Abstract
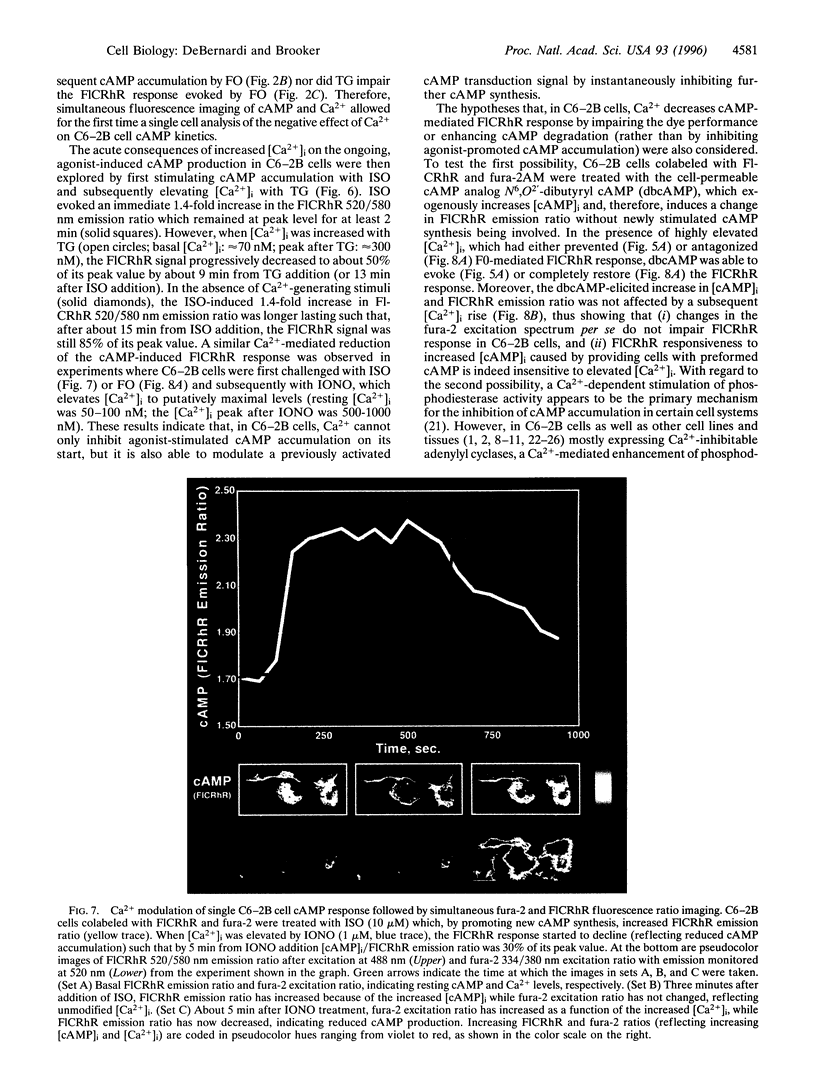
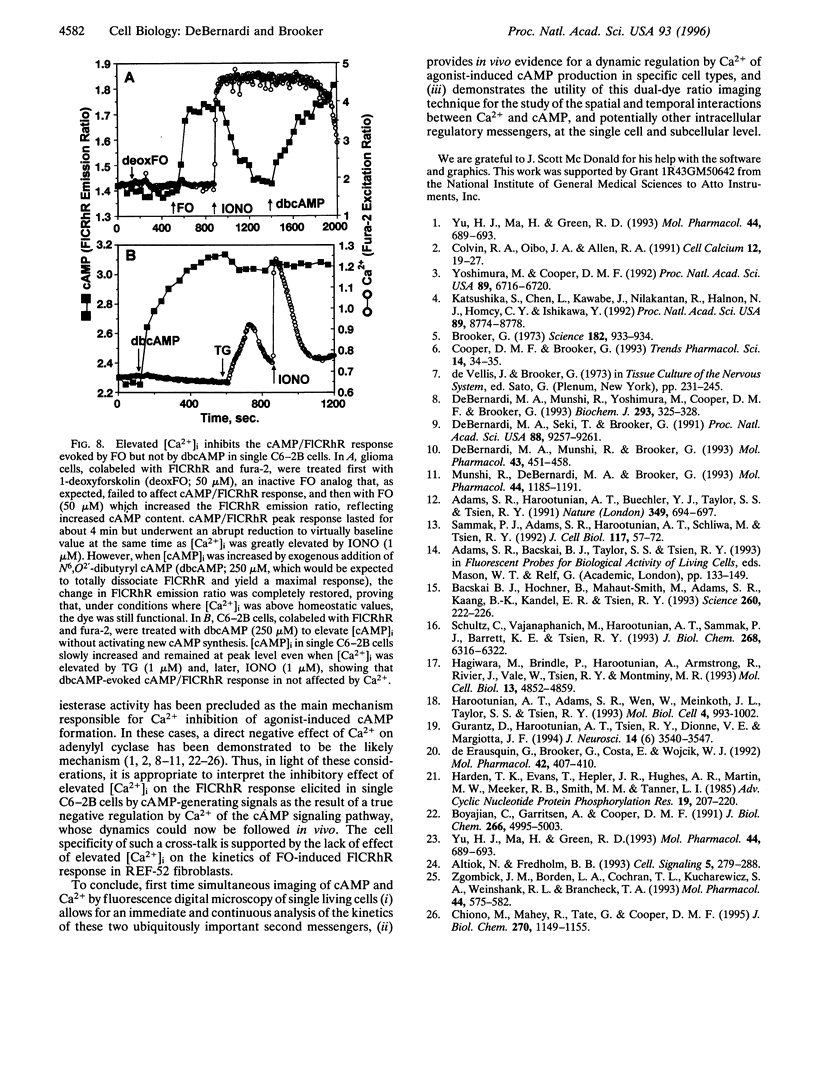
The spatial and temporal dynamics of two intracellular second messengers, cAMP and Ca2+, were simultaneously monitored in living cells by digital fluorescence ratio imaging using FlCRhR, a single-excitation dual-emission cAMP indicator, and fura-2, a dual-excitation single-emission Ca2+ probe. In single C6-2B glioma cells, isoproterenol- or forskolin-evoked cAMP accumulation (measured in vivo as an increased FlCRhR emission ratio) was reduced when cytosolic free Ca2+ concentration was elevated before, simultaneously with, or after cAMP activation. However, in REF-52 fibroblasts, Ca2+ neither prevented nor reduced forskolin-stimulated cAMP production. These results provide novel in vivo evidence for the Ca2+ modulation of the cAMP transduction pathway in C6-2B cells. The simultaneous microscopic measurement of cAMP and Ca2+ kinetics in single cells makes it now possible to study the regulatory interactions between these second messengers at the cellular and even the subcellular level.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. R., Harootunian A. T., Buechler Y. J., Taylor S. S., Tsien R. Y. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991 Feb 21;349(6311):694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- Altiok N., Fredholm B. B. Bradykinin inhibits cyclic AMP accumulation in D384-human astrocytoma cells via a calcium-dependent inhibition of adenylyl cyclase. Cell Signal. 1993 May;5(3):279–288. doi: 10.1016/0898-6568(93)90018-h. [DOI] [PubMed] [Google Scholar]
- Bacskai B. J., Hochner B., Mahaut-Smith M., Adams S. R., Kaang B. K., Kandel E. R., Tsien R. Y. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993 Apr 9;260(5105):222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- Boyajian C. L., Garritsen A., Cooper D. M. Bradykinin stimulates Ca2+ mobilization in NCB-20 cells leading to direct inhibition of adenylylcyclase. A novel mechanism for inhibition of cAMP production. J Biol Chem. 1991 Mar 15;266(8):4995–5003. [PubMed] [Google Scholar]
- Brooker G. Oscillation of cyclic adenosine monophosphate concentration during the myocardial contraction cycle. Science. 1973 Nov 20;182(4115):933–934. doi: 10.1126/science.182.4115.933. [DOI] [PubMed] [Google Scholar]
- Chiono M., Mahey R., Tate G., Cooper D. M. Capacitative Ca2+ entry exclusively inhibits cAMP synthesis in C6-2B glioma cells. Evidence that physiologically evoked Ca2+ entry regulates Ca(2+)-inhibitable adenylyl cyclase in non-excitable cells. J Biol Chem. 1995 Jan 20;270(3):1149–1155. doi: 10.1074/jbc.270.3.1149. [DOI] [PubMed] [Google Scholar]
- Colvin R. A., Oibo J. A., Allen R. A. Calcium inhibition of cardiac adenylyl cyclase. Evidence for two distinct sites of inhibition. Cell Calcium. 1991 Jan;12(1):19–27. doi: 10.1016/0143-4160(91)90081-o. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Brooker G. Ca(2+)-inhibited adenylyl cyclase in cardiac tissue. Trends Pharmacol Sci. 1993 Feb;14(2):34–36. doi: 10.1016/0165-6147(93)90027-h. [DOI] [PubMed] [Google Scholar]
- De Erausquin G., Brooker G., Costa E., Wojcik W. J. Stimulation of high affinity gamma-aminobutyric acidB receptors potentiates the depolarization-induced increase of intraneuronal ionized calcium content in cerebellar granule neurons. Mol Pharmacol. 1992 Sep;42(3):407–414. [PubMed] [Google Scholar]
- DeBernardi M. A., Seki T., Brooker G. Inhibition of cAMP accumulation by intracellular calcium mobilization in C6-2B cells stably transfected with substance K receptor cDNA. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9257–9261. doi: 10.1073/pnas.88.20.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi M. A., Munshi R., Brooker G. Ca2+ inhibition of beta-adrenergic receptor- and forskolin-stimulated cAMP accumulation in C6-2B rat glioma cells is independent of protein kinase C. Mol Pharmacol. 1993 Mar;43(3):451–458. [PubMed] [Google Scholar]
- Debernardi M. A., Munshi R., Yoshimura M., Cooper D. M., Brooker G. Predominant expression of type-VI adenylate cyclase in C6-2B rat glioma cells may account for inhibition of cyclic AMP accumulation by calcium. Biochem J. 1993 Jul 15;293(Pt 2):325–328. doi: 10.1042/bj2930325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurantz D., Harootunian A. T., Tsien R. Y., Dionne V. E., Margiotta J. F. VIP modulates neuronal nicotinic acetylcholine receptor function by a cyclic AMP-dependent mechanism. J Neurosci. 1994 Jun;14(6):3540–3547. doi: 10.1523/JNEUROSCI.14-06-03540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M., Brindle P., Harootunian A., Armstrong R., Rivier J., Vale W., Tsien R., Montminy M. R. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993 Aug;13(8):4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden T. K., Evans T., Hepler J. R., Hughes A. R., Martin M. W., Meeker R. B., Smith M. M., Tanner L. I. Regulation of cyclic AMP metabolism by muscarinic cholinergic receptors. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:207–220. [PubMed] [Google Scholar]
- Harootunian A. T., Adams S. R., Wen W., Meinkoth J. L., Taylor S. S., Tsien R. Y. Movement of the free catalytic subunit of cAMP-dependent protein kinase into and out of the nucleus can be explained by diffusion. Mol Biol Cell. 1993 Oct;4(10):993–1002. doi: 10.1091/mbc.4.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsushika S., Chen L., Kawabe J., Nilakantan R., Halnon N. J., Homcy C. J., Ishikawa Y. Cloning and characterization of a sixth adenylyl cyclase isoform: types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8774–8778. doi: 10.1073/pnas.89.18.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi R., DeBernardi M. A., Brooker G. P2U-purinergic receptors on C6-2B rat glioma cells: modulation of cytosolic Ca2+ and cAMP levels by protein kinase C. Mol Pharmacol. 1993 Dec;44(6):1185–1191. [PubMed] [Google Scholar]
- Sammak P. J., Adams S. R., Harootunian A. T., Schliwa M., Tsien R. Y. Intracellular cyclic AMP not calcium, determines the direction of vesicle movement in melanophores: direct measurement by fluorescence ratio imaging. J Cell Biol. 1992 Apr;117(1):57–72. doi: 10.1083/jcb.117.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz C., Vajanaphanich M., Harootunian A. T., Sammak P. J., Barrett K. E., Tsien R. Y. Acetoxymethyl esters of phosphates, enhancement of the permeability and potency of cAMP. J Biol Chem. 1993 Mar 25;268(9):6316–6322. [PubMed] [Google Scholar]
- Yoshimura M., Cooper D. M. Cloning and expression of a Ca(2+)-inhibitable adenylyl cyclase from NCB-20 cells. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6716–6720. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. J., Ma H., Green R. D. Calcium entry via L-type calcium channels acts as a negative regulator of adenylyl cyclase activity and cyclic AMP levels in cardiac myocytes. Mol Pharmacol. 1993 Oct;44(4):689–693. [PubMed] [Google Scholar]
- Yu H. J., Ma H., Green R. D. Calcium entry via L-type calcium channels acts as a negative regulator of adenylyl cyclase activity and cyclic AMP levels in cardiac myocytes. Mol Pharmacol. 1993 Oct;44(4):689–693. [PubMed] [Google Scholar]
- Zgombick J. M., Borden L. A., Cochran T. L., Kucharewicz S. A., Weinshank R. L., Branchek T. A. Dual coupling of cloned human 5-hydroxytryptamine1D alpha and 5-hydroxytryptamine1D beta receptors stably expressed in murine fibroblasts: inhibition of adenylate cyclase and elevation of intracellular calcium concentrations via pertussis toxin-sensitive G protein(s). Mol Pharmacol. 1993 Sep;44(3):575–582. [PubMed] [Google Scholar]