Abstract
Quality improvement of cereal brans, a health promoting ingredient for functional foods is the emerging research concept due to their low shelf stability and presence of non-nutrient components. A study was conducted to evaluate the storage quality of processed milling industry byproducts so that these can be potentially utilized as a dietary fibre source. Different cereal brans (wheat, rice, barley and oat) were processed by dry, wet, microwave heating, extrusion cooking and chemical methods at variable conditions. Processed brans were stored in high density polyethylene (HDPE) pouches at ambient and refrigeration temperature. Quality assessments (moisture, free fatty acids, water activity and physical quality) of brans were done up to six months, at one month intervals. Free fatty acid content, moisture and water activity of the cereal brans remained stable during the entire storage period. Among treatments, extrusion processing is the most effective for stability. Processing treatments and storage temperature have the positive effect on extending the shelf life of all cereal brans. Therefore, processed cereal brans can be used as a dietary fortificant for the development of value added food products.
Keywords: Storage stability, Cereal brans, Dietary fibre, Quality assessment
Introduction
Dietary fiber is well recognized as one of the important dietary substances needed for a good health (Lee et al. 2004). Dietary fiber is an important constituent due to its functional properties. It can be used in the formulations of foods, resulting in texture modification and enhancement of food stability during production and storage. The hydration properties of dietary fiber which determine its optimum level of use in foods are particularly important because they play a role in retaining a desirable texture (Thebaudin et al. 1997). The term “bran” is applied to a range of products derived from cereal grains and usually is related to the outer layers of the grain (Lebesi and Tzia 2011). Most cereal bran products are characterized by high levels of insoluble fiber, ash, vitamins, lipids, and pigments. Brans are generally composed mainly of insoluble cellulose and hemicelluloses, with only about 5% soluble fibre and have little hypercholesterolemia effect (Kay and Truswell 1980). Bran contributes a pleasing sweet, nutty flavour when added as a flavour enhancer in baked products and pasta. Rice bran is rich in protein, lipids, fiber, minerals (Mg, K, Fe and Mn), B-vitamins, and an excellent source of choline and inositol (Hoffpauer et al. 2005). Barley contains a high level of soluble dietary fibre, making it a desirable food component for health benefits.
A major obstacle in using brans is that it rapidly deteriorates, thus it is necessary to find a good method for preventing the rapid deterioration of brans and to ensure quality material for further processing. Rice bran must be stabilized immediately after milling, by minimizing its free fatty acids (FFA) content to extend the shelf life. It can be done by inactivating lipase and peroxidase enzymes using different heat treatment. Depending on the type of heat treatment, the lipase may be either reversibly inhibited or permanently denatured. The most effective classical methods include, dry heat, moist heat, moist heat stabilization. Other methods include the use of chemicals, such as hydrochloric acid, acetic acid, calcium hydroxide and stabilization by microwave and extrusion. The best stabilization method of rice bran at commercial level is heat treatment at 60 °C for 15 min (Malekian et al. 2000).
Most of the processes involve dry or moist heat treatment. The use of microwave energy is an important new means of heating and is forecast to be used to a greater extent in the future. Microwave heating is efficient, economically superior, and shorter in processing time and has little effect on the original color of the brans. It has little effect on nutritional quality (proximate analysis) and functional property (water and fat absorption capacity, emulsification and foaming) of rice bran (Malekian et al. 2000).
Considering the nutritional and food potential and associated health benefits of brans the primary goal of this investigation was to determine effective methods to minimize the rancidity of brans during storage, by using different heat treatment.
Materials and Methods
Raw materials
Wheat bran was collected from Ludhiana Flour Mill, Ludhiana, India. Rice bran was purchased from ‘Ricela Health Foods Ltd’., Dhuri, Punjab, India. Barley was purchased from the local market and its bran was prepared by milling of barley in a grain pearler (Central Institute of Agricultural Engineering (CIAE), Bhopal, India). Oat bran (Baggry’s India Ltd., New Delhi, India)
Physico-chemical composition of raw materials
Approved methods (AACC 2000) were used to determine ash (method 08–01) and protein (N X 5.7) for durum wheat semolina (N X 6.25) (method 46–10.01) of the cereal brans. Fat content was determined using a 16-hr soxhlet extraction with petroleum ether (AACC 2000). Dietary fiber {acid detergent fiber (ADF), neutral detergent fiber (NDF), Cellulose and Lignins} were estimated by extracting with neutral detergent solution as described by Robertson and Van Soest (1981). The crude fiber of the raw materials was estimated using a Fibertec (FOSS). Per cent carbohydrates were determined as (100–per cent estimated proximate components).
Functional characteristics
The bulk density of the cereal brans was determined by the method of Egan et al. (1981). Water absorption capacities of cereal brans were measured by the method reported by Sosulski (1962). Two grammes of the a cereal bran was dispersed in 20 ml of water in a 50 ml centrifuge tube and the mixture allowed to stand for 15 min at 25 °C and then centrifuge at 3200 rpm for 15 min. The amount of water retained by the solids was measured. The fat absorption of cereal brans was estimated by mixing 2 g sample with 20 ml refined oil allowing the mixture to stand for 15 min at room temperature and then centrifuging at 3200 rpm for 15 min. The fat retained by the solids was measured by the method of Lin et al. (1974). Color analysis was done using a Hunter Lab Colorimeter, MiniScan XE Plus (Hunter Lab, Reston, Virginia). Color readings were expressed as Hunter values for L, a, and b. L values measure black to white (0–100); + a = red, - a = green; + b = yellow; - b = blue. L, a and b color difference was expressed as a single value, ΔE. the larger the ΔE value, larger the color difference.
Storage studies
Treatments were done after conducting extensive trials. The treatments was used [wet heating at 115 °C for 10 and 15 min., dry heating at 110 °C for 25 min., microwave heating at 2450 MHz for 2.5 min., extrusion cooking at 140 °C with 20% moisture content and chemical treatment (1% acetic acid solution @ 22%)]. These samples were further used for storage studies. Processed cereal brans were packed in heat sealed high density polyethylene packs [200 gauges (74 microns)] and stored at ambient temperature (30–35 °C) and refrigeration conditions (5–8 °C) for shelf life study. Subsequent quality analysis was as follows:
Moisture content
Weighed samples (2 g) were dried in a hot air oven at 130 ± 1 °C for 1 hr. and moisture content in percent was calculated from loss of weight (AACC 2000).
Free fatty acids
Standard AOAC procedure (Anon 2001) was followed. Exactly 1 g ground sample was placed in flask. 50 ml benzene was added and then allowed to stand for 30 min. for extraction of free fatty acids. Exactly 1 ml extract was taken in flask to which 5 ml. benzene, 10 ml alcohol and phenolphthalein as indicator was added and titrated against 0.02 N KOH until a light pink color disappeared.
Water activity
The water activity of enriched pasta was estimated using a water activity meter, which a HygroLab 3 bench-top indicator (Rotronic company, Model No: 60266709).
Statistical analysis
Experiments was carried out in triplicate and data was analyzed with the help of factorial designs (Singh et al. 1998).
Results and discussion
Analysis of raw materials
Proximate composition of the raw materials used is presented in Table 1. The protein content of cereal brans ranged from 9.6–15.0 %. Rice and barley brans had 11.9 and 11.9% protein respectively. The lipid content was highest in rice bran and it ranged from 4.1–19.3%. Rice bran is a concentrated source of dietary fibre (38.9%) among all cereal brans. Barley bran had the highest value of crude fibre (14.9%). The highest ash content (6.72%) was observed in rice bran. Similar results have been reported by Khan et al. (2009) for defatted rice bran samples on dry weight basis.
Table 1.
Proximate composition of different cereal brans#
| Type of bran | Crude protein (%) | Ash (%) | Fat (%) | Dietary fiber (%) | Crude fiber (%) |
|---|---|---|---|---|---|
| Full fatted | |||||
| Wheat bran | 9.6 | 4.06 | 4.1 | 33.4 | 7.75 |
| Rice bran | 11.8 | 6.72 | 19.3 | 38.9 | 11.5 |
| Barley bran | 11.9 | 3.07 | 4.2 | 22.6 | 14.9 |
| Oat bran | 15.0 | 1.45 | 10.6 | 14.0 | 3.31 |
| LSD (p ≤ 0.05) | 0.07 | 0.24 | 0.12 | 0.18 | 0.05 |
#Expressed at 14% moisture level
Values are means of three replications
Functional characteristics
The functional characteristics of cereal brans are summarized in Table 2. Bulk density of brans ranged between 0.25 g/ml to 0.59 g/ml. Significant variability was observed with respect to water absorption of cereal brans. Water absorption of brans varied from 148.4 to 383.7%. Wheat bran had the highest water absorption (383.7 g/100 g). Among the type of brans, wheat bran exhibited a different pattern for water and fat absorption. James and Sloan (1984) reported that the water absorption of edible stabilized rice bran was in the range of 182.2–213.1%. The variation in water absorption may be attributed to the source of bran and their processing conditions (Sharma et al. 2004). The hydrophobic nature of fat might have contributed to the reduced water absorption of full-fat brans, while the hydrophyllic nature of crude fibre might have contributed to the increased water absorption in the defatted bran samples (Sekhon et al. 1997). A similar pattern was observed for fat absorption of different cereal brans.
Table 2.
Functional characteristics of different full fatted cereal brans#
| Type of bran | Bulk density (g/ml) | Water absorption (%) | Fat absorption (%) | Colour | Water activity (aw) | Free fatty acids (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | ||||||
| Wheat bran | 0.37 | 383.7 | 302.9 | 58.9 | 3.57 | 15.8 | 61.1 | 0.82 | 7.12 |
| Rice bran | 0.36 | 148.4 | 139.2 | 59.0 | 3.45 | 15.1 | 61.0 | 0.50 | 13.7 |
| Barley bran | 0.25 | 324.1 | 268.6 | 57.2 | 3.91 | 14.8 | 59.2 | 0.50 | 9.14 |
| Oat bran | 0.59 | 115.2 | 138.3 | 52.8 | 3.86 | 15.3 | 55.1 | 0.48 | 5.20 |
| LSD (p ≤ 0.05) | 0.06 | 1.87 | 1.85 | 1.90 | 0.06 | 0.59 | 1.99 | 0.11 | 0.13 |
#Expressed at 14% moisture level
Values are means of three replications
Lightness values (Hunter L values) for wheat, rice, barley and oat brans were 58.9, 59.0, 57.2 and 52.8 respectively. Barley bran had more redness followed by oat bran, wheat bran and rice bran. The b* values (yellowness) was highest in wheat bran (15.8) and lowest in barley bran (14.8). Similar trend in color were reported by Fernandez-Artigas et al. (1999) in different cereal flours. Maximum value of ΔE (61.09) was observed in wheat bran and minimum value of ΔE (55.11) was recorded in oat bran.
Significant variation was noticed in water activity content. Water activity ranged from 0.48–0.82 for all cereal brans. The extent of free fatty acids in different cereal brans varied significantly (5.2–13.7%). The rice bran exhibited significantly higher values for free fatty acids as compared to other brans. This has been attributed to the presence of active inherent lipase system of rice bran, which hydrolyses the oil to free fatty acids and glycerol and renders bran unfit for oil extraction and human consumption (James and Sloan 1984).
Moisture content
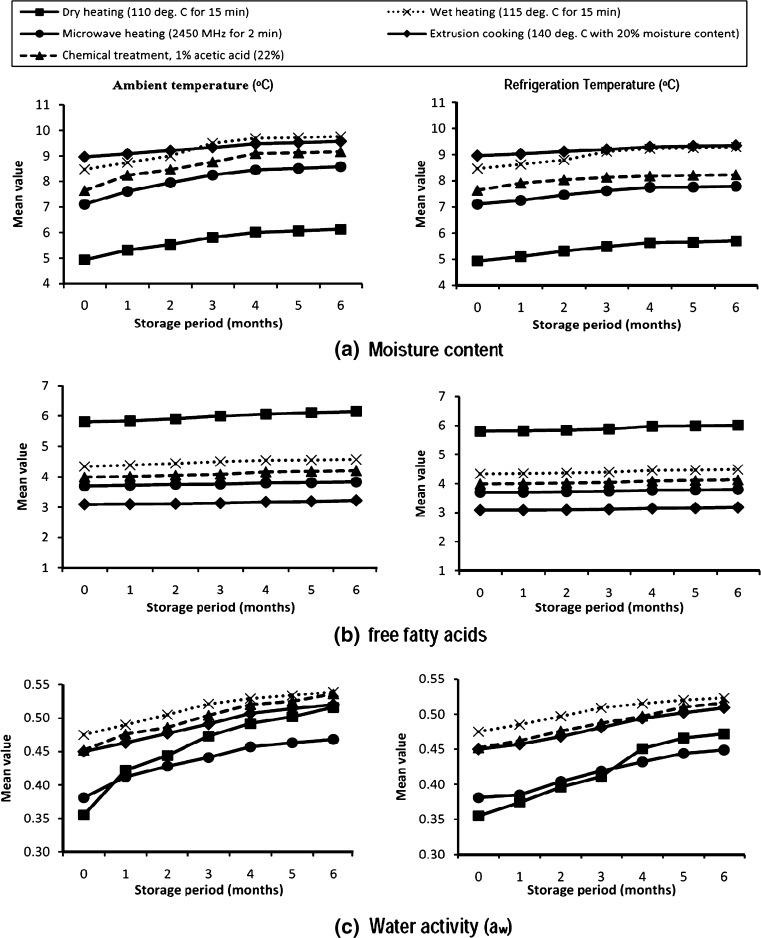
Figure 1(a) shows the effect of storage period and temperature on the moisture content of cereal brans. Moisture content significantly increased during storage period. Brans subjected to different dry processing treatments were found to vary significantly in the moisture content and the mean values of moisture content recorded were 5.54, 7.80, 8.35, 9.12 and 9.25 for dry, microwave, chemical, wet and extrusion treatment, respectively. However, as depicted in Fig. 1(a) temperature was found to have a pronounced effect on the moisture content. The mean moisture content for ambient stored brans were 8.19% while in the case of bran stored under refrigeration conditions the mean moisture content was 7.83%.
Fig. 1.
Effect of temperature, treatment and storage period on (a) moisture content, (b) free fatty acid and (c) water activity (aw) of the processed cereal brans
Storage period had significant impact on the moisture content. The means moisture at the beginning of storage was 7.42%, which increased significantly to 8.36% after six month of storage. The above observations indicated that on the average, there was a moisture gain of 12.51% during six months of progressive storage of brans. Moisture gain during storage is due to hygroscopic properties of brans. The brans packed in HDPE at refrigeration storage recorded lesser moisture uptake during storage than ambient stored brans which might have been due to low temperature conditions. According to Sharma et al. (2004), bran may absorb some moisture during storage, thereby favoring lipolytic activity. Talab et al. (2002) reported that heat treatment, time and temperature method of packaging and storage had significant effect on the bran moisture content. Priyankara and Weerathilake (2009) reported that moisture content of rice bran during microwave heated Poly bag was 6.77%, which was more as compared to microwave heated-vaccum packed rice bran (4.45%). Butt et al. (2009) reported that increase in moisture content of flour was observed during a storage period of 45 days due to high relative humidity and hygroscopic properties of flour.
The results as explained above suggest that refrigeration storage is better suited for storage of brans so as to minimize the moisture gain during storage. The interaction of temperature and storage was observed to be significant (p ≤ 0.05) for the moisture content of brans.
Free fatty acids
The effect of treatment, temperature and length of storage significantly affected the free fatty acid content of the brans (Fig. 1(b)). Different processing treatments (dry, wet, microwave, extrusion and chemical) were found to have a significant effect on the free fatty acid content of cereal brans. The mean free fatty acids (FFA) in the brans subjected to extrusion cooking, microwave heating, chemical treatment, wet and dry heating was 3.14, 3.76, 4.08 and 4.44 and 5.94%, respectively, representing the maximum effect of HTST on inactivation of enzyme.
Storage temperature was found to have a salient effect on the free fatty acid content of brans. Not much variation was observed in free fatty acid content of brans stored at ambient temperature as compared to refrigerated brans. The free fatty acid content (4.29%) of ambient stored brans was slightly higher as compared to the refrigerated stored brans (4.25%). Slight increase in per cent FFA was recorded with the lengthening of storage period. The mean free fatty acid content increased from 4.19% at zero days to 4.36% at the end of six months.
Again it is obvious from Fig. 1 (b) that the temperature and treatment individually had a significant effect on the FFA content of brans.
Similar results were reported by Barber and Benedito-de (1980), Sayre et al. (1982) and Randall et al. (1985). According to Sharma et al. (2004) stabilization methods and storage period significantly affected the rate of production of free fatty acids. The dry heat method of stabilization restricted the development of FFA up to 30 days of storage and therefore a significant increase was observed. This increase in the FFA content of bran may be attributed to the presence of residual lipolytic activity of enzyme lipase, which increased under favorable conditions during storage. Malekian et al. (1999) showed that the FFA content in untreated bran stored in Ziploc bags at 4 °C for six weeks stored under identical conditions. According to Lakkakula et al. (2004) microwave oven which was preheated for 3 min prior to loading the rice bran (21% moisture content) obtained an increased in FFA during six week storage at 25 °C. During extrusion stabilization, the free fatty acid content of rice bran did not increase significantly even at 60 days of storage, indicating that the extrusion processing of rice bran was more effective in destroying the lipolytic system of bran than the dry heat method (Sharma et al. 2004). The capability of the extruder to inactivate inactivating the fat hydrolyzing enzymes of rice bran has also been reported earlier by Randall et al. (1985) and Martin et al. (1993).
There was no significant increase in free fatty acid content of chemically treated bran during the entire storage. Prabhakar and Venkatesh (1986) reported similar results working with acid treated brans. According to Kaur et al. (2011) free fatty acids (%) for enriched pasta increased with storage period, but the values remained in the acceptable range because of which, the taste, flavour and acceptability of pasta was not much affected. Rice bran enriched pasta supplemented at 15 per cent level had maximum values of free fatty acids (%).This may be contributed to the fact that rice bran contained maximum amount of fat per centage than other cereal brans.
Water activity
Figure 1(c) shows the effect of storage, time, temperature, storage period on the water activity of cereal brans. The processing treatments manifested an inconsequential effect on the water activity of the cereal brans. However, storage temperature substantially affected the water activity of cereal brans. The mean water activity observed for brans stored at refrigeration storage was 0.461. This was significantly lower than the water activity of brans stored at ambient storage. The water activity followed the same trend as that of moisture content during the entire storage period. Similarly, during storage a sequential increase in the mean water activity was from initial 0.422 to 0.505 after six months of storage. The increase in water activity perceived during storage is explainable by the increase in moisture content as the storage period progressed. Manthey et al. (2008) reported that during storage, water activity was similar for both traditional pasta and pasta containing Flaxseed flour.
Conclusion
The treatments (heat and chemical) resulted in shelf life extension. The improvement in shelf life during storage was higher for brans stabilized by extrusion technology. Temperature has significant effect on controlling rancidity level of brans during storage. Stabilized brans have excellent keeping quality if stored under proper conditions. Selection of suitable treatment and careful storage of brans would minimize problems of rancidity.
Acknowledgement
The financial support from the Council of Scientific and Industrial Research (CSIR) New Delhi is gratefully acknowledged.
Abbreviations
- AACC
American Association of cereal chemists
- AOAC
Association of Official Analytical Chemists
- ADF
Acid detergent fiber
- NDF
Neutral detergent fiber
- FFA
free fatty acid
- HDPE
High density polyethylene
- g/ml
gram per milliliter
Contributor Information
Savita Sharma, Email: savitasharmans@yahoo.co.in.
Satinder Kaur, Email: satinderpau@yahoo.co.in.
B. N. Dar, Email: darnabi@gmail.com
Baljit Singh, Email: baljitsj@yahoo.co.in.
References
- Approved methods of american association of cereal chemists. 10. Paul: The Association St; 2000. [Google Scholar]
- Official methods of analysis. 16. Washington: The Association of official analytical chemists; 2001. [Google Scholar]
- Barber S, Benedito-de BC. Rice bran: chemistry and technology. In: Luh DS, editor. Rice production and utilization. Westport: AVI Pub. Co.; 1980. [Google Scholar]
- Butt MS, Nasir M, Akhtar S, Sharif K. Effect of moisture and packaging on the shelf life of wheat flour. Internet J Food Safety. 2009;4:1–6. [Google Scholar]
- Egan H, Kirk RS, Sawyer R (1981) Pearson’s chemical analysis of foods, 8th edn. Churchill Livingstone, Logman Inc., New York, USA.
- Fernandez-Artigas P, Guerra-Hernandez E, Garcia-Villanova B. Browning indicators in model system and baby cereals. J Agric Food Chem. 1999;47:2872–2878. doi: 10.1021/jf9808729. [DOI] [PubMed] [Google Scholar]
- Hoffpauer DW, Light Heart LCC, Crowley LA. New application for whole rice bran. Cereal Foods World. 2005;50:173–174. [Google Scholar]
- James C, Sloan S. Functional properties of rice bran in model system. J Food Sci. 1984;49:310–311. doi: 10.1111/j.1365-2621.1984.tb13741.x. [DOI] [Google Scholar]
- Kaur G, Sharma S, Nagi HPS, Dar BN (2011) Functional properties of pasta enriched with variable cereal brans. J Food Sci Technol. doi:10.1007/s13197-011-0294-3 [DOI] [PMC free article] [PubMed]
- Kay RM, Truswell AS. Dietary fibre: effects on plasma and binary lipids in man. In: Spiller GA, Kay RM, editors. Medical aspects of dietary fibre. New York: Plenum Press; 1980. p. 183. [Google Scholar]
- Khan SM, Butt MS, Anjum FM, Jamil A. Antinutritional appraisal and protein extraction from differently stabilized rice bran. Pakistan J Nutr. 2009;8:1281–1286. doi: 10.3923/pjn.2009.1281.1286. [DOI] [Google Scholar]
- Lakkakula NR, Lima M, Walker T. Rice bran stabilization and rice bran oil extraction using Ohmic heating. Bioresour Technol. 2004;92:157–161. doi: 10.1016/j.biortech.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Lebesi DM, Tzia C. Effect of the addition of different dietary fiber and edible cereal bran sources on the baking and sensory characteristics of cupcakes. Food Bioprocess Technol. 2011;4(5):710–722. doi: 10.1007/s11947-009-0181-3. [DOI] [Google Scholar]
- Lee S, Inglett GE, Carriere CJ. Effect of Nutrim oat bran and flaxseed on rheological properties of cakes. Cereal Chem. 2004;81:637–642. doi: 10.1094/CCHEM.2004.81.5.637. [DOI] [Google Scholar]
- Lin MJ, Humbert ES, Sosuliski FW. Certain functional properties of sunflower meal products. J Food Sci. 1974;39:368. doi: 10.1111/j.1365-2621.1974.tb02896.x. [DOI] [Google Scholar]
- Malekian F, Rao RM, Priyawiwatkul W, Marshall WE, Windhauser M, Ahmedna M (1999) Lipase and lipoxygenase activity, functionality, and nutrient losses in rice bran during storage. Bulletin number 870, LSU Agcenter, Baton Rouge, LA 70803.
- Malekian F, Rao RM, Priyawiwatkul W, Marshall WE, Windhauser M and Ahmedna M (2000) Lipase and lipoxygenase activity, functionality, and nutrient losses in rice bran during storage. Bulletin Number 820, Louisiana Agricultural Experimental Station Deparment of Food Science, Louisiana State University Baton Rouge, LA, 70803, pp. 4–40.
- Manthey FA, Sinha S, Wolf Hall CE, Hall CA. Effect of flaxseed flour and packaging on shelf life of refrigerated pasta. J Food Proc Preserv. 2008;32:75–87. doi: 10.1111/j.1745-4549.2007.00166.x. [DOI] [Google Scholar]
- Prabhakar JV, Venkatesh KVL. A simple chemical method for stabilization of rice bran. J Am Oil Chem Soc. 1986;63:644–646. doi: 10.1007/BF02638229. [DOI] [Google Scholar]
- Priyankara SKA, Weerathilake WADV (2009) Effect of vaccum and polybag packing on simple and microwave heat treated parboiled rice bran. J Anim Sci 9–16. Number 1253105818.
- Randall JM, Sayre RN, Schultz WG, Fong RY, Mossman AP, Tribelhorn RE, Saunders RM. Rice bran stabilization by Extrusion Cooking for extraction of edible oil. J Food Sci. 1985;50:361–365. doi: 10.1111/j.1365-2621.1985.tb13402.x. [DOI] [Google Scholar]
- Robertson JB, Van Soest PJ. The detergent system of analysis and its application to human foods. In: James WPT, Thrander O, editors. The analysis of dietary fiber. New York: Marcell Dekker; 1981. pp. 123–158. [Google Scholar]
- Sayre RN, Saunders RM, Enochian RV, Schultz WG, Beagle EC. Review of rice bran stabilization systems with emphasis on extrusion cooking. Cereal Foods World. 1982;27:317–322. [Google Scholar]
- Sekhon KS, Dhillon SS, Singh N, Singh B. Functional suitability of commercially milled rice bran in India for use in different food products. Plant Food Hum Nutr. 1997;50:127–140. doi: 10.1007/BF02436032. [DOI] [PubMed] [Google Scholar]
- Sharma HR, Chauhan GS, Agarwal K. Physico-chemical characteristics of rice bran processed by dry heating and extrusion cooking. Int J Food Prop. 2004;7:603–614. doi: 10.1081/JFP-200033047. [DOI] [Google Scholar]
- Singh S, Bansal ML, Singh TP, Kumar R. Statistical methods for research workers. New Delhi: Kalyani Publishers; 1998. [Google Scholar]
- Sosulski FW. The centrifuge method for the flour water absorption in hard red spring wheat. Cereal Chem. 1962;39:344–350. [Google Scholar]
- Talab KT, Shabedi M, Shokrani R, Dokhani S. Stabilization of rice bran oil by inactivation of its lipase and lipoxidase. Iran J Agric Sci. 2002;33:593–603. [Google Scholar]
- Thebaudin JY, Lefebvre AC, Harrington M, Bourgeois CM. Dietary fibers: nutritional and technological interest. Trends Food Sci Technol. 1997;8:41–48. doi: 10.1016/S0924-2244(97)01007-8. [DOI] [Google Scholar]