Abstract
Guar gum is a novel agrochemical processed from endosperm of cluster bean. It is largely used in the form of guar gum powder as an additive in food, pharmaceuticals, paper, textile, explosive, oil well drilling and cosmetics industry. Industrial applications of guar gum are possible because of its ability to form hydrogen bonding with water molecule. Thus, it is chiefly used as thickener and stabilizer. It is also beneficial in the control of many health problems like diabetes, bowel movements, heart disease and colon cancer. This article focuses on production, processing, composition, properties, food applications and health benefits of guar gum.
Keywords: Cholesterol, Cluster bean, Dietary fiber, Guar gum, Hydration rate, Viscosity
Introduction
Guar gum is derived from the seeds of the drought tolerant plant Cyamopsis tetragonoloba, a member of Leguminosae family (Whistler and Hymowitz 1979; Kay 1979; Prem et al. 2005). The common names used in the scientific literature for the bean, guar gum flour and the galactomannan fraction are Indian cluster bean, guar and guaran, respectively. There is lack of general consensus with regard to the origins of this plant (Whistler and Hymowitz 1979), although the concept of transdomestication was originally proposed by Hymowitz (1972). This hypothesis explained how the domesticated guar plant, C. tetragonoloba, developed from the drought-tolerant wild Africa species C. senegalensis. The latter species was originally taken from Africa to South Asian subcontinent by Arab traders as fodder for horses probably some time between the 9th and 13th centuries A.D. The domesticated species is normally associated with India and Pakistan, where the plant has been grown for centuries as food for both human and animals (Whistler and Hymowitz 1979). Guar gum industry developed in the 1940s and 1950s in United States (BeMiller 2009). Guar was brought into the United States before World War I primarily as a green manure but was not used in industrial applications until 1943 and probably it was the main reason why it has been studied to a limited scale. At that time, the supply of locust bean gum, which was widely used in the paper and textile industries and imported from Europe and North Africa had declined and was difficult to get. Therefore, the Institute of Paper Chemistry, Appleton, Wisconsin and United States Department of Agriculture made an effort to find a domestic plant that could provide a substitute for locust bean gum. This search led to the reexamination of guar gum and it was found to be the best solution of the problem. The commercial development was made at the University of Arizona during World War II. At the close of the war the gum was examined by Whistler (1948) at Purdue. He worked on the molecular structure and, in examining the properties of the pure polysaccharide, guaran, visualized its wide industrial potential and recommended development of the guar plant as a domestic crop for industry (Whistler 1948; Whistler and Hymowitz 1979). Studies had revealed that the gum is a valuable paper maker's adjunct in obtaining temporary wet strength in sheets, such as paper toweling and this gum facilitates hydration during the beating of various pulps. The services of the Soil Conservation Commission of the U. S. Department of Agriculture were enlisted and as a result, numerous seeds were investigated for their potentialities. The most satisfactory results were found from those of guar or Cyamopsis tetragonolobus, an annual drought-resistant leguminous plant, three to six feet tall, which has been cultivated in certain sections of India for centuries as fodder for cattle and horses.
Guar gum resembles locust bean gum in being composed essentially of the complex carbohydrate polymer of galactose and mannose, but with different proportions of these two sugars. It is reported that guar flour is of value as a beater additive for improving the strength of certain grades of paper. It has also been reported that guar possesses properties which might be useful in warp sizing, printing pastes, and in certain finishing operations. In order to obtain the gum it is necessary to separate the gum-containing endosperm of the seed from the outer and largely fibrous portions.
Consumption of guar gum rapidly increased but it was the development of anionic and cationic guar gum derivatives and their use in oil and gas well stimulation that gave guar gum its present commercial importance. In textile and carpet printing, guar gum thickens the dye solutions which allow more sharply printed patterns to be produced. Guar gum has been used in explosives for over 25 years as an additive to dynamite for water blocking. In recent years, it has become the primary gelling agent in water based slurry explosives. The production of paper is enhanced by an addition of small amounts of guar gum to the pulp. It serves as a fiber deflocculent and dry-strength additive.
Guar seed endosperm is a source of water soluble gum which is used as stabilizer, emulsifier and thickener in various food products and contributes to soluble dietary fiber (SDF) portion of seed total dietary fiber (TDF). TDF and SDF, respectively, made up 52–58% and 26–32% of seed dry weight (Kays et al. 2006). As a food additive, it emulsifies, binds water, prevents ice crystals in frozen products, moisturizes, thickens, stabilizes and suspends many liquid–solid systems. It is used in ice cream, sauces, cake mixes, cheese spreads, fruit drinks and dressings usually in amount of <1% of the food weight (Whistler and Hymowitz 1979; Parija et al. 2001).
Production
Guar gum is a gel-forming galactomannan obtained by grinding the endosperm portion of Cyamopsis tetragonolobus, a leguminous plant grown for centuries mainly in India and Pakistan where it is a most important crop that has long been used as food for humans and animals (Chandirami 1957). The guar plant is essentially a sun-loving plant, tolerant of high environmental temperatures but very susceptible to frost (Whistler and Hymowitz 1979; Kay 1979). For maximum growth the plant requires a soil temperature of 25–30 °C and ideally, a dry climate with sparse but regular rainfall. Guar plant requires rain for optimum growth before planting and again to induce maturation of seeds (Anderson, 1949). Excess of moisture during early phase of growth and after maturation of seeds results in lower quality guar beans (Heyne & Whistler 1948; Venkateswarlu et al. 1982). The rain pattern of the monsoons in the northern parts of India and Pakistan generally provides ideal growing conditions for guar. Almost 90% of world’s guar is grown in India and Pakistan. Unique requirement of right amount of rain at a particular time of growth and maturation makes this crop largely dependent on annual rainfall pattern and causes occasional wide swings in guar supply and prices. Guar is also cultivated in the southern hemisphere in semi-arid zones in Brazil, Australia, South Africa and Southern part of the USA like Texas or Arizona. The total production of guar seed in these countries is estimated at 15,000 MT annually. The agro-climatic conditions in Australia are also quite conducive to the cultivation of guar. Efforts have been made to promote cultivation of guar in Australia by the Department of Agriculture and Rural Industrial Development Agency. Similarly, it is reported that countries like China and Thailand are also trying to grow guar. Therefore, in future guar may not remain monopoly of India and Pakistan.
India accounts for 80% of the total guar produced in the world and 70% of it is cultivated in Rajasthan. India is the world leader for production of guar, which is grown in the northwestern parts of country encompassing states of Rajasthan, Gujrat, Haryana and Punjab. During 1970s guar was also grown regularly in the State of Uttar Pradesh (U.P.), Madhya Pradesh (M.P.) and Orissa. As the processing facilities have been closed down in U.P. and M.P., the cultivation in these states is negligible now. In Orissa too guar is not cultivated any more. The annual production of guar during last 3 years ranged from 11, 00,000 to 12, 87,000 MT.
In Pakistan, before 90s, about 80% of the guar was grown under irrigated conditions therefore per hectare yield was higher. During that period guar was grown in Punjab, Multan, Muzaffargarh, Mianwali and Sargoda. The other areas include Bahawalpur, Banawalnagar and Sind Province. The annual production of guar during this period ranged between 180,000 and 250,000 MT annually. List of Indian guar products importing countries is as given in Table 1.
Table 1.
Country wise export data of Indian guar gum from 2008 to 2010 (APEDA 2011)
| Country | Percent Export | ||
|---|---|---|---|
| 2007–08 | 2008–09 | 2009–10 | |
| United States | 38.7 | 37.6 | 32.9 |
| China | 16.2 | 15.2 | 11.6 |
| Germany | 8.5 | 8.7 | 9.4 |
| South Africa | 4.2 | 1.7 | 1.4 |
| Malaysia | 2.9 | 3.8 | 3.8 |
| Italy | 2.3 | 2.3 | 3.1 |
| Netherland | 2.1 | 1.5 | 1.5 |
| Australia | 1.8 | 2.5 | 1.8 |
| Russia | 1.8 | 2.6 | 2.8 |
| Vietnam | 1.4 | 4.1 | 3.7 |
| Others | 20.1 | 30.0 | 28.0 |
Processing
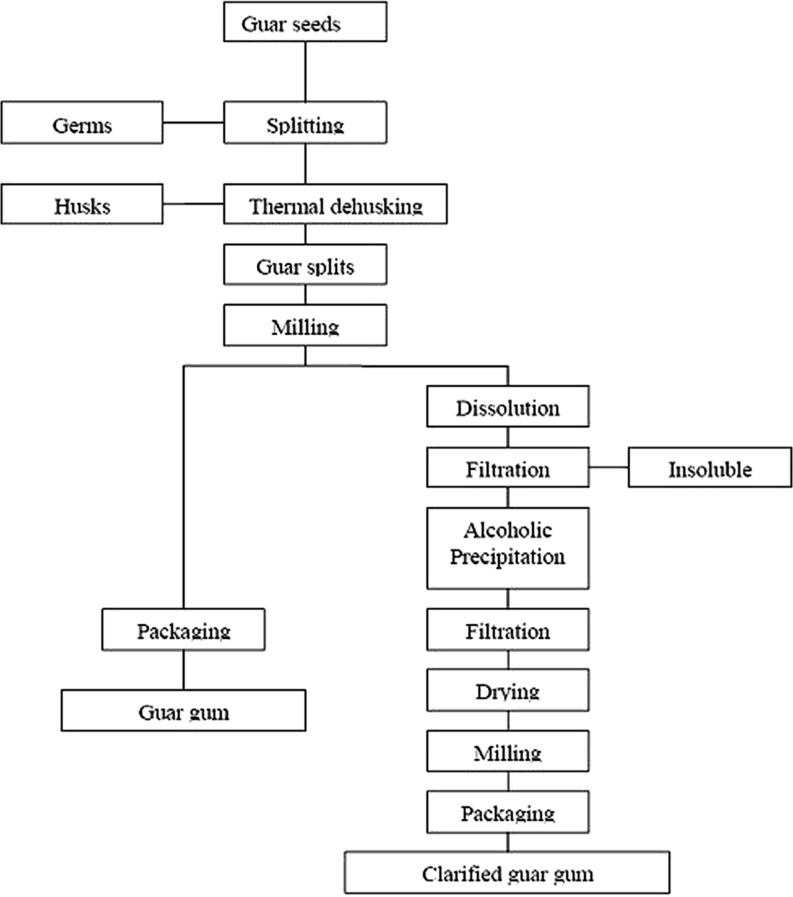
Guar gum processing varies from plant to plant. The general outline of the manufacturing process of guar gum is shown in Fig. 1. When guar seeds are removed from their pods these are spherical in shape, brownish in color, smaller than pea seeds in size.
Fig. 1.
Flow diagram for industrial manufacturing of guar gum
The gum is commercially extracted from seeds essentially by a mechanical process of roasting, differential attrition, sieving and polishing. The seeds are broken and the germ is separated from the endosperm. Two halves of the endosperm are obtained from each seed and are known as undehusked guar split. When the fine layer of fibrous material, which forms the husk, is removed and separated from the endosperm halves by polishing, refined guar splits are obtained. The hull (husk) and germ portion of guar seed are termed as guar meal which is a major byproduct of guar gum powder processing and is utilized as cattle feed. The refined guar splits are then treated and finished into powders (known as guar gum) by a variety of routes and processing techniques depending upon the end product desired. The pre hydrated guar splits are crushed in flacker mill and then uniformly moved to ultra fine grinder, which grinds the splits without producing too much heat. The grinded material is dried and passed through screens for grading of the material according to the particle size. Various grades are available depending upon color, mesh size, viscosity potential and rate of hydration (Chudzikowski 1971). In industrial processing of guar gum extrusion is also included before hydration and flaking. After these steps grinding and drying are done. Inclusion of extrusion gives guar gum powder with improved hydration rate (Chowdhary 2002). The byproducts of guar gum industry are Churi and Korma which are utilized for cattle feed.
Non-food applications
Demand of guar gum has increased during last few decades due to the development of different derivatives of guar gum like anionic and cationic derivatives. Present commercial importance of guar gum is because of its use in oil and gas well stimulation specifically hydraulic fracturing in which high pressure is used to crack rock. Guar gum makes the fracturing fluid thicker so that it can carry sand into fractured rock. This fracture remains open due to presence of sand which creates a path for gas or oil to flow to well bore. Guar derivatives for use in fracturing fluids are hydroxypropyl guar (HPG) and carboxymethyl hydroxypropyl guar (CMHPG). In textile and carpet printing, guar gum thickens the dye solutions which allow more sharply printed patterns to be produced. Guar gum has been used in explosives for over 25 years as an additive to dynamite for water blocking. In recent years, it has become the primary gelling agent in water based slurry explosives. Water blocking, swelling and gelling property of guar gum make it enable to use as an additive in explosive industry. Explosive property is maintained by mixing of ammonium nitrate, nitroglycerine and oil explosives with guar gum even in wet conditions. The production of paper is enhanced by an addition of small amounts of guar gum to the pulp. It serves as a fiber deflocculent and dry-strength additive. It provides denser surface to the paper used in printing. Research investigation shows that high viscosity guar gum derivatives can be obtained by treatment of guar gum with complexing agents like organic titanates, chromium salts and aluminum salts. These agents react with guar gum to form complexes with high viscosity gel.
Composition and structure
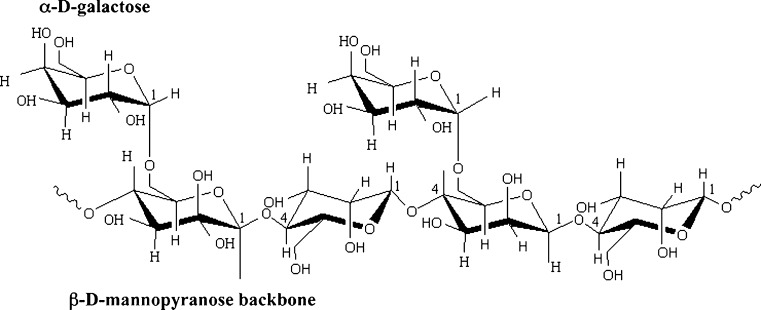
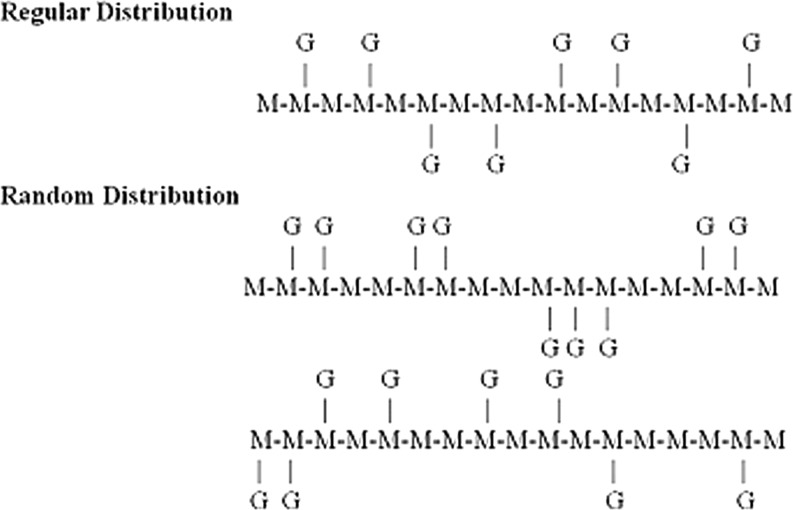
The guar kernel is composed of several layers, namely the outer husk (16–18%), the germ (43–46%) and the endosperm (34–40%). The germ portion of its seed is predominantly protein and the endosperm predominantly galactomannan. Guar gum mainly consists of the high molecular weight polysaccharides of galactomannans which are linear chain of (1 → 4)-linked β-D-mannopyranosyl units with (1 → 6)-linked α-D-galactopyranosyl residues as side chains as shown in Fig. 2. These galactose and mannose groups constitute the galactomannan portion of seed endosperm. General composition of guar gum is given in Table 2. It was first believed that the sidegroups were substituted at regular intervals along the mannan backbone (Whistler and Hymowitz 1979). However, experiment using enzyme degradation of guar (McCleary 1979), spectroscopic methods (Grasdalen and Painter, 1980) and computer simulation (McCleary et al. 1985), indicate more random distribution of galactose side groups as given in Fig. 3.
Fig. 2.
Structure of guar gum molecule
Table 2.
General composition of guar gum (Chudzikowski 1971)
| Constituent | Percentage |
|---|---|
| Galactomannan | 75–85 |
| Moisture | 8.0–14 |
| Protein (N x 6.25) | 5.0–6.0 |
| Fiber | 2.0–3.0 |
| Ash | 0.5–1.0 |
Fig. 3.
Sequence of galactose and mannose in guar
One such model proposes a guar galactomannan in which the galactosyl units are randomly arranged mainly in pairs and triplets (Hoffman and Svensson 1978). The ratio of mannose to galactose units has historically been reported as 2:1 (Garti and Leser 2001). Various research studies support ratios in the range of 1.6:1 to 1.8:1 (Grasdalen and Painter 1980; McCleary et al. 1985; Hoffman and Svensson 1978; Barth and Smith 1981; Vijayendran and Bone 1984; McCleary 1981; Mathur and Mathur 2005). Current data also suggest that galactomannans from different guar varieties have the same galactose/mannose arrangement (McCleary et al. 1985). The greater branching of guar is believed to be responsible for its easier hydration properties as well as its greater hydrogen bonding activity (Whistler 1954). It is also reported that aggregates are prominent in guar systems and may have important role in viscoelastic behavior of solution, depending on how they are interlinked (Gittings et al. 2000)
Guar is a polysaccharide with one of the highest molecular weights of all naturally occurring water soluble polymers. The viscosifying effect of commercial guar gum preparations can vary enormously depending on the molecular weight of the galactomannan. Early publications reported that average molecular weight of guar gum vary enormously, depending on what method is used, but these are typically in the range of 0.25–5.0 million.
Absolute methods have also been used to determine molecular weight, including light scattering techniques, which are also useful for providing structural information on the polysaccharide (Robinson et al. 1982; Burchard 1994; Ross-Murphy et al. 1998). One relatively simple and reliable way of estimating molecular weight is to use intrinsic viscosity measurements, calibrated by light scattering or some other absolute method using the Mark-Houwink equation. But more recent results obtained with size exclusion chromatography and low angle laser light scattering show the average molecular weight in the range of 106 to 2 x 106 (Barth and Smith 1981; Vijayendran and Bone 1984)
Physico-chemical properties
The biological properties of guar galactomannan and other such polysaccharides are dependent on their behavior in an aqueous medium. Guar gum swells and or dissolves in polar solvent on dispersion and form strong hydrogen bonds. In nonpolar solvents it forms only weak hydrogen bonds. The rate of guar gum dissolution and viscosity development generally increases with decreasing particle size, decreasing pH and increasing temperature. Hydration rates are reduced in the presence of dissolved salts and other water-binding agents such as sucrose (Bemiller and Whistler 1993).
Rheology
Rheology is the study of flow and deformation of material when external force is applied. Guar gum in aqueous solutions shows pseudoplastic or shear-thinning behavior which means reduction in viscosity with increasing shear rate as shown by many high molecular weight polymers. This shear-thinning behavior of guar gum aqueous solution increases with polymer concentration and molecular weight. Guar gum aqueous solutions also do not show yield stress properties (Whistler and Hymowitz 1979). Aqueous solutions of guar gum at 1% concentration show a typical behavior of macromolecular biopolymer with dominating loss modulus (G″) over storage modulus (G′) in lower frequency range. However, in high frequency range storage modulus dominates the loss modulus (Shobha and Tharanathan 2009). With time guar gum aqueous solutions showed a decrease in storage modulus (G′) and loss modulus (G″) (Chenlo et al. 2010).
Viscosity
The most significant characteristic of guar gum is its ability to hydrate rapidly in cold water systems to give highly viscous solutions. Guar gum forms a viscous colloidal dispersion when completely hydrated which is a thixotropic rheological system. Dilute solution of less than 1% concentration of guar gum are less thixotropic than solutions of concentration of 1% or higher (Glicksman 1969). As like the other gums, viscosity of guar gum is dependent on time, temperature, concentration, pH, ionic strength and also on type of agitation. Schlakman and Bartilucci (1957) examined thirteen different commercial samples, and found great variation in the viscosity property, particle size and rate of hydration. A 1% aqueous dispersion of good quality guar gum may show a high viscosity value of 10000 cP (Parija et al. 2001).
Hydration rate
Rate of hydration of guar gum varies. Hydration of about 2 h is required in practical applications in order to reach maximum viscosity. Hydration rate largely depend on particle size of guar gum powder. Hence, for quick initial viscosity, very fine mesh guar gums are available (Glicksman 1969). However, a considerable time interval is still desired for maximum hydration and viscosity to be achieved.
Hydrogen bonding activity
Hydrogen bonding activity of guar gum is due to the presence of hydroxyl group in guar gum molecule. Guar gum shows hydrogen bonding with cellulosic material and hydrated minerals. With slight addition of guar gum, there is alteration in electrokinetic properties of any system markedly (Glicksman 1969). Substitution of hydroxyl groups in guar gum with hydroxypropyl causes steric hindrance that decreases the stability of hydrogen bonds (Cheng and Prud’homme 2002).
Factors affecting viscosity and hydration rate
Viscosity and hydration rate of guar gum does not remain constant but changes with conditions like temperature, pH, solute, concentration, etc.
Temperature
Temperature is the most significant factor that affects the rate of hydration and maximum viscosity. Guar solutions reach maximum viscosity much faster when prepared at higher temperatures than those at lower temperatures. But the prolonged heat is also considered to have degradative effect. In most of the cases, guar gum solutions prepared by heating have a lower final viscosity than the same solutions prepared with cold water and allowed to hydrate slowly. Temperature range of 25–40 °C is desirable for maximum viscosities of guar gum dispersion. The viscosity of 0.5% (w/w) guar solution at 25 °C is significantly higher than that of 37 °C (Srichamroen 2007).
Concentration
Guar gum solution shows very high viscosity even at very low concentration. In most of the food applications it is recommended at below 1% concentration. Guar gum solution viscosities increase proportionally with increases in guar gum concentration (Morris et al. 1981; Robinson et al. 1982). This is due to the interaction of galactose side chain of guar molecule with water molecule. Increase in concentration of guar gum enhances the inter-molecular chain interaction or entanglement which leads to increase in viscosity (Zhang et al. 2005).On doubling the concentration guar gum shows tenfold increase in viscosity (Carlson et al. 1962). Upto 0.5% concentration, guar gum solutions behave as Newtonian system whereas above this concentration level guar solutions behave as non-Newtonian and thixotropic systems. It is also reported that viscosities of different concentration of guar gum at constant temperature reduces with increase in shear rate (Srichamroen 2007).
pH
Guar gum solutions are stable over a wide pH range of about 1.0–10.5. This is due to its non-ionic and uncharged behaviour. Final viscosity of guar gum is not affected by the pH, but the hydration rate shows variation with any change in pH. Fastest hydration is achieved at pH 8–9, however slowest hydration rate occurs at pH above 10 and below 4 (Carlson et al. 1962).
Sugar
In guar-sugar solution, sugar competes with guar gum molecule for the water available in the solution, hence presence of sugar in guar gum solution causes delay in hydration of guar gum molecules. The viscosity of guar-sugar solution decreases gradually and is inversely proportional to the sugar concentration (Carlson and Ziegenfuss 1965). Sweeteners like aspartame, acesulfame-k, cyclamate and neotame do not affect intrinsic viscosity of guar gum solutions significantly (Samavati et al. 2008).
Salt
Salt is most widely used ingredient in foods other than water, its effect on guar gum has been extensively studied (Carlson et al. 1962). Guar gum solutions in brine behave same as in water. Hydration rate is not influenced by salt; however, the presence of sodium chloride slightly increases the final viscosity of fully hydrated guar gum. Physiological buffer i.e. Krebs bicarbonate decreases the viscosity of 0.25% guar gum solution as compared to gaur gum in water alone (Srichamroen 2007). Salts restrict the hydration of guar gum solution (Doyle et al. 2006). Srichamroen demonstrated that viscosity of 0.5% guar gum solution increases with added salts. Presence of salts can help the intermolecular interactions due to change in the charge density and conformation of gum (Gittings et al. 2001).
Food applications
In food industry, guar gum is used as a novel food additive in various food products for food stabilization and as fiber source (Morris 2010). It is liked by both manufacturer and consumer because it is economical as well as natural additive. It is used in variety of foods as an additive because it changes the behaviour of water present as a common component in various foods. Some of the most common food applications of guar gum are shown in Table 3. Permissible use levels and limitations in various products are covered under Title 21 CFR 184.1339, affirming guar’s “generally recognized as safe” (GRAS) status.
Table 3.
Food applications of guar gum
| Food | Dose level | Function | Reference |
|---|---|---|---|
| Chapati | 0.75% | Softness | Ghodke 2009 |
| Bread | 0.5% | Softness, loaf volume | Keskin et al. 2007; Ribotta et al. 2001 |
| Fried Products | 0.5–1.0% | Oil uptake reduction | Sakhale et al. 2011 |
| Yoghurt | 2.0% | Texture improver | Brennan and Tudorica 2008 |
| Cake | 0.15% | Fat replacer, Firmness | Zambrano et al. 2004 |
| Sausage | 0.13–0.32% | Softness | Andres et al. 2006 |
| Pasta | 1.5% | Texture improver | Raina et al. 2005 |
| Ice cream | 0.5% | Smaller ice crystals | Sutton and Wilcox 1998 |
| Baked goods | 1.0% | Dough improver | Kohajdova and Karovicova 2008 |
| Tomato Ketchup | 0.5–1.0% | Consistency improver, | Gujral et al. 2002 |
| Serum loss reduction | Koocheki et al. 2009 |
Beverages
Guar gum is used in beverages for thickening and viscosity control because of its several inherent properties. The important property of guar gum is its resistance to breakdown under low pH conditions present in beverages. Guar gum is soluble in cold water which makes it easy to use in beverage processing plants. It improves the shelf life of beverages.
Processed cheeses
In cheese product, syneresis or weeping is a problem of serious concern. Guar gum prevents syneresis or weeping by water phase management and thus also improves the texture and body of the product (Klis 1966). In cheese products it is allowed upto 3% of the total weight. Guar gum in the soft cheeses enhances the yield of curd solids and gives a softer curve with separated whey. Low-fat cheese can be produced with addition of guar gum (at concentration 0.0025–0.01% w/v) without changing the rheology and texture compared with full-fat cheese.
Dairy products
Main purpose of using guar gum in frozen products is stabilization. Guar gum has important role in ice cream stabilization because of its water binding properties. Its use in high temperature short time (HTST) processes is very favorable because such processes require hydrocolloids that can fully hydrate in a short processing time. According to McKiernan (1957) locust bean gum has all the properties of an ideal gum but it hydrates slowly which is not favorable in HTST process. Julien (1953) obtained satisfactory results with guar as stabilizer in continuous ice cream processing. Guar gum should be used in ice cream mix at a concentration level of 0.3% (Goldstein & Alter 1959a, b). It was also used in combination with carrageenan in a mixed guar-carrageenan system developed for HTST process. Like locust bean gum its performance can be improved when used in combination with other stabilizers (Weinstein 1958). Guar gum in ice cream improves the body, texture, chewiness and heat shock resistance. Partially hydrolyzed guar gum (at 2–6% concentration level) decreases syneresis and improves the textural and rheological properties of low-fat yoghurt comparable with full-fat yoghurt (Brennan and Tudorica 2008).
Processed meat products
Guar gum has strong water holding capacity in both hot and cold water. Hence, it is very effectively used as a binder and lubricant in the manufacturing of sausage products and stuffed meat products. It performs specific functions in processed meat products like syneresis control, prevention of fat migration during storage, viscosity control of liquid phase during processing and cooling and control of accumulation of the water in the can during storage. Guar gum also enhances the creaming stability and control rheology of emulsion prepared by egg yolk (Ercelebi and Ibanoglu 2010)
Bakery products
Addition of guar gum in cake and biscuit dough improves the machinability of the dough that is easily removed from the mold and can be easily sliced without crumbling. At 1% addition of in batter of doughnuts, it gives desirable binding and film-forming properties that decreases the penetration of fats and oils. Guar gum in combination with starch is found to be effective in prevention of dehydration, shrinking and cracking of frozen-pie fillings (Werbin 1950). In wheat bread dough, addition of guar gum results in significant increase in loaf volume on baking (Cawley 1964). Guar gum along with xanthan gum retard staling in gluten-free rice cakes by decreasing the weight loss and retrogradation enthalpy (Sumnu et al. 2010). Similarly, guar gum also retards staling in chapati at room temperature as well as refrigerated temperature by controlling retrogradation of starch (Shaikh et al. 2008).
Salad dressings and sauces
Its cold water dispersibility and compatibility with high acidic emulsions enable it to use as thickener in salad dressing at about 0.2–0.8% of total weight. In salad dressings, it acts as an emulsion stabilizer by enhancing the viscosity of water phase and hence decreasing the separation rate of the water and oil phase (Goldstein and Alter 1959a, b). Guar gum has been found useful as a thickener in place of tragacanth in pickle and relish sauces (Burrell 1958). Guar gum enhances the consistency of tomato ketchup more prominently than other hydrocolloids like carboxy methyl cellulose, Sodium alginate, gum acacia and pectin. On addition of guar gum serum loss and flow values of tomato ketchup decreases which makes it a novel thickener for tomato ketchup (Gujral et al. 2002).
Health benefits
Various studies have been conducted on animals to test for both harmful and beneficial effect of guar gum. Guar is completely degraded in the large intestine by Clostridium butyricum (Hartemink et al. 1999). Harmful effects are observed only when the guar gum is given to the animals at a high concentration of about 10–15% on weight basis. This high concentration will reduce growth of animal due to decreased feed intake and impaired digestion. It is considered that the high viscosity of the intestinal tract contents, resulting from intake of guar gum at higher concentration, is the major cause of the negative effects. Hence, guar gum can only be used for its beneficial effects at lower concentration of about 0.5–1.0%. Above this concentration it will show negative effects of higher viscosity, decreased protein efficacy and lipid utilization. High viscosity of guar gum when used at a higher concentration, above 1.0% will not only interfere with nutritional properties of the food but also with the physicochemical and sensory properties of the food product which is not accepted by the consumer. Partial hydrolysis of guar gum (PHGG) reduces the chain length and molecular weight of the polymer and ultimately the lower viscosity makes it a novel soluble fiber that resembles in basic chemical structure with native guar gum and has various applications in clinical nutrition associated with ingestion of dietary fiber. It solves all the problems of high viscosity of guar gum. With hydrolyzed guar gum it is possible to increase the dietary fiber content of various food products like beverages without disturbing the nutritional and sensory properties of the food products. PHGG supplementation to the diet also reduces the laxative requirement, incidence of diarrhea and symptoms of irritable bowel syndrome (Greenberg and Sellman 1998; Slavin and Greenberg 2003). For treatment of irritable bowel syndrome water soluble non-gelling fibers are preferred. Due to its water solubility and non-gelling behavior, partially hydrolyzed guar gum decreased the symptoms in both forms of irritable bowel syndrome i.e. constipation predominant and diarrhea predominant (Giannini et al. 2006).
In vitro study shows that presence of guar gum significantly decreases the digestion of starch. It acts as a barrier between starch and starch hydrolyzing enzymes (Dartois et al. 2010).
Guar gum shows cholesterol and glucose lowering effects because of its gel forming properties. It also helps in weight loss and obesity prevention. Due to gel forming capacity of guar gum soluble fiber, an increased satiation is achieved because of slow gastric emptying. Diet supplemented with guar gum decreased the appetite, hunger and desire for eating (Butt et al. 2007). Mechanism behind cholesterol lowering by guar gum is due to increase in excretion of bile acids in faecus and decrease in enterohepatic bile acid which may enhances the production of bile acids from cholesterol and thus hepatic free cholesterol concentration is reduced (Rideout et al. 2008). Hypotriacylglycerolaemic effects are due to decrease in absorption of dietary lipids and reduced activity of fatty acid synthase in liver (Yamamoto 2001). Toxicity study on partially hydrolyzed guar gum has revealed that it is not mutagenic upto dose level of 2500 mg/day (Takahashi et al.,1994). Adequate intake of guar gum as dietary fiber helps in the maintenance of bowel regularity, significant reductions in total and LDL-cholesterol, control of diabetes, enhancement of mineral absorption and prevention of digestive problems like constipation (Yoon et al. 2008).
Conclusion
Guar gum is an important agrochemical derived from the seed endosperm of guar plant i.e. Cymopsis tetragonolobus which is cultivated in India and Pakistan from ancient times. Guar gum is a useful material to investigate. It has a strong hydrogen bond forming tendency in water which makes it a novel thickener and stabilizer. Aqueous solutions of guar gum are very viscous in nature. Because of these properties it has wide applications in the industries like food, pharmaceutical, textile, oil, paint, paper, explosive and cosmetics. Another reason for its popularity in the industry is its low cost. Its economical nature makes it popular in gums and stabilizers industry. In food industry, it has wide applications in ice cream, sauce, beverages, bakery and meat industry. It is also used in food products for supplementation as dietary fiber. Its consumption reduces the risk of heart diseases by reducing the cholesterol level in body, control diabetes and maintains the bowel movement in human beings.
References
- Anderson E. Endosperm mucilages of legumes: occurrence and composition. Ind Eng Chem. 1949;41:2887–2890. doi: 10.1021/ie50480a056. [DOI] [Google Scholar]
- Andres S, Zaritzky N, Califano A. The effect of whey protein concentrates and hydrocolloids on the texture and color characteristics of chicken sausages. Int J Food Sci Technol. 2006;41:954–961. doi: 10.1111/j.1365-2621.2005.01152.x. [DOI] [Google Scholar]
- APEDA (2011) APEDA annual export report. http://agriexchange.apeda.gov.in/index/product_description_32head.aspx?gcode=0502. Accessed June 2011.
- Barth HG, Smith DA. High-performance size-exclusion chromatography of guar gum. J Chromatogr. 1981;206:410–415. doi: 10.1016/S0021-9673(00)82558-3. [DOI] [Google Scholar]
- BeMiller JN. One hundred years of commercial food carbohydrates in the United States. J Agric Food Chem. 2009;57:8125–8129. doi: 10.1021/jf8039236. [DOI] [PubMed] [Google Scholar]
- Bemiller JN, Whistler RL (1993) Industrial gums: polysaccharides and their derivatives. Academic Press
- Brennan CS, Tudorica CM. Carbohydrate-based fat replacers in the modification of the rheological, textural and sensory quality of yoghurt: comparative study of the utilisation of barley beta-glucan, guar gum and inulin. Int J Food Sci and Technol. 2008;43:824–833. doi: 10.1111/j.1365-2621.2007.01522.x. [DOI] [Google Scholar]
- Burchard W. Light scattering. In: Murphy R, editor. Physical techniques for the study of food biopolymers S B. London: Blackie Academic and Professional; 1994. pp. 151–213. [Google Scholar]
- Burrell JR. Pickles and sauces. Food Manuf. 1958;33:10–13. [Google Scholar]
- Butt MS, Shahzadi N, Sharif MK, Nasir M. Guar gum: a miracle therapy for hypercholesterolemia, hyperglycemia and obesity. Crit Rev Food Sci Nutr. 2007;47:389–396. doi: 10.1080/10408390600846267. [DOI] [PubMed] [Google Scholar]
- Carlson WA, Ziegenfuss EM. The effect of sugar on guar gum as a thickening agent. Food Technol. 1965;19:64–68. [Google Scholar]
- Carlson WA, Ziegenfuss EM, Overton JD. Compatibility and manipulation of guar gum. Food Technol. 1962;16:50–54. [Google Scholar]
- Cawley RW. The role of wheat flour pentosans in baking. II. Effect of added flour pentosans and other gums on gluten starch loaves. J Sci Food Agr. 1964;15:834–838. doi: 10.1002/jsfa.2740151204. [DOI] [Google Scholar]
- Chandirami Guar gum. Paintindia. 1957;7:34–35. [Google Scholar]
- Cheng Y, Prud’homme RK. Measurement of forces between galactomannan polymer chains: effect of hydrogen bonding. Macromolecules. 2002;35:10155–10161. doi: 10.1021/ma020887e. [DOI] [Google Scholar]
- Chenlo F, Moreira R, Silva C. Rheological behaviour of aqueous systems of tragacanth and guar gums with storage time. J Food Eng. 2010;96:107–113. doi: 10.1016/j.jfoodeng.2009.07.003. [DOI] [Google Scholar]
- Chowdhary M (2002) Guar gum powder processing improved hydration characteristics. U.S. Patent 20020052298
- Chudzikowski RJ. Guar gum and its applications. J Soc Cosmet Chem. 1971;22:43–60. [Google Scholar]
- Dartois A, Singh J, Kaur L, Singh H. Influence of guar gum on the in vitro starch digestibility-rheological and microstructural characteristics. Food Biophysics. 2010;5:149–160. doi: 10.1007/s11483-010-9155-2. [DOI] [Google Scholar]
- Doyle JP, Giannouli P, Martin EJ, Brooks M, Morris ER. Effect of sugars, galactose content and chainlength on freeze-thaw gelation of galactomannans. Carbohydr Polym. 2006;64:391–401. doi: 10.1016/j.carbpol.2005.12.019. [DOI] [Google Scholar]
- Ercelebi EA, Ibanoglu E. Stability and rheological properties of egg yolk granule stabilized emulsions with pectin and guar gum. Int J Food Prop. 2010;13:618–630. doi: 10.1080/10942910902716984. [DOI] [Google Scholar]
- Garti N, Leser ME. Emulsification properties of hydrocolloids. Polym Adv Technol. 2001;12:123–135. doi: 10.1002/1099-1581(200101/02)12:1/2<123::AID-PAT105>3.0.CO;2-0. [DOI] [Google Scholar]
- Ghodke SK (2009) Effect of guar gum on dough stickiness and staling in Chapatti-An Indian unleavened flat bread. Int J Food Eng 5: Issue 3, Article 7
- Giannini EG, Mansi C, Dulbecco P, Savarino V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition. 2006;22:334–342. doi: 10.1016/j.nut.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Gittings MR, Cipelletti L, Trappe V, Weitz DA, In M, Marques C. Structure of guar in solutions of H2O and D2O: an ultra-small-angle light scattering study. J Phys Chem. 2000;104:4381–4386. [Google Scholar]
- Gittings MR, Cipelletti L, Trappe V, Weitz DA, In M, Lal J. The effect of solvent and ions on the structure and rheological properties of guar solutions. J Phys Chem. 2001;105:9310–9315. doi: 10.1021/jp0121825. [DOI] [Google Scholar]
- Glicksman M (1969) Gum technology in the Food Industry Academic Press; New York
- Goldstein AM, Alter EN (1959a) Gum Karaya. In: Whistler (ed) Industrial gums, polysaccharides and their derivatives. Academic Press, New York, pp 343–360
- Goldstein AM, Alter EN (1959b) Guar Gum. In: Whistler (ed) Industrial gums, polysaccharides and their derivatives. Academic Press, New York, pp 321
- Grasdalen H, Painter TJ. NMR studies of composition and sequence in legume seed galactomannans. Carbohydr Res. 1980;81:59–66. doi: 10.1016/S0008-6215(00)85677-3. [DOI] [Google Scholar]
- Greenberg NA, Sellman D. Partially hydrolyzed guar gum as a source of fiber. Cereal Foods World. 1998;43:703–707. [Google Scholar]
- Gujral HS, Sharma A, Singh N. Effects of hydrocolloids, storage temperature and duration on the consistency of tomato ketchup. Int J Food Prop. 2002;5:179–191. doi: 10.1081/JFP-120015600. [DOI] [Google Scholar]
- Hartemink R, Schoustra SE, Rombouts FM. Degradation of guar gum by intestinal bacteria. Bioscience Microflora. 1999;18:17–25. [Google Scholar]
- Heyne E, Whistler RL. Chemical composition and properties of guar polysaccharides. J Am Chem Soc. 1948;70:2249–2252. doi: 10.1021/ja01186a075. [DOI] [PubMed] [Google Scholar]
- Hoffman J, Svensson S. Studies of the distribution of the d-galactosyl side chains in guaran. Carbohydr Res. 1978;65:65–71. doi: 10.1016/S0008-6215(00)84213-5. [DOI] [Google Scholar]
- Hymowitz T. The transdomestication concept as applied to guar. Econ Bot. 1972;26:49–60. doi: 10.1007/BF02862261. [DOI] [Google Scholar]
- Julien JP. A study of some stabilizers in relation to HTST pasteurization of Ice cream. Ice Cream Trade J. 1953;49:44. [Google Scholar]
- Kay DE. Crop and product digest no 3-food legumes. London: Tropical Products Institute; 1979. pp. 72–85. [Google Scholar]
- Kays SE, Morris JB, Kim Y. Total and soluble dietary fiber variation in cyamopsis tetragonoloba (L.) Taub. (Guar) genotypes. J Food Quality. 2006;29:383–391. doi: 10.1111/j.1745-4557.2006.00080.x. [DOI] [Google Scholar]
- Keskin SO, Sumnu G, Sahin S. A study on the effects of different gums on dielectric properties and quality of breads baked in infrared-microwave oven. Eur Food Res Technol. 2007;224:329–334. doi: 10.1007/s00217-006-0334-9. [DOI] [Google Scholar]
- Klis JB. Woody’s Chunk O’Gold cold-pack cheese food weeps no more. Food Processing Marketing. 1966;27:58–59. [Google Scholar]
- Kohajdova Z, Karovicova J. Influence of hydrocolloids on quality of baked goods. Acta Sci Pol Technol Aliment. 2008;7:42–49. [Google Scholar]
- Koocheki A, Gandhi A, Razavi SMA, Mortazavi SA, Vasiljevic T. The rheological properties of ketchup as a function of different hydrocolloids and temperature. Int J Food Sci Technol. 2009;44:596–602. doi: 10.1111/j.1365-2621.2008.01868.x. [DOI] [Google Scholar]
- Mathur V, Mathur NK. Fenugreek and other lesser known legume galactomannan-polysaccharides: scope for developments. J Sci Ind Res. 2005;64:475–481. [Google Scholar]
- McCleary BV. Enzymic hydrolysis, fine structure and gelling interaction of legume seed D-galacto-D-mannans. Carbohydr Res. 1979;71:205–230. doi: 10.1016/S0008-6215(00)86071-1. [DOI] [Google Scholar]
- McCleary BV. Galactomannan quantitation in guar varieties and seed fractions. Lebensm. Wiss. Technol. 1981;14:188–191. [Google Scholar]
- McCleary BV, Clark AH, Dea ICM, Rees DA. The fine structures of guar and carob galactomannans. Carbohydr Res. 1985;139:237–260. doi: 10.1016/0008-6215(85)90024-2. [DOI] [Google Scholar]
- McKiernan BJ (1957) The role of gums in stabilizers. Paper presented at the Michigan Dairy Manufacturer’s Annual Conference, Michigan State University, East Lansing, Michigan
- Morris JB. Morphological and reproductive characterization of guar (cyamopsis tetragonoloba) genetic resources regenerated in Georgia, USA. Genet Resour Crop Ev. 2010;57:985–993. doi: 10.1007/s10722-010-9538-8. [DOI] [Google Scholar]
- Morris ER, Cutler AN, Ross-Murphy SB, Rees DA, Price J. Concentration and shear-rate dependence of viscosity in ‘random coil’ polysaccharide solutions. Carbohydr Polym. 1981;1:5–21. doi: 10.1016/0144-8617(81)90011-4. [DOI] [Google Scholar]
- Parija S, Misra M, Mohanty AK. Studies of natural gum adhesive extracts: an overview. Polym Rev. 2001;41:175–197. [Google Scholar]
- Prem D, Singh S, Gupta PP, Singh J, Kadyan SPS. Callus induction and de novo regeneration from callus in guar (cyamopsis tetragonoloba) Plant Cell Tiss Org. 2005;80:209–214. doi: 10.1007/s11240-004-0738-9. [DOI] [Google Scholar]
- Raina CS, Singh S, Bawa AS, Saxena DC. Textural characteristics of pasta made from rice flour supplemented with proteins and hydrocolloids. J Texture Stud. 2005;36:402–420. doi: 10.1111/j.1745-4603.2005.00024.x. [DOI] [Google Scholar]
- Ribotta PD, Leon AE, Anon MC. Effect of freezing and frozen storage of doughs on bread quality. J Agric Food Chem. 2001;49:913–918. doi: 10.1021/jf000905w. [DOI] [PubMed] [Google Scholar]
- Rideout TC, Harding SV, Jones PJH, Fan MZ. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: current understandings and future research priorities. Vasc Health Risk Manag. 2008;4:1023–1033. doi: 10.2147/vhrm.s3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Ross-Murphy SB, Morris ER. Viscosity-molecular weight relationships, intrinsic chain flexibility and dynamic solution properties of guar galactomannan. Carbohydr Res. 1982;107:17–32. doi: 10.1016/S0008-6215(00)80772-7. [DOI] [Google Scholar]
- Ross-Murphy SB, Wang Q, Ellis PR. Structure and mechanical properties of polysaccharides. Macromol Symp. 1998;127:13–21. doi: 10.1002/masy.19981270105. [DOI] [Google Scholar]
- Sakhale BK, Badgujar JB, Pawar VD, Sananse SL (2011) Effect of hydrocolloids incorporation in casing of Samosa on reduction of oil uptake. J Food Sci Technol. doi:10.1007/S13197-011-0333-0 [DOI] [PMC free article] [PubMed]
- Samavati V, Razavi SH, Mousavi SM. Effect of sweeteners on viscosity and particle size of dilute guar gum solutions. Iranian J Chem Chem Eng. 2008;27:23–31. [Google Scholar]
- Schlakman IA, Bartilucci AJ. A comparative study of commercially available guar gums. Drug Stand. 1957;25:149–154. [Google Scholar]
- Shaikh MI, Ghodke SK, Ananthanarayan L. Inhibition of staling in chapati (Indian unleavened flat bread) J Food Process Pres. 2008;32:378–403. doi: 10.1111/j.1745-4549.2008.00185.x. [DOI] [Google Scholar]
- Shobha MS, Tharanathan RN. Rheological behaviour of pullulanase-treated guar galactomannan on co-gelation with xanthan. Food Hydrocolloid. 2009;23:749–754. doi: 10.1016/j.foodhyd.2008.04.006. [DOI] [Google Scholar]
- Slavin JL, Greenberg NA. Partially hydrolyzed guar gum: clinical nutrition uses. Nutrition. 2003;19:549–552. doi: 10.1016/S0899-9007(02)01032-8. [DOI] [PubMed] [Google Scholar]
- Srichamroen A. Influence of temperature and salt on viscosity property of guar gum. Naresuan University Journal. 2007;15:55–62. [Google Scholar]
- Sumnu G, Koksel F, Sahin S, Basman A, Meda V. The effects of xanthan and guar gums on staling of gluten-free rice cakes baked in different ovens. Int J Food Sci Technol. 2010;45:87–93. doi: 10.1111/j.1365-2621.2009.02107.x. [DOI] [Google Scholar]
- Sutton RL, Wilcox J. Recrystallization in ice cream as affected by stabilizers. J Food Sci. 1998;63:104–107. doi: 10.1111/j.1365-2621.1998.tb15686.x. [DOI] [Google Scholar]
- Takahashi H, Yang SI, Fujiki M, Kim M, Yamamoto T, Greenberg NA. Toxicity studies of partially hydrolyzed guar gum. Int J Toxicol. 1994;13:273–278. doi: 10.3109/10915819409140599. [DOI] [Google Scholar]
- Venkateswarlu B, Raikhy NP, Aggarwal RK. Effect of inoculation and cobalt application on nodulation and nitrogen uptake in guar (Cyamopsis tetragonoloba L.) J Indian Soc Soil Sci. 1982;30:550–551. [Google Scholar]
- Vijayendran BR, Bone T. Absolute molecular weight and molecular weight distribution of guar by size exclusion chromatography and low-angle laser light scattering. Carbohydr Polym. 1984;4:299–311. doi: 10.1016/0144-8617(84)90005-5. [DOI] [Google Scholar]
- Weinstein B (1958) Stabilizers for ice cream type desserts. U.S. Patent 2856289
- Werbin SJ (1950) Natural Gums in the Baking Industry, Paper presented at the American Association of Cereal Chemists, Dec. 5
- Whistler RL. Guar-a new industrial crop. Chem Ind. 1948;62:60–61. [Google Scholar]
- Whistler RL (1954) Guar gum, locust bean gum and other. In: Staff of Ind. Eng. Chem. (ed) Natural plant hydrocolloids. American Chemical Society, Washington, DC, pp 45–50
- Whistler RL, Hymowitz T. Guar: agronomy, production, industrial use, and nutrition. West Lafayette: Purdue University Press; 1979. [Google Scholar]
- Yamamoto Y. Hypolipidemic effects of a guar gum-xanthan gum mixture in rats fed high sucrose diet. Journal of Japanese Society of Nutrition and Food Science. 2001;54:139–145. doi: 10.4327/jsnfs.54.139. [DOI] [Google Scholar]
- Yoon SJ, Chu DC, Juneja LR. Chemical and physical properties. Safety and application of partially hydrolyzed guar gum as dietary fiber. J Clin Biochem Nutr. 2008;42:1–7. doi: 10.3164/jcbn.2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano F, Despinoy P, Ormenese RCSC, Faria EV. The use of guar and xanthan gums in production of ‘light’ low fat cakes. Int J Food Sci Technol. 2004;39:959–966. doi: 10.1111/j.1365-2621.2004.00864.x. [DOI] [Google Scholar]
- Zhang LM, Zhou JF, Hui PS. A comparative study on viscosity behavior of water-soluble chemically modified guar gum derivatives with different functional lateral groups. J Sci Food Agric. 2005;85:2638–2644. doi: 10.1002/jsfa.2308. [DOI] [Google Scholar]