Abstract
In this study, juvenile beluga (Huso huso) was fed by the diets containing different carbohydrate to lipid ratios for 5 months. At the end of culture period, proximate compositions of the fish carcasses (moisture, protein, lipid, and ash) were measured. Then, qualitative changes in the fishes were evaluated during 6 months frozen storage (−20 °C) along with recording changes in their Total Volatile Bases Nitrogen (TVN), Thiobarbituric Acid (TBA), Free Fatty Acids (FFA), pH, and muscle texture profile analysis. The results of proximate analysis of the carcasses showed that moisture, protein, and ash of the carcasses increased significantly (p < 0.05) with higher carbohydrate to lipid ratio, but lipid content of the carcasses decreased significantly (p < 0.05). Also, during frozen storage, TVN, TBA, and FFA increased significantly in all the samples (p < 0.05). Significant differences were detected in pH of the treatments (p < 0.05), but these changes didn’t follow a regular pattern in all the treatments. The results of muscle texture profile analysis showed lower chewiness, hardness, and gumminess during the first 3 months of frozen storage; however, after 6 month, the values increased significantly compared to those in 3 months. Different characteristics of texture showed significant differences in the treatments during frozen storage (p < 0.05), although these changes didn’t follow an identical pattern in all the treatments. It can be concluded that carbohydrate higher than 27% in diet has had adverse effect on quality of fillets during frozen storage. However, lipid levels used in the present study haven’t had significant influence on quality of the fillets during the preservation.
Keywords: Huso huso, Carbohydrate to lipid ratio, Proximate composition, Frozen storage and texture
Introduction
Fish is one of the most important sources of animal protein available in the topic and has been widely accepted as a good source of protein and other elements for the maintenance of healthy body (Arannilewa et al. 2005). Fish tissue protein is characterized by a very desirable composition of amino acids and also is a rich source of group B vitamins and is rich in vitamins A and D (Zmijewski et al. 2006). Fish lipids have gained more importance because of the presence of health beneficial omega-3 polyunsaturated fatty acids (PUFA). These PUFA viz, eicosapentanoeic acid (EPA) and decosahexanoic acid (DHA) play a crucial role in the prevention of atherosclerosis, heart attack and etc. (Stephen et al. 2010).
Qualitative factors of fish involve various aspects such as color and appearance, texture, nutritional value, i.e. protein, essential amino acids, lipid, fatty acids and their composition, vitamins, minerals, trace elements, and more importantly, resistance against oxidation. Quality of fish and products prepared from, it is affected by various factors such as fish size and diet. Of course, some factors, such as genotype, age, sex, physiologic status, inherent composition of fish, and some environmental factors are also of importance (Lie 2001).
Sturgeons are considered as the most valuable fish in the world, among which beluga can play a crucial role in production of meat and caviar thanks to its rapid growth and high adaptation capability. Following high production of young sturgeons during early 1980s, sturgeons culture has been founded in many countries such as Italy, the US, etc. (Hung and Deng 2002).
During the past few years, sturgeon culture has gained higher importance. Considering long culture period of these fishes, economical preparation of diet is of prominent issues. To obtain cheaper diet, non-protein energy sources, e.g. carbohydrates and lipid, which are cheaper, should be used. On the other hand, one of the effective factors on quality of fish flesh, which has received a high attention, is fish nutrition, because this factor can be said to affect all qualitative factors of carcasses and also affect fillets’ shelf life (Lie 2001).
It is noteworthy that the higher lipid level and lesser carbohydrate level in diet, the more lipid accumulation in fish body. Since lipid decay rapidly due to their unsaturated fatty acids, they would lead to a faster decay of fish muscle during preservation (Pirini et al. 2004).
Decaying results in unfavorable odor, taste, and color and alteration in fish fillets’ tissue (Hernandez et al.2009). Although several studies have shown that lipid content of diet affect qualitative characteristics of fish carcass during preservation, relative data about sturgeons is limited.
Shelf life of fresh fish depends on bacteria flora, preservation temperature, characteristics of transportation, and physiological properties of fish (Abbas et al.2008). Because the environment negatively affects fish, it must be consumed immediately after fishing or must be stored under suitable conditions to reach consumers without losing its nutritional value (Izgi and Ciftcioglu 1997).
Bacteria growth, enzymatic changes, and biochemical reactions are greatly affected by preservation temperature. Preservation of fish at low temperature leads to reduced rate of both enzymatic and chemical reactions. As a result, freezing is broadly used to preserve sensory properties and nutritional value of fish (Lugasi et al. 2007), also the demand for using frozen products has increased in the last years (Nielsen et al.1994).
Freezing is one of the best methods for fish preservation and has been employed increasingly both on shore and fishing vessels (Begona 1999). The purpose of frozen storage of seafood is to extend its shelf life and to limit microbial and enzymatic activity which causes deterioration (Atayeter and Ercoskun 2011). However, fish and fish products undergo several chemical and physical changes during frozen storage. These changes adversely affect frozen-fish product quality and storage stability (Al-Bulushi et al. 2011). Although, microbial deterioration of fish muscle can be inhibited by frozen storage, fish proteins undergo a number of changes that affect the flavor and texture of flesh (Aubourg et al. 1998). Also, lipid decay has been proved to occur during frozen storage (Badii and Howell 2001). Frozen fish are usually kept in the form of fillets. Filleting, also, can harmfully effect on quality of frozen fish (Ciarlo et al. 1985).
Knowledge about sensory and chemical variations in sturgeon fillets and shelf life for the fish is confined to a few studies. Therefore, the present study aimed at evaluation of some qualitative variations in frozen fillets of beluga (Huso huso) fed by different carbohydrate to lipid ratios.
Materials and methods
Fish sample
Juvenile beluga was obtained from Voshmgir Dam Proliferation and Culture Center for sturgeons placed in Golestan Province, Iran and then they were taken to Aquaculture Researches Center of Agricultural Sciences and Natural Resources University of Gorgan. The fish was 1-year-old and average weight of fish was 300 ± 7.3 g. The fish were kept in the lab for 4 weeks for adaptation to the lab condition. During this time, they were fed by commercial diet.
Diet
Six experimental diets with identical protein and gross energy contents were prepared. Averages of protein and energy contents of the diet were 40.2% and 16.56 kJ/g, respectively. Fish powder and gelatin, and corn starch were used as protein and carbohydrate sources, respectively. Equal amounts of fish oil and canola oil were used as lipid source.
Before use, nutritional ingredients were analyzed and the data resulted from proximate analysis of diet was used for diet balancing. For preparation of the diet, the ingredients were first mixed thoroughly and next, pellets (4 mm in diameter) were produced. The foods were dried in room temperature and then they were kept in freezer at −20 °C. After 4 weeks adaptation, the fish were fed by the experimental diet for 5 months. Ingredients and nutrient composition of the experimental diets are given in Table 1.
Table 1.
Formulation (g/100 g diet) and chemical composition (% DM) of the experimental diets
| Experimental diets | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Ingredients (g 100 g−1) | ||||||
| Kilka Fish meal | 48 | 48 | 48 | 48 | 48 | 48 |
| Gelatin | 10.51 | 10.51 | 10.51 | 10.51 | 10.51 | 10.51 |
| Corn starch | 0 | 6.4 | 13.3 | 19.9 | 27 | 34.2 |
| Cellulose | 18.4 | 15.5 | 12.4 | 8.5 | 4.3 | .06 |
| Vitamin mixturea | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Mineral mixtureb | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| L- methionine | 2 | 2 | 2 | 2 | 2 | 2 |
| L- lysine | 2 | 2 | 2 | 2 | 2 | 2 |
| Canola oil | 7.9 | 6.1 | 4.2 | 2.9 | 1.4 | 0 |
| Fish oil | 7.9 | 6.1 | 4.2 | 2.9 | 1.4 | 0 |
| Chemical composition | ||||||
| Dry matter (%) | 89.6 | 88.5 | 88.1 | 89.3 | 88.7 | 88.6 |
| Crud protein(% DM) | 40.2 | 40.2 | 40.2 | 40.2 | 40.2 | 40.2 |
| Crud lipid | 23.5 | 20 | 16.5 | 13.8 | 10.9 | 8.1 |
| Ash | 8.8 | 8.7 | 9.4 | 8.9 | 9.3 | 8.9 |
| Crude fiber | 23.4 | 21.7 | 18.3 | 16 | 12.5 | 9.8 |
| NFEc | 4.1 | 9.4 | 15.6 | 21.1 | 27.1 | 33 |
| Carbohydrate: lipid ratio | 0.17 | 0.47 | 0.94 | 1.52 | 2.47 | 4.07 |
| Gross energy (Mjkg−1)d | 16.96 | 16.66 | 16.45 | 16.44 | 16.46 | 16.51 |
aSupplied (IU or mg kg−1 diet): vitamin A, 1800 IU; vitamin D3, 1200 IU; vitamin E, 120 mg; vitamin B12, 24 mg; riboflavin, 15 mg; niacin, 90 mg; D-pantothenic acid, 27 mg; menadione, 3 mg; folic acid, 4.8 mg; pyridoxine, 9 mg; thiamine, 9 mg; D-biotin, 0.48 mg; choline chloride 360 mg; cobalamin 24 mg; ascorbic acid 156 mg; nicotinic acid 90 mg; inositol 72; antioxidant 15 mg
bSupplied (mg kg−1 diet): Zn, 18 mg; I, 0.6 mg; Mg, 7.8 mg; Co, 0.15 mg; Se, 0.15 mg; CU, 1.8 mg; Fe, 12 mg
cNFE (nitrogen-free extract) = 100-(% crude protein +% crude lipid +% ash +% crude fiber)
dCalculated on the basis of the physiological fuel values of 4.4 and 9 kcal g−1 (16.7, 16.7, 37.7 kj/g−1) for protein, carbohydrate and fat respectively (Garling and Wilson 1977)
Experimental design
After 4 weeks adaptation, 72 fish sample with a primary weight of 355 ± 1.31 g were randomly divided into six treatments. Eighteen tanks were used and each treatment consisted of three replicates. Each replicate was represented by one tank and four fish were placed in each tank. Five hundred-liters fiberglass tank were used for culture.
Feeding
The fish were fed twice a day (0800 and 1700 h) based on 2% of body weight for 5 months by hand until they reached mean weight of 1300 ± 13.7 g. The feed intake was checked at each feeding: all the supplied feed was consumed and no rejection was recorded during the trial. The fish biometry was done monthly and given food was calculated according to body weight gain. Water in each tank was exchanged daily at a rate of 80% volume and supplemental aeration was provided to maintain the dissolved oxygen level near saturation. Water quality parameters were monitored during the experimental period. Water temperature, dissolved oxygen and pH were measured periodically in each experimental tank by a water checker (Horiba u 10, Japan). The Average water temperature, dissolved oxygen and pH in all treatments were 21 ± 1.5 °C, 6.4 ±0.5 mg L−1 and 8.1 ± 0.5, respectively.
Sampling methods
At the end of feeding period, 24 h after the last feeding, six fish of each treatment (two fish from each of the iterations) were sampled and slaughtered. Then, they were skinned and filleted after gutting. The samples of each treatment were separately packed in polyethylene bags and they were frozen for 5 month at −20 °C. Proximate composition of fish carcasses were estimated in the first day of sampling. Changes in qualitative parameters of the sampled fillets (TVN, TBA, FFA, pH, and and muscle texture profile analysis) were evaluated in various frozen times (0, 1, 3 and 6 months).
Proximate composition of carcasses
In order to specify proximate composition of fish carcasses, all fish of each treatment were mixed together and then they were homogenized. Of these homogenized tissues, three samples were chosen as experimental replicates. After that, carcasses’ compounds were estimated for each of the treatments on the basis of standard method of AOAC (1995) as follows: Crude protein through khjeldal method using digestion and dilution set (Gerhardt, type VAP.40, Germany), Crude lipid through petroleum ether and Soxhlet set (Gerhardt, type SE-416, Germany), Moisture using oven set (Binder, USA) for 24 h at 105 °C, Ash through electric kiln (Nabertherm, Germany) for 8 h at 550 °C.
TVN
TVN content of the samples was measured through dilution and titration method (Watabe et al. 1991). The resulted concentrate from the dilution was titrated with sulfuric acid 0.1 N and concentration of volatile nitrogenous bases as mg nitrogen per 100 g of the sample (Hasegawa 1987).
TBA
TBA was measured through colorimetric method and absorption was measured in 538 nm and compared to control sample (malonaldehyde standard) (Kirk and Sawyer 1991).
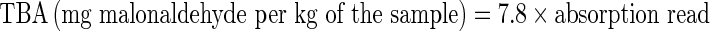 |
FFA
In order to estimate free fatty acids, Egan et al. (1997) method was employed and free fatty acid content was obtained in terms of percentage of oleic acid.
pH measurement
Two grams of fish muscle was agitated and homogenized in 10 ml of distilled water. Then, the samples’ pH was measured via digital pH-meter (type 1/713.0010, Switzerland) set in room temperature.
Muscle texture profile analysis
The fillets were first taken out of freezer so as to reach ambient temperature in sufficient time. Then, three cubes (1 × 1 × 1 cm) were separated from each fillet. Each cube was pressed in two stages by TPA, Texture Profile Analyzer, (Lfra 4500, U.S.A.) set. For this end, plastic cylindrical probe, 2.54 cm in diameter (TA 1000), speed of 0.8 mm per second, 25% of deformation, and trigger-point of 22.5 was used. Load cell of the set was 5 kg.
Hardness is defined as the peak force during the first compression cycle (first bite) in Newtons. Cohesiveness is defined as the ratio of the positive force area during the second compression portion to that during the first compression, excluding the areas under the decompression portion in each cycle. Cohesiveness is dimensionless. Springiness (originally called elasticity) is defined as the higher that the food recovers during the time that elapses between the end of the first bite and the start of the second bite. Results are expressed commonly in mm. Adhesiveness is defined as the negative force area for the first bite. Representing the work (N × mm) necessary to pull the plunger away from the food sample. Gumminess is defined as the product of hardness × cohesiveness in Newtons. Gumminess only applies to semisolid products. Chewiness is defined as the product of gumminess × springiness (which is equivalent to hardness × cohesiveness × springiness). Chewiness measured a work (N × mm) and only applies for solid products (Sanchez-Alonso et al. 2010).
Statistical analysis
Statistical analysis of the data, except proximate composition analysis was done on split-plot experimental design through SAS software. In order to find significant differences in 5% significance level between the data resulted from each index in different times, LSD test was used. Proximate composition was analyzed by One-Way ANOVA using SPSS program version 16. All data were expressed as mean ± standard deviation in triplicate groups.
Results and discussion
The data derived from analyzing beluga’s carcasses were shown in Table 2. The results show that fish body’s compounds were significantly affected (p < 0.05) by carbohydrate to lipid ratio of diet. Moisture, protein, and ash content of the carcasses increased significantly with increase in carbohydrate to lipid ratio, while lipid content of the carcasses decreased significantly.
Table 2.
Average Body composition of beluga juvenile fed with experimental diets (Mean ± SD)
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Body composition% | 1 | 2 | 3 | 4 | 5 | 6 |
| Moisture | 74.9 ± 1.57a | 77.4 ± 1.60ab | 77.6 ± 1.57ab | 77.0 ± 2.08ab | 79.0 ± 0.89b | 79.6 ± 0.61b |
| Protein | 14.9 ± 0.73a | 15.7 ± 0.40ab | 15.9 ± 0.40b | 16.2 ± 0.16b | 15.7 ± 0.70ab | 16.0 ± 0.50b |
| Lipid | 8.1 ± 0.63e | 6.1 ± 0.58d | 4.6 ± 0.37c | 4.2 ± 0.54c | 3.2 ± 0.50b | 2.4 ± 0.18a |
| Ash | 3.7 ± 0.20a | 4.1 ± 0.10ab | 4.4 ± 0.20ab | 4.2 ± 0.15ab | 4.3 ± 0.05b | 5.3 ± 0.32b |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different superscript letters within each row represent significant differences (P < 0.05)
In this study, moisture content of the carcasses increased with increase in carbohydrate to lipid ratio. This is consistent with the result obtained from the study of Hu et al. (2007) on Sea Bream and Gao et al. (2009) on Grass Carp. Protein and ash content of carcasses increased significantly with increase in carbohydrate to lipid ratio but there is a slight trend to increase. It probably is only a reflection of the lower lipid content in the muscle. Lipid content of the carcasses increased with higher lipid level in the diet. Lipid level of the carcasses is affected by lipid and carbohydrate contents of the diet and lipid content of the carcasses increase with higher lipid level of the diet. The same results were derived about rainbow trout (Lee and Putnam 1973; Reinitz and Hitzel 1980; Brauge et al.1993), channel catfish (Garling and Wilson 1977), tilapia (El-sayed and Garling 1988), reddrum (Ellis and Reigh 1991; Serrano et al.1992), walking catfish (Erfanullah and Jafri 1998), yellowfin seabream (Hu et al.2007), and grass carp (Gao et al.2009). There is a reverse correlation between carbohydrate levels of the diet and lipid content of the carcasses (Hilton and Atkinson 1982) so that increase in carbohydrate suppresses unfavorable lipids accumulation in the carcasses (Erfanullah and Jafri 1998).
During frozen storage, muscle tissue protein denatures due to formation of ice crystals which is a consequence of dehydration (Simeonidou et al. 1997). Lower quality protein of frozen fish can be attributed to preservation temperature, temperature fluctuations during the preservation, moisture variations, duration of preservation, and enzymatic decomposition (Bauchart et al. 2007).
Measurement of TVN level shows proteins breakage owing to enzymatic and bacterial activities which results in production of amines and lower nutritional value (Kerr et al. 2002) and is generally considered as one of spoilage indices in meats. The data related to TVN was given in Table 3. The results showed that TVN amounts in the samples had a significant increase (p < 0.05) during the preservation. TVN amounts of different treatments showed significant difference (p < 0.05) in all treatments. The treatments 5 and 6 showed the highest values of TVN. The results of the present study showed that TVN values in the samples in time 0 ranged between 17.7 and 20.09. According to Connell (1995), when TVN value exceeds 30 mg/100 g tissue, the fillet is not suitable to use. In this study, the results indicated that after the fillets were preserved in freezer for 6 months, the highest TVN value was 25.74 mg N/100 g tissue which is lower than permissible value reported by Connell (1995). Similarly, Simeonidou et al. (1997) reported that TVN value of horse mackerel increased during frozen storage. Same results have been reported for other fish species preserved in freezer (Ciarlo et al.1985; Reddy et al.1992; Erickso and Thed 1994).
Table 3.
Change in the mean values of TVN (based on milligrams of nitrogen per 100 g tissue) in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer
| Treatment | Time storage (month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 17.7 ± 0.50Aa | 17.8 ± 1.09Aa | 23.7 ± 0.74Ab | 24.7 ± 0.58Ab |
| 2 | 21.0 ± 0.66Ba | 22.7 ± 0.36Bb | 23.1 ± 1.43Ab | 25.7 ± 0.74Ac |
| 3 | 20.8 ± 0.65Ba | 19.8 ± 1.00Ca | 25.3 ± 0.70Bb | 25.5 ± 1.08Ab |
| 4 | 20.4 ± 0.48Ba | 21.8 ± 0.34Ca | 23.4 ± 0.85Ab | 24.6 ± 0.58Ab |
| 5 | 20.4 ± 0.23Ba | 23.0 ± 1.60Cb | 25.2 ± 1.68Bc | 25.6 ± 1.13Ac |
| 6 | 20.4 ± 0.56Ba | 23.9 ± 0.64Cb | 25.0 ± 0.14Bb | 25.2 ± 0.42Ab |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
TVN levels among the treatments were different during various storage times and generally increased from the treatment 1 to the treatment 6, but this increase was significant only in the treatment 6. The obtained results from carcasses analyses showed that protein level of the carcass had an increasing rate from the treatment 1 to the treatment 6.
Lipid oxidation, which occurs in raw materials during preservation, processing, and also in final product during other steps of preservation, is one of the main processes that brings forth sourness of foods (Selmi and Sadok 2008). Lipid oxidation in frozen fishes was measured through measurement of TBA index. This index is usually used so as to evaluate Lipid oxidation level and secondary products resulted from Lipid oxidation, which usually involved aldehydes or carbonyl which results in unfavorable odor and taste of Lipid or product (Lin and Lin 2005). Lakshmanan (2000) proposed the range of 1–2 mg malonealdehyde per kg Lipid as redline of TBA in fish.
The data related to TBA was given in Table 4. The results showed that TBA levels increase significantly (p < 0.05) in all the treatments during the preservation. Among the treatments, TBA values increased with increased carbohydrate to lipid ratios from treatment 1 to treatment 6 and the highest value was seen in treatment 6. According to Lakshmanan (2000), TBA values in treatment 5 and 6 of the present study after 3 months of frozen storage were higher than permissible value; however, in other treatments, this value exceeded the permissible level after 6 months of preservation. Similarly, Ng and Bahurmiz (2009) reported that after 30 weeks of frozen storage at −20 °C, TBA values in tilapia fillets increased. Also, after 6 months of storage at −20 °C, Stephan et al. (1995) found that TBA values in turbot fillets increased .TBA value in Atlantic salmon preserved in freezer for 4 months significantly increased (Regost et al. 2004) .
Table 4.
Change in the mean values of TBA (based on mg malonaldehyde in a kg of meat) in beluga fillet fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 0.46 ± 0.08Aa | 0.62 ± 0.04Ab | 1.4 ± 0.07Bc | 2.5 ± 0.03Ad |
| 2 | 0.65 ± 0.02Ba | 0.74 ± 0.07ABa | 1.2 ± 0.05Ab | 2.5 ± 0.06Ad |
| 3 | 0.67 ± 0.03BCa | 0.85 ± 0.04Bb | 1.4 ± 0.08Bc | 2.7 ± 0.07Bd |
| 4 | 0.72 ± 0.00BCa | 1.5 ± 0.08Cb | 1.8 ± 0.04Cc | 2.8 ± 0.08Bd |
| 5 | 0.83 ± 0.03Ca | 1.4 ± 0.07Cb | 2.3 ± 0.03Dc | 3.3 ± 0.26Cc |
| 6 | 1.0 ± 0.05Da | 1.4 ± 0.04Cb | 2.3 ± 0.11Dc | 4.0 ± 0.09Dd |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
FFA is one of the indices of fat spoilage whose increase after fish death and during preservation indicates hydrolytic spoilage of Lipid (Shewfelt 1981). FFA, resulting from lipid hydrolysis, may accumulated during storage and accelerate quality deterioration (Saeed and Howell 2002). However, FFA has not been reported to directly cause quality defects. Also, FFA greatly affects proteins denaturation which leads to lower quality of tissue (Losada et al. 2004). FFA value depends on duration and temperature of storage (Aubourg and Medina 1999; Roldan et al. 2005). FFA has been found to produce some compounds with low molecular weight during oxidation leading to unfavorable flavor in fish and final products (Refsgaard et al.2000).
Glycerides, glycolipids, and phospholipids are hydrolyzed by lipase enzymes and converted to free fatty acids. They are converted to aldehydes and ketones as lipid oxidation continues. Thus, determination of FFA levels can be named as a good index so as to indicate influence of lipolytic enzymes on fish Lipid and other meat products (Aubourg 2001). Accumulation of free fatty acids in fish flesh brings about unfavorable taste and tissue damages due to bonding to protein of flesh (Mai and Kinsella 1980). The data related to FFA was given in Table 5. FFA levels increased significantly (p < 0.05) among all the treatments during the preservation. The results showed that among different treatments, increased FFA levels were seen with increased carbohydrate to lipid ratio of diet and the treatment 6 showed the highest FFA value.
Table 5.
Change in the mean values of FFA in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 0.19 ± 0.00Aa | 0.19 ± 0.01Aa | 1.0 ± 0.06Bb | 1.3 ± 0.05Ac |
| 2 | 0.21 ± 0.01Aa | 0.30 ± 0.00BCb | 0.81 ± 0.03Ac | 1.5 ± 0.05Bd |
| 3 | 0.21 ± 0.00Aa | 0.22 ± 0.00ABa | 1.5 ± 0.08Cb | 1.9 ± 0.02Cc |
| 4 | 0.22 ± 0.01Aa | 0.33 ± 0.01Cb | 1.6 ± 0.03Cc | 2.0 ± 0.12Dd |
| 5 | 0.32 ± 0.02Ba | 0.37 ± 0.01Ca | 1.0 ± 0.03Bb | 2.5 ± 0.16Ec |
| 6 | 0.34 ± 0.02Ba | 0.61 ± 0.04Db | 2.1 ± 0.08Dc | 2.5 ± 0.03Ed |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Phospholipids are readily decomposed during frozen storage and produce free fatty acids. The produced FFA is probably resulted from decomposition of phospholipids which is due to low stock Lipid content in the carcasses in treatment 6. Considering higher sensitivity of this group of Lipids to hydrolytic spoilage, it resulted in higher FFA level in this treatment. This is consistent with the results obtained from the study of Simeonidou et al. (1997) on horse mackerel and Mediterranean hake preserved in freezer. Also, Aubourg and Medina (1999) found that FFA values in cod and haddock increased during frozen storage.
Huss (1988) stated that primary pH of fish after death depends on species, catching method, and season. Physiologic status or activity or stress degree before death can have a significant influence on speed and development of autolytic changes and consequently, on pH of flesh after death (Hiltz and Dyer 1971). After fish death, due to formation of lactic acid through glycolysis, pH decreases (Woywoda et al. 1986). After occurrence of glycolysis, autolytic changes, e.g. denaturation and protein breakage, prepare suitable situation for growth and development of microbes which may increase pH (Parkin and Brown 1983). Fresh fish were used in the present study. The pH of samples didn’t increase which is possibly due to lower contamination of the fish. Table 6 shows relative data about pH levels. The pH ranged between 6.27 and 6.98. Although a significant difference (p < 0.05) was seen between pH levels during different preservation times in different treatments, there was no regular pattern in these changes.
Table 6.
Change in the mean values of pH in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 6.9 ± 0.01Ec | 6.6 ± 0.00Db | 6.4 ± 0.00Ca | 6.3 ± 0.01ABa |
| 2 | 6.5 ± 0.02Db | 6.6 ± 0.02Dc | 6.4 ± 0.02Ca | 6.4 ± 0.01Ca |
| 3 | 6.4 ± 0.09Bb | 6.5 ± 0.02Cc | 6.2 ± 0.02Aa | 6.3 ± 0.03Aa |
| 4 | 6.4 ± 0.04BCb | 6.5 ± 0.02BCbc | 6.3 ± 0.02ABa | 6.5 ± 0.07Cc |
| 5 | 6.3 ± 0.02Aa | 6.4 ± 0.02Ab | 6.2 ± 0.01Aa | 6.3 ± 0.01ABab |
| 6 | 6.4 ± 0.07Cc | 6.4 ± 0.01ABbc | 6.3 ± 0.01Ba | 6.4 ± 0.02Bab |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Simeonidou et al. (1997) reported that pH of horse mackerel preserved at −18 °C significantly increased during the preservation period. Same results were obtained in Ciarlo et al. and Hennigar et al. (1989).
Rostamzad et al. (2011) reported that pH of Persian sturgeon didn’t change significantly during the storage period. Other studies, also, showed that frozen storage has no significant effect on pH variations during the storage (Aubourg et al. 2004).
Characteristics of texture of fish flesh depend on some inherent biological factors which are related to density of muscle fiber which is consisted of both Lipid and collagen. Fish death and microbiological processes result in softness of fish tissue and lower elasticity. In the present study, hardness was significantly correlated with preservation duration (Olafsdottir et al. 2004). Although measurement texture characteristics are not a reliable index of spoilage, they can be used so as to confirm the results obtained from physicochemical, microbiological and sensory analyses. Among different parameters of texture, hardness reflects muscle texture in a better manner and so, it can better describe freshness of fish fillet.
Some characteristics, e.g. gumminess and chewiness, are more affected by rigor mortis rather than decomposition of muscle structure. The data related to texture measurement, involving hardness, adhesiveness, cohesiveness, chewiness, springiness, and gumminess, were respectively given in Tables 7, 8, 9, 10, 11, 12. The results showed that hardness, chewiness and gumminess levels decreased significantly during the first 3 months of freezing, but the values increased with continuation of freezing to 6 months however, This increase can be attributed to loss of fillets’ moisture. Adhesiveness and cohesiveness values didn’t obey a regular pattern during the preservation. Springiness value increased after 6 months of the preservation. Regost et al. (2001) state increasing in fillet lipid of brown trout (Salmo trutta) has no effect on texture properties.
Table 7.
Change in the mean values of hardness (N) in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 11.3 ± 0.08Dd | 7.0 ± 0.05Dc | 4.5 ± 0.06Ca | 5.3 ± 0.17Bb |
| 2 | 5.2 ± 0.17Ab | 4.2 ± 0.07Ba | 5.6 ± 0.04Dc | 5.6 ± 0.13Bc |
| 3 | 12.5 ± 0.18Ec | 6.8 ± 0.13Db | 6.2 ± 0.22Ea | 6.6 ± 0.16Cb |
| 4 | 20.4 ± 0.30Fd | 8.5 ± 0.02Ec | 4.2 ± 0.30BCa | 4.9 ± 0.40Ab |
| 5 | 6.3 ± 0.16Bc | 3.9 ± 0.14Ab | 3.1 ± 0.24Aa | 7.0 ± 0.07Dd |
| 6 | 9.8 ± 0.02Cd | 5.1 ± 0.06Cc | 4.0 ± 0.34Ba | 4.7 ± 0.29Ab |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Table 8.
Change in the mean values of gumminess (N) in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 6.3 ± 0.06Ec | 5.4 ± 0.08Eb | 1.8 ± 0.13Ba | 1.9 ± 0.08Aa |
| 2 | 2.0 ± 0.03Aa | 2.0 ± 0.08Aa | 2.3 ± 0.06CDb | 2.9 ± 0.13Cc |
| 3 | 5.5 ± 0.21Dc | 3.2 ± 0.08Cb | 2.7 ± 0.19Ea | 2.6 ± 0.21Ba |
| 4 | 10.5 ± 0.26Fd | 3.5 ± 0.09Dc | 2.1 ± 0.06Ca | 2.4 ± 0.11Bb |
| 5 | 2.7 ± 0.19Bc | 2.2 ± 0.10Ab | 1.4 ± 0.08Aa | 3.0 ± 0.08Cd |
| 6 | 4.8 ± 0.24Cb | 2.6 ± 0.16Ba | 2.5 ± 0.26DEa | 2.6 ± 0.17Ba |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Table 9.
Change in the mean values of adhesiveness (NS) in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (Month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 0.00Bb | −0.02 ± 0.00Ba | −0.02 ± 0.00Ba | −0.02 ± 0.00Ba |
| 2 | −0.02 ± 0.00Ab | −0.02 ± 0.00Bb | −0.03 ± 0.00Aa | −0.01 ± 0.00Cc |
| 3 | −0.02 ± 0.00Ab | −0.03 ± 0.00Aa | −0.02 ± 0.00Bb | −0.02 ± 0.00Bb |
| 4 | 0.00 ± 0.00Bc | −0.03 ± 0.00Aa | −0.02 ± 0.00Bb | −0.02 ± 0.00Bb |
| 5 | 0.00 ± 0.00Bc | −0.01 ± 0.00Cb | −0.01 ± 0.00Cb | −0.03 ± 0.00Aa |
| 6 | 0.00Bc | −0.01 ± 0.00Cb | −0.01 ± 0.00Cb | −0.03 ± 0.00Aa |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Table 10.
Change in the mean values of cohesiveness in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (Month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 0.52 ± 0.02Cd | 0.47 ± 0.00Cc | 0.42 ± 0.02Bb | 0.37 ± 0.00Aa |
| 2 | 0.38 ± 0.00Aa | 0.52 ± 0.01Cb | 0.42 ± 0.00Ba | 0.51 ± 0.02Cb |
| 3 | 0.46 ± 0.01Bbc | 0.43 ± 0.02Bb | 0.35 ± 0.03Aa | 0.47 ± 0.03BCc |
| 4 | 0.45 ± 0.02Bab | 0.48 ± 0.05Cab | 0.50 ± 0.02Cb | 0.43 ± 0.02Bc |
| 5 | 0.45 ± 0.03Bb | −0.51 ± 0.02Aa | 0.41 ± 0.04Bb | 0.43 ± 0.02Bb |
| 6 | 0.48 ± 0.02Bb | −0.51 ± 0.04Aa | 0.51 ± 0.05Cb | 0.47 ± 0.06BCb |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Table 11.
Change in the mean values of chewiness (Nmm) in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (Month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 5.4 ± 0.15Ec | 4.3 ± 0.16Cb | 1.8 ± 0.12Ba | 1.9 ± 0.02Aa |
| 2 | 1.8 ± 0.02Aa | 2.4 ± 0.16Ab | 2.7 ± 0.22Db | 4.1 ± 0.22DEc |
| 3 | 4.8 ± 0.21Dc | 3.3 ± 0.17Bb | 2.6 ± 0.19CDa | 4.5 ± 0.28Ec |
| 4 | 12.1 ± 0.61Fc | 5.0 ± 0.25Db | 2.6 ± 0.09CDa | 2.6 ± 0.31Bc |
| 5 | 3.0 ± 0.17Ba | 2.4 ± 0.15Ab | 1.1 ± 0.28Ac | 3.8 ± 0.41Dd |
| 6 | 4.2 ± 0.59Cc | 2.8 ± 0.26ABb | 2.1 ± 0.23BCa | 3.2 ± 0.53Cb |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Table 12.
Change in the mean values of springiness (mm) in beluga muscle fed with different carbohydrates to lipid ratios storage in the freezer (Mean ± SD)
| Treatment | Time storage (Month) | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| 1 | 0.91 ± 0.03Aa | 1.3 ± 0.04Cb | 0.97 ± 0.07ABa | 1.0 ± 0.03Aa |
| 2 | 0.93 ± 0.04Aa | 1.1 ± 0.08ABb | 1.1 ± 0.09Cb | 1.4 ± 0.06Cc |
| 3 | 0.84 ± 0.03Aa | 1.1 ± 0.06ABb | 1.1 ± 0.09Cb | 1.4 ± 0.06Cc |
| 4 | 1.1 ± 0.01Ba | 1.2 ± 0.09Cb | 1.2 ± 0.03Cb | 1.2 ± 0.03Bb |
| 5 | 1.0 ± 0.05Bb | 1.2 ± 0.05BCc | 0.90 ± 0.07Aa | 1.2 ± 0.09Bc |
| 6 | 0.88 ± 0.08Aa | 1.0 ± 0.12Ab | 1.0 ± 0.02Bb | 1.4 ± 0.05Cc |
All values are the means ± standard deviations of three replicates
Treatments 1 to 6 show different carbohydrate to lipid ratios (0.17, 0.47, 0.94, 1.52, 2.47 and 4.07 respectively). Different Capitalized letters in each column indicate significant differences among different treatments at any time and Different lowercase letters in each row indicate significant differences at any treatment in different time
Variations of hardness, adhesiveness, cohesiveness, chewiness, springiness, and gumminess values didn’t follow a clear pattern. Therefore, various carbohydrates to lipid ratios in diet can be said not to have special effect on beluga fillets during frozen storage.
Conclusion
Because nearly 60% of fish culture cost is related to fish food, use of suitable and inexpensive diet is of great importance for economic aquaculture. Use of energetic non-protein resources (carbohydrates and lipid) in fish diet, which are cheaper than protein, can play a prominent role in food cost reduction and fish production. Considering long period for beluga culture and other sturgeons, use of carbohydrate and lipid in diet is effective in reducing the relative costs. It is noteworthy that fish diet affects quality of fish fillet. On the basis of the results obtained from the present study, carbohydrate higher than 27% of diet negatively affects the quality of fillet during frozen storage; however, dietary lipid has no effect on quality of beluga fillet during the preservation. In conclusion, carbohydrate higher than 27% of beluga diet is not recommended; however, more studies have to be done.
Acknowledgement
The authors thank Dr. Majid Azim Mohseni for his help in statistical analysis and Voshmgir Dam Proliferation and Culture Centre for Sturgeon.
References
- Abbas KA, Mohamed A, Jamilah B, Ebrahimian M. A review on correlation between fish freshness and ph dring cold storage. Am J Biochem Biotechnol. 2008;4(4):416–421. doi: 10.3844/ajbbsp.2008.416.421. [DOI] [Google Scholar]
- Al-Bulushi Im, Kasapis S, Dykes GA, Al-Wili H, Guizani N, Al-Oufi H (2011) Effect of frozen storage on the characteristics of a develop and commercial fish sausages. J Food Sci Technol. doi:10.1007/s13197-011-0441-x [DOI] [PMC free article] [PubMed]
- Official methods of analysis. 16. Arlington: AOAC; 1995. p. 1141. [Google Scholar]
- Arannilewa T, Salawu SO, Sorungbe AA, Ola-Salawu BB. Effect of frozen period on the chemical, microbiological and sensory quality of frozen tilapia fish (sarotherodun galiaenus) Afr J Biotechnol. 2005;4:852–855. doi: 10.1177/026010600601800210. [DOI] [PubMed] [Google Scholar]
- Atayeter S, Ercoskun H. Chemical composition of European squid and effects of different frozen storage temperatures on oxidative stability and fatty acid composition. J Food Sci Technol. 2011;48(1):83–89. doi: 10.1007/s13197-010-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubourg S. Damage detection in horse mackerel (Trichurus trachurus) during chilled storage. J Am Oil Chem Soc. 2001;78:857–862. doi: 10.1007/s11746-001-0355-3. [DOI] [Google Scholar]
- Aubourg SP, Medina I. Influence of storage time and temperature on lipid deterioration during cod (gadus morhua) and haddock (melanogrammus aeglefinus) frozen storage. J Sci Food Agric. 1999;94:1948–1973. [Google Scholar]
- Aubourg SP, Stoleo C, Perez-Martin R. Assessment of quality changes in frozen sardinae (sardine pilchardus) by flouresence detection. J Am Oil Soc. 1998;75:575–580. doi: 10.1007/s11746-998-0068-x. [DOI] [Google Scholar]
- Aubourg S, Alenso F, Gallardo M. Studies on rancidity inhibition in frozen horse mackerel (trichurus trachurus) by citric and ascorbic acids. Eur J Lipid Sci Technol. 2004;106:232–240. doi: 10.1002/ejlt.200400937. [DOI] [Google Scholar]
- Badii F, Howell A comparison of biochemical changes in cod (Gadus morha) and haddoch (Melnogrammus aeglefinus) fillets during frozen storage. J Sci Food Agric. 2001;82:87–97. doi: 10.1002/jsfa.998. [DOI] [Google Scholar]
- Bauchart C, Chambon C, Patureau P, Savary-Auzeloux I, Remond D, Morzel M. Peptides in rainbow trout (onchorhynchus mykiss) muscle subjected to ice storage and cooking. J Food Chem. 2007;100:1566–1572. doi: 10.1016/j.foodchem.2005.12.023. [DOI] [Google Scholar]
- Begona B. Chemical changes and visual appearance of albacore tuna as related to frozen storage. J Food Sci. 1999;64:20–24. doi: 10.1111/j.1365-2621.1999.tb09853.x. [DOI] [Google Scholar]
- Brauge C, Corraze G, Medale F (1993) Combined effect of dietary lipid carbohydrate ratioand environmental factors on growth and nutritional balance in rainbow trout. In: From Discovery to Commercialization Carlio M, Danle L, Morales J, Sorgeloos P, Svennevig N, Wyban J (eds) Spec publ European Aquaculture Society 19, Oostende, Belgium, p 209
- Ciarlo AS, Boeri RL, Giannini DH. Storage life of froen blocks of Patagonian hake (merluccius hubbsi) filleted and minced. J Food Sci. 1985;50:723–726. doi: 10.1111/j.1365-2621.1985.tb13782.x. [DOI] [Google Scholar]
- Connell JJ. Control of fish quality. 4. London: Fishing News Books; 1995. [Google Scholar]
- Egan H, Krik RS, Sawyer R (1997) Pearsons chemical analysis of foods, 9th edn. pp 609–634
- Ellis DJ, Reigh RC. Effect of dietary lipid and carbohydrate levels on growth andbody composition of juvenile red drum (Scianop socellatus) Aquaculture. 1991;97:383–394. doi: 10.1016/0044-8486(91)90330-A. [DOI] [Google Scholar]
- El-Sayed AM, Garling DL. Carbohydrate-to-lipid ratios in diet for Tilapia zilli fingerling. Aquaculture. 1988;73:157–163. doi: 10.1016/0044-8486(88)90050-6. [DOI] [Google Scholar]
- Erfanullah, Jafri AK. Effect of dietary carbohydrate-to-lipid ratio on growth and body composition of walking catfish (Clarias batrachus) Aquaculture. 1998;16:159–168. doi: 10.1016/S0044-8486(97)00267-6. [DOI] [Google Scholar]
- Erickso MC, Thed ST. Int J Food Sci Tech. 1994;29:581–585. [Google Scholar]
- Gao W, Liu YJ, Tian LX, Mai KS, Liang GY, Yang HJ, Huai MY, Luo WJ. Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngdon idella) Aquacult Nutr. 2009;16:327–333. doi: 10.1111/j.1365-2095.2009.00668.x. [DOI] [Google Scholar]
- Garling DL, Wilson RP. Effect of carbohydrate-to-lipid ratio on growth and body composition of fingerling channel catfish. Prog Fish-Cult. 1977;39:43–47. doi: 10.1577/1548-8659(1977)39[43:EODCRO]2.0.CO;2. [DOI] [Google Scholar]
- Hasegawa H. Laboratory manual on analytical methods and procedures for fish and fish products. Singapore: Marine Fisheries Research Department Center; 1987. [Google Scholar]
- Hennigar CJ, Buck EM, Hultin HO, Peleg M, Vareltzis K. Mechanical properties of fish and beef gels prepared with and without washing and sodium chloride. J Food Qual. 1989;12:155–166. doi: 10.1111/j.1745-4557.1989.tb00318.x. [DOI] [Google Scholar]
- Hernandez MD, Lopez MB, Alvarez A, Ferrnandini E, Garcia Garcia B, Garrido MD. Sensory, physical, chemical and microbiological changes in aquacultured meager (Argyrosomu sregius) fillets during ice storage. J Food Chem. 2009;114(1):237–245. doi: 10.1016/j.foodchem.2008.09.045. [DOI] [Google Scholar]
- Hilton JW, Atkinson JL. Response of rainbow trout to increased levels of available carbohydrate in practical trout diets. Br J Nutr. 1982;47:597–607. doi: 10.1079/BJN19820071. [DOI] [PubMed] [Google Scholar]
- Hiltz DF, Dyer WJ. Octopin in adductor muscle of the sea scallop (Placopectenma gellanicus) J Fish Res Bd Can. 1971;28:869. doi: 10.1139/f71-127. [DOI] [Google Scholar]
- Hu YH, Liu YJ, Tian LX, Yang HG, Liang GY, Gao W. Optimal dietary carbohydrate to lipid ratio for juvenile yellowfinseabream (Sparu slatus) Aquacult Nutr. 2007;13:291–297. doi: 10.1111/j.1365-2095.2007.00476.x. [DOI] [Google Scholar]
- Hung SSO, Deng DF. Sturgeon, Acipenser spp. In: Webster CD, Lim C, editors. Nutrition requirement and feeding of finfish for aquaculture. New York: CABI; 2002. pp. 344–357. [Google Scholar]
- Huss HH (1988) Fresh fish quality quality changes. Training manual rom: United Nations. Food and agriculture organization and Danish international development agency: FAO/DANIDA
- Izgi S, Ciftcioglu G. Investigations on the shelf life of trout packed under modified atmosphere. Istanbul Universitesi Veterinerlik Fakultesi Dergisi. 1997;23:231–254. [Google Scholar]
- Kerr M, Lawicki P, Aguirre S, Rayner C. Effect of storage conditions on histamine formation in fresh and canned Tuna. Werribee: State Chemistry Laboratory- Food Safety Unit, Department of Human Service; 2002. pp. 5–20. [Google Scholar]
- Kirk RS, Sawyer R. Pearson’s composition and analysis of foods. 9. London: Longman Sci Tech Engl; 1991. pp. 607–617. [Google Scholar]
- Lakshmanan PT (2000) Fish spoilage and quality assessment. In: Lyre TSG, Kandoran MK, Thomas M, Mathew PT (eds) quality assurance in seafood processing, Cochin: society fisher techno (india), pp 26–40
- Lee DJ, Putnam GB. The response of rainbow trout to varying protein/energy ratioin a test diet. Aquacult Nutr. 1973;103:916–922. doi: 10.1093/jn/103.6.916. [DOI] [PubMed] [Google Scholar]
- Lie Q. Flesh quality—the role of nutrition. Aquac Res. 2001;32:341–348. doi: 10.1046/j.1355-557x.2001.00026.x. [DOI] [Google Scholar]
- Lin C, Lin C. Enhancement of the storage quality of frozen Bonito fillets by glazing with tea extracts. Food Control. 2005;16:169–175. doi: 10.1016/j.foodcont.2004.01.007. [DOI] [Google Scholar]
- Losada V, Barros-Velazquez J, Gallardo JM, Aubourge SP. Effect of advanced chilling methods on lipid damage during sardine (sardina pilchardus) storage. Eur J Lipids Sci Technol. 2004;106:844–850. doi: 10.1002/ejlt.200400991. [DOI] [Google Scholar]
- Lugasi A, Losada V, Hovari J, Lebovisc V, Jakoczi I, Aubourg S. Effect of pre-soaking whole pelagic fish in a plant extract on sensory and biochemical changes during subsequent frozen storage. LWT. 2007;40:930–936. doi: 10.1016/j.lwt.2005.09.021. [DOI] [Google Scholar]
- Mai J, Kinsella JE. Composition and lipids and proteins of deboned minced and fillet white sucker (Catostomus commersoni) J Food Biochem. 1980;3:229–239. doi: 10.1111/j.1745-4514.1980.tb00779.x. [DOI] [Google Scholar]
- Ng WK, Bahurmiz OM. The impact of dietary oil source and frozen storage on the physical, chemical and sensorial quality of fillets from market-size red hybrid tilapia, Oreochromis sp. J Food Chem. 2009;113:1041–1048. doi: 10.1016/j.foodchem.2008.08.060. [DOI] [Google Scholar]
- Nielsen J, Boknaes N, Jessen K (1994) In: WEFTA (ed) quality index method for frozen fish. Proceeding of 24th annual meeting of western europen fish technologists association, September 1994, Nantes, France pp 60–62
- Olafsdottir G, Nesvadba P, Natale C, Careche M, Oehlenschlager J, Tryggvadottir SV. Multisensors for fish quality determination. Trends Food Sci Tech. 2004;15:86–93. doi: 10.1016/j.tifs.2003.08.006. [DOI] [Google Scholar]
- Parkin KL, Brown WD. Modified atmosphere storage of Dungeness crab (Cancer magister) J Food Sci. 1983;48:370. doi: 10.1111/j.1365-2621.1983.tb10745.x. [DOI] [Google Scholar]
- Pirini M, Gatta PP, Testi S, Trigari G, Monetti PG. Effect of refrigerated storage on muscle lipid quality of sea bass (Dicentrachus labrax) fed on diets containing different levels of vitamin E. J Food Chem. 2004;68:289–293. doi: 10.1016/S0308-8146(99)00190-9. [DOI] [Google Scholar]
- Reddy GVS, Srikar LN, Sudhakara NS. Int J Food Sci Tech. 1992;27:271–276. doi: 10.1111/j.1365-2621.1992.tb02028.x. [DOI] [Google Scholar]
- Refsgaard H, Brochoff P, Jensen B. Free polyunsaturated fatty acids cause taste deterioration of salmon during frozen storage. J Agric Food Chem. 2000;48:3280–3285. doi: 10.1021/jf000021c. [DOI] [PubMed] [Google Scholar]
- Regost C, Arzel J, Cardinal M, Laroche M, Kaushik SJ. Fat deposition and flesh quality in seawater reared, triploid brown trout (Salmo trutta) as affected by dietary fat levels and starvation. Aquaculture. 2001;193:325–345. doi: 10.1016/S0044-8486(00)00498-1. [DOI] [Google Scholar]
- Regost C, Jv J, Roar AMB. Flesh quality of raw and smoked fillets of Atlantic salmon as influenced by dietary oil sources and frozen storage. Food Res Int. 2004;37:259–271. doi: 10.1016/j.foodres.2003.12.003. [DOI] [Google Scholar]
- Reinitz G, Hitzel F. Formulation of practical diets for rainbow trout based on desired performance and body composition. Aquaculture. 1980;19:243–252. doi: 10.1016/0044-8486(80)90048-4. [DOI] [Google Scholar]
- Roldan HA, Roura SI, Montecchia CL, Borla OP, Crupkin M. Lipid changes in frozen stored fillets from pier and post spawned hake (merluccius hubbsi marini) J Food Biochem. 2005;29:187–204. doi: 10.1111/j.1745-4514.2005.00006.x. [DOI] [Google Scholar]
- Rostamzad H, Shabanpour B, Shabani A, Shahiri H. Enhancement of storage quality of frozen Persian sturgeon fillets by using of ascorbic acid. Int Food Res J. 2011;18:109–116. [Google Scholar]
- Saeed S, Howell NK. Effect of lipid oxidation and frozen storage on muscle of Atlantic mackerel (scomber scombrus) J Sci Food Agric. 2002;82:579–586. doi: 10.1002/jsfa.1080. [DOI] [Google Scholar]
- Sanchez-Alonso I, Barroso M, Careche M (2010) Instrumental texture. In: Handbook of seafood and seafood products analysis. CRC Press, p 430
- Selmi S, Sadok S. The effect of natural antioxidant (thymus vulgaris Linnaeus) on flesh quality of tuna (thunnus thynnus (Linnaeus)) during chilled storage. Pan-Am J Aquat Sci. 2008;3(1):36–45. [Google Scholar]
- Serrano JA, Nematipour GR, Galtin DM. Dietary protein requirement of the red drum (Scianops ocellats) and relative use of dietary carbohydrate and lipid. Aquaculture. 1992;101:283–291. doi: 10.1016/0044-8486(92)90031-F. [DOI] [Google Scholar]
- Shewfelt RL. Fish muscle lipolysis: a review. J Food Biochem. 1981;5:79–100. doi: 10.1111/j.1745-4514.1981.tb00663.x. [DOI] [Google Scholar]
- Simeonidou A, Govaris A, Vareltzis K. Effect of frozen storage on the quality of whole fish and fillets of horse mackerel (Trachuru strachurus) and Mediterranean hake (merluccius meditrraneus) Z Lebensmuntersforscha. 1997;204:405–410. [Google Scholar]
- Stephan G, Guillaume J, Lamoure F. Lipid peroxidation in turbot (scophthalmus maximus) tissue: effect of dietary vitamin e and dietary n-6 or n-3 polyunsaturated fatty acids. Aquaculture. 1995;130:251–268. doi: 10.1016/0044-8486(94)00322-F. [DOI] [Google Scholar]
- Stephen NM, Jeya Shakila R, Jeyasekaran G, Sukumar D. Effect of different types of heat processing on chemical change in tuna. J Food Sci Technol. 2010;47(2):174–181. doi: 10.1007/s13197-010-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe S, Kamal M, Hashimoto K. Post mortem changes in ATP, creatinine phosphate, and lactate in sardine muscle. J Food Sci. 1991;56:151–153. doi: 10.1111/j.1365-2621.1991.tb07998.x. [DOI] [Google Scholar]
- Woywoda AD, Shaw SJ, Ke PJ, Burns BG. Recommended laboratory methods for assessment of fish quality. Can Tech Rep Fish Aquat Sci. 1986;1448:73–82. [Google Scholar]
- Zmijewski T, Kujawa R, Jankowska B, Kwiatkowska A, Mamcarz A. Slaughter yield proximate and fatty acid composition and sensory properties of rapfen (Aspius aspius L.) with tissue of bream (Abramis brama L.) and pike (Exox lucius L.) J Food Composit Anal. 2006;19:176–181. doi: 10.1016/j.jfca.2005.03.006. [DOI] [Google Scholar]


