Abstract
Chicken meat emulsions prepared using food processor (FP), an indigenous meat cutter (MC) and bowl chopper (BC) were evaluated for physicochemical, texture and electron microscopic studies (SEM). Product yield, emulsion stability, hydration properties and gel strength (N) were significantly (P < 0.05) higher in BC. Total fluid release (TFR), water release (WR) and fat release (FR) was lowest in BC. Significantly (P < 0.05) higher lightness (L) in BC and redness (a) in FP emulsion were observed. Higher firmness, gumminess, chewiness and cohesiveness were observed in BC emulsion. SEM studies revealed a dense and compact protein matrix characteristic of heat induced protein gels. All micrographs showed structures that are compatible with fat globules, muscle fiber, meat protein matrix and heat induced gel/protein matrix. Sensory evaluation showed no significant difference between three treatments for colour, flavour, texture and acceptability scores. Thus, food processor and indigenously developed meat cutter found suitable for producing a stable chicken meat emulsion required for indigenous meat products.
Keywords: Chicken emulsion, Gel strength, Meat cutter, SEM, Texture profile analysis, Sensory scores
Introduction
Finely comminuted meat products are the major group of processed meat products marketed in India. There is a rising demand for convenience processed meats and further experts believe that demand will continue to grow in the future. The demand for further processed meat products is mainly a consequence of the fast progress in urbanization and increased income among city dwellers. Most popular meat products like burger patties, balls (kofta), kebabs, frankfurter type sausages and nuggets are produced from finely chopped meat emulsion. Emulsion type cooked meat products are composed of water, muscle proteins, fat particles, salt and small amounts of non-meat ingredients, where the meat proteins serve as natural emulsifier. In this group of processed meat products, fat and protein concentration and their chemical interactions, especially those occurring during the emulsification process, exert a marked impact on final product quality (Mittal and Usborne 1985; Barbut 2006). To form a stable meat emulsion, meat proteins must surround the finely chopped fat particles before cooking. Myosin is the most important of the proteins for fat emulsification and water holding capacity of processed meats that may bridge the oil—water interface during the emulsification steps (Xiong 2000). Thus, as fat particle size decreases during chopping the emulsion stability will increase, provided there is sufficient protein to coat the entire fat particle. Provided optimum emulsion stability, a high quality finely comminuted meat product would also require adequate gelation of myofibrillar proteins during cooking. If the meat emulsion is unstable, larger fat and water separation will occur during cooking (Townsend et al. 1971; Su et al. 2000; Tornberg 2005). Increased water and fat separation will reduce both yield and quality of the final product promoting economical losses and consumer rejection. Chopping duration has an optimum where stability of the product is maximized and the separation of water and fat during the cooking process is minimized. It is well established that fat stabilization during chopping is due to the formation of a protein film around the fat particles that allows fat to be retained inside the protein matrix (Pietrasik 1999). Obtaining an optimum, stable meat emulsion depends on many factors and requires, (a) reducing fat and meat particle sizes, (b) extracting and dispersing the myofibrilar proteins from the cellular structures, and (c) keeping the degree of myofibrilar proteins denaturation to a minimum during chopping to ensure optimum coating of the fat globules with proteins before cooking. These three main requirements for optimum emulsion stability directly depend on the chopping process and impact the final product yield and quality (Jones and Mandigo 1982; Allais et al. 2004).
Therefore, chopping is one of the most important steps in meat emulsion manufacturing. During this process, the raw materials are extensively comminuted in a bowl chopper/meat cutter in order to reduce the size of the particles and obtain a stable and homogeneous emulsion. Extraction of meat proteins is important to meat processing because proteins are able to hold more water as they become more soluble (Andersson et al. 1997; Tornberg 2005). Inadequate soluble protein extraction or an inadequate fat to protein ratio as well as an excessive reduction of fat particles size could lead to reduced emulsification ability and other qualities of finished products (Jones and Mandigo 1982). As chopping continues, increased emulsion temperature causes the surface tension of the fat particles to decrease. This decrease in surface tension further enhances the particle size reduction process and rapidly increases the surface area of the fat particles and consequently, more protein is required to surround the fat globules. During chopping, certain attractive forces contribute to holding the raw materials together, creating a homogeneous matrix structure (Allais et al. 2004). When this mixture is then cooked, this protein solution denatures and coagulates to form a gel around the muscle bundles and fat particles. At the latest stage of the cooking process, when the temperature reaches 70–75 °C, fat tends to melt and expand, collagen is transformed into liquid-like gelatin and myofibril proteins coagulate (Samejima et al. 1976), salt soluble protein denaturation after cooking turns the emulsion into a viscoelastic gel matrix. Formation of glutamyl-lysine bonds in myosin, myosin and actin, myosin and fibronectin and fibrin and actin are responsible for formation emulsion/gel structure (Tseng et al. 2000). Further formation of cross links of ε- (γ-glutamyl)-lysyl contribute to the gelation of the meat emulsion (Nio et al. 1986).
The bowl chopper/meat cutter is the commonly used meat chopping equipment designed to produce small or very small (“finely comminuted”) lean meat and fat particles. Bowl cutters consist of a horizontally revolving bowl and a set of curved knives rotating vertically on a horizontal axle at required speeds. Bowl cutters are equipped with a strong cover. This lid protects against accidents and its design plays a crucial role in the efficiency of the chopping process by routing the mixture flow. Number, shape, arrangement, and speed of knives are the main factors determining the performance of the cutter. Bowl cutters are used to chop and mix fresh or frozen lean meat, fat (and/or edible offal, if required) together with water (often used in form of ice), functional ingredients (salt, curing agents, additives) and extenders (fillers and/or binders). Fine chopping of meat and fat particles during emulsification increases the protein extraction, binding, yield and juiciness of the final products (Schwartz and Mandigo 1976).
Increased consumer demand for ready to eat meat products in India has motivated small and medium scale entrepreneurs enter into meat processing. Further rapid growth in the processed meat sector has increased demand for basic equipment like meat cutter/chopper. Currently these meat processing equipment have to be imported from Germany, Italy, U.S and South Korea. Small scale entrepreneurs can not afford this equipment mainly because of higher cost and un-availability. Hence Indian meat industry is searching for indigenous meat processing equipment to produce good quality processed meats. Therefore, the aim of this study was to evaluate the potential use a domestic food processor (FP) and an indigenous meat cutter (MC) in comparison to a commercial imported bowl chopper (BC) for producing a stable and good quality chicken meat emulsion required for the manufacture of popular indigenous meat products.
Materials and methods
Materials
Freshly dressed broiler chicken breast meat was obtained from a commercial meat processing unit and stored at −2 to −4 °C until further processing. Three different equipments used for emulsion preparation were food processor/FP (INALSA, India), meat cutter/MC (Kalsi, India) and bowl chopper/BC (Herman Scharfen, Germany). Technical description of these equipments is given in Table 1.
Table 1.
Description of food processor (FP), meat cutter (MC) and bowl chopper (BC) used in the preparation of model chicken meat emulsion
| Machine parameters | FP | MC | BC |
|---|---|---|---|
| Manufacturer | Inalsa, India | Kalsi, India | Hermanscharfen, (TC-11), Germany |
| Bowl Capacity (L) | 3 | 5 | 11 |
| Power requirement (kW) | 0.45 | 0.74 | 1.2 |
| Number of blades/knife | 2 | 2 | 3 |
| Shaft-knife speed (rpm) | 250 | 320 | 2840 cuts per min |
| Bowl rotation | Fixed | Fixed | 14 rpm |
| Sound level (dB) | 85 | 80 | 70 |
| Chopping time (min) | 6 | 4 | 6 |
Preparation of chicken meat emulsion
Three different emulsions were prepared using FP, MC and BC as chopping equipments. Each group (3 kg) was consisting of 85% broiler breast meat, 10% vegetable oil and 5% chilled water. Each formulation also contained (w/w) 2.0% salt and 0.4% sodium tripolyphosphate. The frozen broiler (−2 to −4 °C) meat was ground through a 8 mm plate using a meat mincer (Sirman, Italy).The ground meat was emulsified either using FP, MC and BC as per the treatment requirement. In general, ground meat was mixed with salt and phosphate for 2 min. Vegetable oil was added and mixed further for 2 min and chilled water was used during this final mixing to lower the end point temperature of meat batter to less than 10 °C. Thus prepared emulsion was shaped into balls (approximately 20 g) and loaf (800 g) and cooked in a water bath at 85 °C for 30 min to an internal temperature of 78 °C.
Yield and moisture determination
Cooked meat balls were cooled (4 °C) for 60 min and cooking yield was calculated as the weight of cooked meat ball divided by weight of the raw meat ball, expressed as percentage. Moisture percentage of cooked emulsion was measured using hot air oven (AOAC 2000).
Measurement of instrumental colour
Instrumental colour of raw and cooked and emulsions was measured using a Hunter Colorimeter. Colorimetric analysis on raw and cooked cooled meat emulsion was performed using a Hunter Lab Miniscan XE Plus colorimeter (Hunter Associates Laboratory Inc., Reston, VA, USA) with 25 mm aperture set for illumination D65, 10° standard observer angle. Hunter L (lightness), a (redness) and b (yellowness) values were measured on cut surface of raw and cooked emulsion.
Determination of water and fat binding properties and gel strength
The batters (10 g each) were stuffed into plastic tubes (2.0 cm diameter) and centrifuged (3000 rpm, 4 °C, 15 min) (MP 400 R, ElteK, India) to homogenize and eliminate air bubbles. The tubes with 10 g batter were hermetically closed and heated in a water bath for 30 min at 75 °C. For measuring binding properties, the tubes were opened and left to stand upside down for 60 min to release the exudates into glass plate. Total fluid release (TFR) was calculated as percentage of weight loss after heating. The fluid released in glass plate was oven dried at 105 °C for 16 h. Water released (WR) was determined as weight loss during 16 h and fat release (FR) was calculated as the difference between TFR and WR (Cofrades et al. 2008).
A method described by Kuraishi et al. (1997) with suitable modification was used to measure the gel strength of above samples. A texturometer (TA-HDi, Stable Microsystems UK) attached with a blade set with knife to cut the gel placed horizontally on the platform was used to measure the maximum force (N) required to cut the gel into two pieces.
Texture profile analysis
The texture profile analysis (TPA) was conducted using a texturometer (TA-HDi, Stable Microsystems UK) attached to a software, texture expert (Bourne 1976). Six samples from each cooked emulsion (1.5 cm height and 2.5 cm diameter) were compressed twice to 30% of their original height. Force-time deformation curves were derived with 50 N load cell applied at a constant crosshead speed of 1 mm/s. The parameters determined were: Firmness (N)—maximum force required to compress the sample, springiness (cm)—ability of the sample to recover to its original shape after the deforming force was removed, cohesiveness- extent to which the sample could be deformed prior to rupture, gumminess (N)—force to disintegrate a semisolid meat sample for swallowing (firmness × cohesiveness) and chewiness (N-cm)—work to masticate the sample for swallowing.
Microstructure of emulsion
Microstructure of three emulsions was analysed by scanning electron microscopy (SEM) as described by Ashie et al. (1997). The cooked samples were fixed with (1:1, v/v) a mixture of paraformaldehyde (4%) and glutarldehyde (0.2%), post fixed with OsO4, washed, dehydrated using a lypholyzer. The lyophilized samples were put onto a double slided adhesive tape, mounted on aluminium stubs and gold coated and subjected to scanning electron microscope analysis (Hitachi S-3400 N, Japan). A large number of micrographs were taken in order to select the most representative ones.
Sensory evaluation
A sensory panel of eight members evaluated the different sensory attributes of chicken nuggets prepared from three emulsions. The panellists rated each sample for colour, flavor, texture and overall acceptability on nine point descriptive scale (Keeton 1983). Samples were warmed in a microwave oven for 20 s just before sensory evaluation and coded samples were served at room temperature. Water was served for cleansing the mouth between samples.
Statistical analysis
Six samples (n = 6) from every treatment were analysed for each parameter. Data were analyzed using Statgraphics Plus 2.1 (STSC Inc. Rockvile, MD, USA) for one way analysis. Least square difference was used for comparison of mean values among treatments and significant difference was identified at 95% level (P < 0.05).
Results and discussion
Physicochemical properties
Mean values of physicochemical properties of different emulsions are summarised in Table 2. Products yield was significantly (p < 0.05) higher in BC and it was in the order of BC > MC > FP. However the difference between BC and MC treatments was not statistically significant. Binding properties were significantly (P < 0.05) higher in BC than MC and FP emulsions. TFR, WR and FR were lowest in BC followed by MC and highest in FP. However, analysis of variance studies indicated no significant difference. Similarly, moisture content also did not show any significant difference. The differences in binding properties of three emulsions is probably due to the fact that in the BC, the higher amounts of myofibrillar proteins extracted create a dense protein network that holds more water (Andersson et al. 1997). It has been reported that under-chopping usually results in minimal binding of fat particles while over-chopping results in massive fat and water separation during the cooking process (Barbut 2006). The consequences of using different meat cutting equipment on meat emulsion systems are harder to determine. There have been no studies intended to assess the effect of different equipment on emulsion stability and other properties of meat emulsion. In general use of salt and chopping are responsible for solubilization of meat proteins, thus contributing to the formation of a protein gel/emulsion matrix with good water and fat binding properties upon heating leading to higher product yield (Jones and Mandigo 1982 ; Su et al. 2000). Among three equipment, bowl chopper was more effective in solubilization of meat proteins and formation of good protein matrix and formation of more stable emulsion upon cooking. Higher product yield suggested better emulsion stability and hydration properties in emulsion prepared using bowl chopper as compared to others. Optimum chopping thus is important for better emulsion stability and cooking yield. The smaller particle size of meat proteins and fat meant a higher surface/volume ratio and hence more opportunity for its components to interact with the medium, fully distribution throughout the meat system thus helping to form emulsion system with good water-and-fat binding properties (Pietrasik 1999).
Table 2.
Physicochemical, instrumental texture, colour and sensory characteristics of chicken meat emulsions/ nuggets prepared using different equipment
| Parameter | Treatments | |||
|---|---|---|---|---|
| FP | MC | BC | Mean standard error | |
| Yield (%) | 85.1a | 90.8b | 92.3b | 0.54 |
| Total fluid released (%) | 19.2 | 18.3 | 17.8 | 1.85 |
| Water released (%) | 17.2 | 16.6 | 16.6 | 1.84 |
| Fat released (%) | 2.0 | 1.6 | 1.2 | 0.35 |
| Gel strength (N) | 3.7a | 4.1a | 5.2b | 0.17 |
| Moisture (%) | 71.8 b | 71.3a | 71. 9 b | 0.15 |
| Hunter colour values of raw emulsions | ||||
| L | 60.1a | 68.0b | 72.3 | 0.49 |
| a | 9.8b | 7.6a | 6.9a | 0.23 |
| b | 18.7 | 19.1 | 19.0 | 0.30 |
| Hunter colour values of cooked emulsions | ||||
| L | 77.4a | 78.9b | 79.0b | 0.47 |
| a | 2.4 | 3.1 | 2.9 | 0.44 |
| b | 15.9 | 15.7 | 15.7 | 0.18 |
| Texture profile analysis | ||||
| Firmness (N) | 16.5b | 12.8a | 19. 8b | 1.29 |
| Gumminess (N) | 9.2b | 6.1a | 11.2b | 0.66 |
| Cohesiveness | 0.54b | 0.48a | 0.56 b | 0.08 |
| Springiness (cm) | 0.98 | 0.99 | 0.99 | 0.01 |
| Chewiness (N cm−1) | 9.1b | 6.2a | 11.1b | 0.66 |
| Sensory Scores | ||||
| Colour and appearance | 7.8 | 7.7 | 8.0 | 0.17 |
| Taste and flavour | 7.6 | 7.8 | 8.0 | 0.18 |
| Texture | 7.9 | 7.7 | 7.7 | 0.16 |
| Overall acceptability | 7.7 | 7.6 | 7.8 | 0.21 |
N Newton; FP emulsion prepared in domestic food processor; MC emulsion prepared in meat cutter; BC emulsion prepared in bowl chopper
Means bearing different superscripts within a row are significantly different (P < 0.05)
n (Number of sample) =6 for all parameters but n = 20 for sensory scores
Sensory scores were measured on 9 point descriptive scale where 9= highly desirable scores and 1= undesirable scores
Chicken nuggets were the product on which sensory evaluation was carried out
Gel strength
It expressed as force (N) required to cut emulsion gel into two pieces was significantly higher in BC, followed by MC and FP emulsions (Table 2). There are several factors that can influence the strength of gel formed by meat proteins. During thermal processing of meat batter, salt soluble protein denaturation turns the emulsion into a viscoelastic gel matrix. Collagen is transformed into liquid-like gelatin and myofibrillar proteins coagulate (Samejima et al. 1976). The solublization of muscle proteins to form a gel or emulsion matrix upon cooking significantly affects the characteristics of meat products including its water and fat biding properties (Comfort and Howell 2003). This is evident in meat emulsion prepared from bowl chopper in which an optimum level of chopping and size reduction of both meat proteins and fat globules occur. Gel strength depends upon possibility of association between molecules (water, fat and proteins), so that particle size is a factor to be taken into account when considering the technological behaviour of meat batter (Hermansson 1982; Nio et al. 1986). In a gel/emulsion system, lower gel strength implies less solublized proteins, and hence there may be insufficient protein aggregation to form a strong protein network. The gel emulsion systems studied in this investigation contain similar meat protein and fat content, this means that the emulsion stability and gel strength difference among the treatments are attributed to the equipment used. Consistency of meat products is influenced by formation of three dimensional networks of meat proteins capable of modifying rheological properties of the continuous phase of emulsions. Therefore explanation for differences in gel strength and binding properties of emulsion systems studied may lie in the differences in protein solubility during emulsification in different equipment.
Colour values
The hunter colour values of raw emulsion are shown in Table 2. Lightness value (L) was significantly (P < 0.05) higher in BC followed by MC and FP. Redness (a) of FP emulsion was significantly higher than MC and BC which did not vary significantly. Yellowness (b) did not vary among three emulsions. Cooking of meat emulsion further increased the lightness (L) in all samples and significant higher lightness values were observed in MC and MCI as compared to FP. In contrast, cooking decreased redness and yellowness values of three emulsions and further no significant difference was seen between three cooked emulsions for redness and yellowness. In general, cooking significantly (P < 0.05) increased lightness (L) and decreased redness (a) and yellowness (b) values of the emulsion. This might be due to protein denturation and conversion of myoglobin into metmyoglobin during thermal treatment (Tornberg 2005). Similarly, Tseng et al. (2000) reported Hunter colour values of 71.16 to 74.66 (L), 1.86 to 2.42, (a) and 11.0 to 13.46 (b) in cooked chicken meat balls. In emulsion based meat products colour changes were attributed to the content of fat, water, meat pigments and proteins (Pietrasik 1999). It has been further reported that increase in protein concentration in a meat emulsion was responsible for increase in lightness (L) and decrease in red (a) values. (Su et al. 2000).The changes in colour observed in this study could be attributed to different level of solubilization of meat proteins during chopping. Swatland and Barbut (1990) reported that in comminuted meat products composed of water, fat, protein, and salt, every constituent plays at least a minor role in the scattering of light. The functional state of the myofibrillar proteins, myoglobin and the content of connective tissue proteins such as collagen and elastin are considered the most important proteins affecting the optical reflectance (Tornberg 2005).
Texture profile
Results of texture profile analysis are given in Table 2. Firmness, gumminess and chewiness were significantly (P < 0.05) higher in BC than MC and FP emulsions. Higher firmness and gumminess can be related to better protein extraction in BC (Barbut 2006). Among MC and FP, texture parameters were significantly higher in FP. Higher protein extraction and better binding priorities might be responsible for increased firmness and gumminess in BC. Springiness showed no significant difference in three emulsions. Cohesiveness was also significantly (P < 0.05) higher in BC and FP emulsions than MC emulsion. Overall, bowl chopper provided the highest firmness, indicating the strong gel structure formation in BC than MC and FP. It has been reported that extractable meat proteins are mainly responsible for typical characteristics of meat batter. (Hung and Zayas 1992; Su et al. 2000). These results further suggested that the gel strength and emulsion stability were optimum in MC and FP indicating that these can be employed in small scale production of emulsion meat products.
Microstructure
Micrographs of meat batter system showed that use of different equipment affected some characteristics of gel/emulsion structure. All samples presented dense and compact protein matrix (Fig. 1) characteristic of heat induced protein gels. In general, all batters showed a homogenous and spongy bulk meat protein matrix, but some more morphological aspects were dependant upon protein solubilization during emulsification. Emulsion prepared in meat cutter showed more free fat globules (Fig. 1e) as compared to others emulsion in which fat globules were uniformly distributed within meat protein matrix. All micrographs showed some structure that are compatible with fat globules, muscle fiber, meat protein matrix and heat induced gel/protein matrix. The degree to which fat globules and muscle fibers chopped influences the protein solubilization and subsequent formation of strong protein gel matrix and which in turn influences the physicochemical properties of meat batter (Jones and Mandigo 1982; Su et al. 2000; Barbut 2006). Free fat globules and muscle fibres which are not integrated may interfere in the formation of a thermal gel matrix producing softer structure (Fig. 1e).Three equipments adequately produced smaller particles of fat globules and muscle fibre which favoured the formation of adequate strong protein gel responsible for important emulsion properties like water-and–fat binding and textural characteristics. Hammer (1998) investigated the properties of raw sausage batters and suggested that linking of meat proteins occurs during comminution of meat batter which then results in a finer protein network structure after cooking. They further suggested that improved meat /gel structure was probably due to the formation of ε- (γ-glutamyl) lysyl bonds between myofibrillar proteins. These micrographs also revealed that many fat globules were entrapped physically within the hydrated muscle proteins (Fig. 1b, f, and h–i) in the meat batters, which stabilise the meat emulsion during cooking and contributed to physicochemical and textural properties of meat batters. Hermansson (1982) found that gelation of meat proteins begins during cooking and produces gels consisting of linear polymer chains and stabilized by non-covalent and hydrophobic linkages. Previous studies on meat emulsion have attributed the typical microstructure of meat batter to this mechanism of heat induced gel formation of muscle proteins (Hammer 1998; Pietrasik and Li-Chan 2002; Pietrasik et al. 2007; Barbut 2006; Cofrades et al. 2008). Comminution in combination with salt addition and subsequent heating, used in the making of emulsion drastically alter the structure of the meat system. The higher amounts of myofibrillar proteins extracted during chopping and emulsification on heating create a dense protein network, a gel that holds water efficiently by capillary forces. The amount of myofibrillar proteins that are extracted into the water-phase during comminution and blending is generally considered to be the most important factor for the quality of the meat network (Tornberg 2005). Moreover, the type of gel matrix formed is related to the dispersed or aggregated state of the protein prior to gelation. The minor differences in micrographs were presumably due to differences in the particle size of fat globules and muscle fibers. Tornberg (2005) reported that complex meat system consists of, not only dissolved proteins, but also insoluble components like meat fibres, connective tissue and fat. The amount and state of these components have a large impact on the gelation properties. The properties of the gel are also influenced by the heating process. Results of this experiment indicated that all three equipment were adequate to produce an integrated meat batter system which formed harder gel/emulsion network after cooking.
Fig. 1.
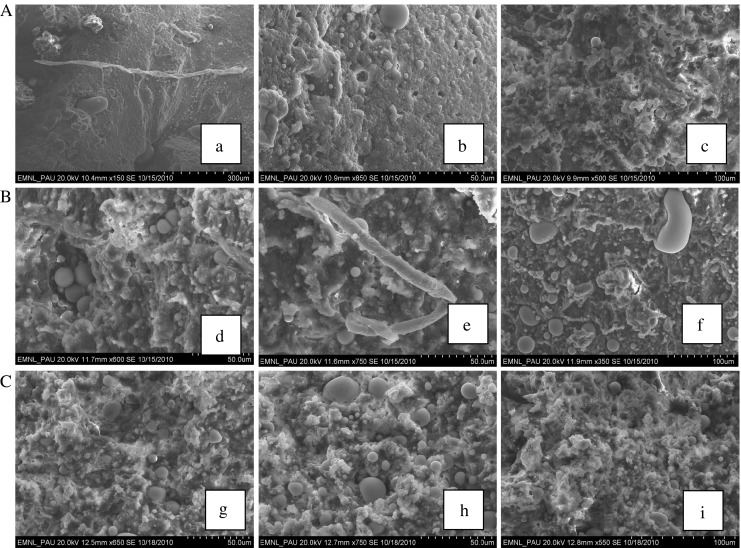
Scanning electron micrographs (X 50 magnification) of chicken meat emulsion prepared using (A) food processor (FP), (B) indigenous meat cutter (MC) and (C) commercial bowl chopper (BC). a Dense protein matrix characteristics of protein induced gel. b Fat globules entrapped physically within hydrated muscle proteins. c Dense protein matrix characteristics of protein induced gel. d Dense protein matrix characteristics of protein induced gel. e. Protein matrix and free small fat globules. f Fat globules entrapped physically within hydrated muscle proteins. g Protein matrix characteristics of protein induced gel. h Fat globules entrapped physically within hydrated muscle proteins. i Protein matrix characteristics of meat emulsion
Sensory evaluation
Results of sensory evaluation showed no significant difference among three treatments for sensory scores. Although flavour and acceptability scores were slightly higher in nuggets prepared from emulsion prepared in bowl chopper the difference was statistically non significant. These results indicated that acceptable chicken products can be prepared from meat emulsion prepared using meat cutter and food processors that are indigenously available and imported bowl chopper could easily be replaced by these indigenous equipment.
Conclusion
Overall, small scale meat processors searching for low cost equipment can use these equipments for regular production of emulsion based meat products. The most cost effective equipment evaluated in this study provided desired cooking yield, emulsion stability and textural properties required for emulsion based indigenous meat products. Thus food processor and indigenously developed meat cutter seemed suitable for producing emulsion based meat products.
Acknowledgements
This research was supported by the National Agricultural Innovation Project (NAIP), Indian Council of Agricultural Research (ICAR) through its subproject entitled “A Value Chain on Novelty Pork Products under Organized Pig Farming System”.
References
- Allais I, Christophe V, Pierre A, Dufour E. A rapid method based on front-face fluorescence spectroscopy for the monitoring of the texture of meat emulsions and frankfurters. Meat Sci. 2004;67:219–229. doi: 10.1016/j.meatsci.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Andersson K, Andersson A, Tornberg E. Microstructures related to the water-holding, textural and sensory properties of emulsion sausages. In: Dickinson E, Bergenstahl B, editors. Food colloids—Proteins, lipids and polysaccharides. Cambridge: Royal Society of Chemistry; 1997. pp. 29–42. [Google Scholar]
- Official methods of analysis of AOAC International. 17. Maryland: Association of Official Analytical Chemistry; 2000. [Google Scholar]
- Ashie IN, Simpson BK, Ramaswamy HS. Changes in texture and microstructure of pressure-treated fish muscle tissue during chilled storage. J Muscle Foods. 1997;8:13–32. doi: 10.1111/j.1745-4573.1997.tb00375.x. [DOI] [Google Scholar]
- Barbut S. Effect of caseinate, whey and milk powders on the texture and microstructure of emulsified chicken meat batters. LWT Food Sci Technol. 2006;39:660–664. doi: 10.1016/j.lwt.2005.03.017. [DOI] [Google Scholar]
- Bourne MC. Texture profile analysis. Food Technol. 1976;32:62–66. [Google Scholar]
- Cofrades S, Lopez-Lopez FP, Solas MT, Bravo L, Jimenez-Colmenero F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008;79:767–776. doi: 10.1016/j.meatsci.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Comfort S, Howell NK. Gelation properties of salt soluble meat protein and soluble wheat protein mixtures. Food Hydrocolloids. 2003;17:149–159. doi: 10.1016/S0268-005X(02)00047-4. [DOI] [Google Scholar]
- Hammer GF. Microbial transglutaminase and diphosphate in finely comminuted cooked sausage. Fleischwirtschaft. 1998;78:1155–1162. [Google Scholar]
- Hermansson AM. Gel characteristics–structure as related to texture and water binding of blood plasma gels. J Food Sci. 1982;47:1965–1972. doi: 10.1111/j.1365-2621.1982.tb12924.x. [DOI] [Google Scholar]
- Hung SC, Zayas JF. Functionality of milk proteins and corn germ protein flour in comminuted meat products. J Food Qual. 1992;15:139–152. doi: 10.1111/j.1745-4557.1992.tb00982.x. [DOI] [Google Scholar]
- Jones KW, Mandigo RW. Effects of chopping temperature on the microstructure of meat emulsions. J Food Sci. 1982;47:1930–1935. doi: 10.1111/j.1365-2621.1982.tb12916.x. [DOI] [Google Scholar]
- Keeton JT. Effect of fat and sodium chloride/phosphate level on the chemical and sensory properties of pork patties. J Food Sci. 1983;48:878–881. doi: 10.1111/j.1365-2621.1983.tb14921.x. [DOI] [Google Scholar]
- Kuraishi C, Sakamoto J, Yamazaki K, Susa Y, Kuhara C, Soeda T. Production of restructured meat using microbial transglutaminase without salt or cooking. J Food Sci. 1997;62:488–490. doi: 10.1111/j.1365-2621.1997.tb04412.x. [DOI] [Google Scholar]
- Mittal GS, Usborne WR. Meat emulsion extenders. Food Technol. 1985;39:121–130. [Google Scholar]
- Nio N, Motoki M, Takinami K. Gelatin of protein emulsion by transglutaminase. Agric Biol Chem J. 1986;50:1409–1412. doi: 10.1271/bbb1961.50.1409. [DOI] [Google Scholar]
- Pietrasik Z. Effect of content of protein, fat and modified starch on binding, textural characteristics, and colour of comminuted scalded sausages. Meat Sci. 1999;51:17–25. doi: 10.1016/S0309-1740(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Pietrasik Z, Li-Chan ECY. Binding and textural properties of beef gels as affected by protein, k-carrageenan and microbial transglutaminase addition. Food Res Int. 2002;35:91–98. doi: 10.1016/S0963-9969(01)00123-5. [DOI] [Google Scholar]
- Pietrasik Z, Jarmoluk A, Shand PJ. Effect of non-meat proteins on hydration and textural properties of pork meat gels enhanced with microbial transglutaminase. LWT Food Sci Technol. 2007;40:915–920. doi: 10.1016/j.lwt.2006.03.003. [DOI] [Google Scholar]
- Samejima K, Takahashi K, Yasui T. Heat induced denaturation of myosin total rod. Agric Biol Chem. 1976;40:2455–2460. doi: 10.1271/bbb1961.40.2455. [DOI] [Google Scholar]
- Schwartz WC, Mandigo RW. Effect of salt, sodium tripolyphosphate and storage on restructured pork. J Food Sci. 1976;41:1266–1269. doi: 10.1111/j.1365-2621.1976.tb01148.x. [DOI] [Google Scholar]
- Su YK, Bowers JA, Zayas JF. Physical characteristics and microstructure of reduced-fat frankfurters as affected by salt and emulsified fats stabilized with non meat. proteins. J Food Sci Technol. 2000;65:128–132. [Google Scholar]
- Swatland HJ, Barbut S. Fibre-optic spectrophotometry for predicting lipid content, pH and processing loss of comminuted meat slurry. Int J Food Sci Technol. 1990;25:519–526. doi: 10.1111/j.1365-2621.1990.tb01111.x. [DOI] [Google Scholar]
- Tornberg E. Effect of heat on meat proteins-Implications on structure and quality of meat products. Meat Sci. 2005;70:493–508. doi: 10.1016/j.meatsci.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Townsend WE, Ackerman SA, Witnauer LP, Palm WE, Swift CE. Effects of types and levels of fat and rates and temperatures of comminution on the processing and characteristics of frankfurters. J Food Sci. 1971;36:261–265. doi: 10.1111/j.1365-2621.1971.tb04038.x. [DOI] [Google Scholar]
- Tseng TF, Liu DC, Chen MT. Evaluation of transglutaminase on the quality of low salt chicken meat balls. Meat Sci. 2000;55:427–431. doi: 10.1016/S0309-1740(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Xiong YL. Meat processing. In: Nakai S, Modler HW, editors. Food proteins: processing applications. New York: Wiley–VCH; 2000. pp. 89–145. [Google Scholar]


