp. 133–160. [Google Scholar]
- Lajolina P, Laine J, Linko P (1983) Quality changes in minced fish during cold and frozen storage. In: Zerthan et al (eds) Thermal processing and quality of foods. Elsvier Applied Science Publishers Ltd.
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. doi: 10.1016/S0308-8146(01)00251-5. [DOI] [Google Scholar]
- Mandeville S, Yaylayan V, Simpson BK. Proximate analysis, isolation and identification of amino acids and sugars from raw and cooked commercial shrimp waste. Food Biotechnol. 1992;6:51–64. doi: 10.1080/08905439209549821. [DOI] [Google Scholar]
- Meyers SP. Utilization of shrimp processing waste. Infofish Marketing Digest. 1986;4:18–19. [Google Scholar]
- Mutilangi WAM, Panyam D, Kilara A. Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J Food Sci. 1996;61(270–274):303. [Google Scholar]
- Nawar WW. Lipids. In: Fennema OR, editor. Food Chemistry. 3. New York: Marcel Dekker; 1996. pp. 225–319. [Google Scholar]
- Pan BS. Recovery of shrimp waste for flavorant. In: Voigt MN, Botta JR, editors. Advances in fisheries technology and biotechnology for increased profitability. Basel: Technomic Pub; 1990. pp. 437–447. [Google Scholar]
- Pokorny J, Schmidt S. Natural antioxidant functionality during food processing. In: Pokorny J, Yanishlieva N, Gordon M, editors. Antioxidant in food practical applications. Abington: Woodhead; 2001. pp. 331–351. [Google Scholar]
- Quaglia GB, Orban E. Enzymic solubilisation of proteins of sardine (Sardina pilchardus) by commercial proteases. J Sci Food Agric. 1987;38:263–269. doi: 10.1002/jsfa.2740380310. [DOI] [Google Scholar]
- Reddy GVS, Srikar LN. Preprocessing ice storage effects on functional properties of fish mince protein. J Food Sci. 1991;56:965–968. doi: 10.1111/j.1365-2621.1991.tb14617.x. [DOI] [Google Scholar]
- Ruttanapornvareesakul Y, Somjit K, Otsuka A, Hara K, Osatomi K, Osako K, Kongpun O, Nozaki Y. Cryoprotective effects of shrimp head protein hydrolysate on gel forming ability and protein denaturation of lizardfish surimi during frozen storage. Fish Sci. 2006;72(2):421–428. doi: 10.1111/j.1444-2906.2006.01166.x. [DOI] [Google Scholar]
- Sathivel S, Bechtel P, Babbitt J, Smiley S, Crapo C, Reppon K. Biochemical and functional properties of herring (Clupea harengus) byproducts hydrolysates. J Food Sci. 2003;68:2196–2200. doi: 10.1111/j.1365-2621.2003.tb05746.x. [DOI] [Google Scholar]
- Shahidi F, Synowiecki J, Naczk M. Utilization of shellfish processing discards. In: Graham BE, editor. Seafood science andtechnology. Canada: Fishing News Books; 1992. pp. 300–304. [Google Scholar]
- Shih MC, Yang KT, Kuo SJ. Quality and antioxidative activity of black soybean tofu as affected by Bean Cultivar. J Food Sci. 2002;67:480–484. doi: 10.1111/j.1365-2621.2002.tb10623.x. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) walp.) seed extracts. Food Chem. 2007;101:10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Sikorski ZE, Naczk M. Modification of technological properties of fishprotein concentrate. Crit Rev Food Sci Nutr. 1981;14:201–230. doi: 10.1080/10408398109527305. [DOI] [PubMed] [Google Scholar]
- Spinelli J, Mahnken C. Carotenoid deposition in pen reared salmonids fed diets containing oil extracts of red crab (Pleuronnocodes planipes) Aquaculture. 1978;13:213–216. doi: 10.1016/0044-8486(78)90003-0. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principle and procedure of statistics. New York: MacGraw-Hill; 1980. [Google Scholar]
- Suetsuna K. Antioxidant peptides from the protease digest of prawn (Penaeus Japonicus) muscle. Mar Biotechnol. 2000;2:5–10. doi: 10.1007/s101269900002. [DOI] [PubMed] [Google Scholar]
- Takagi T, Hayashi K, Itabashi Y. Toxic effect of free unsaturated fatty acid in mouse assay diarluric shell fish toxin by intraperitoinal injection. Bull Jap Soc Sci Fish. 1984;50(8):1413–1418. doi: 10.2331/suisan.50.1413. [DOI] [Google Scholar]
- Wu HC, Chen HM, Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Yen G, Wu J. Antioxidant and radical scavenging properties of extract from Ganoderma tsugae. Food Chem. 1999;65:375–379. doi: 10.1016/S0308-8146(98)00239-8. [DOI] [Google Scholar]
- Yu TC, Sinnhuber RO. An improved 2-thiobarbituric acid (TBA) procedure for the measurement of autoxidation in fish oil. J Am Oil Chem Soc. 1975;44:256–258. doi: 10.1007/BF02639270. [DOI] [PubMed] [Google Scholar]


