Abstract
Objective(s)
We aimed to evaluate the predictive value of amniotic fluid index (AFI) (<5) for adverse perinatal outcome in terms of cesarean section for fetal distress, birth weight, meconium staining, Apgar scores, and cord pH at birth.
Method(s)
This was a prospective study of 200 antenatal women booked at Ram Manohar Lohia (RML) Hospital during the years 2009–2011 with gestational age between 34 and 41 weeks. The women’s history, clinical examination recorded, and AFI were measured and the perinatal outcome was compared between two groups, i.e., AFI < 5 and >5.
Result(s)
The cesarean section rate for fetal distress and low birth weight babies, <2.5 kg, was higher in patients with oligohydramnios (p = 0.048, 0.001, respectively). There was no significant difference in meconium staining, Apgar score at 5 min <7, and cord pH at birth between the two groups (p = 0.881, 0.884, 0.764, respectively).
Conclusions
Oligohydramnios has a significant correlation with cesarean section for fetal distress and low birth weight babies.
Keywords: Meconium staining, Cesarean delivery, Apgar scores, Birth weight, Cord pH
Introduction
Modern obstetrics is concerned with the health and well-being of both the mother and the unborn child. Recognition of a fetus at risk for death or damage in utero, quantifying the risk, balancing the fetal risk against the risk of neonatal complications from immaturity, and determining the optimal time and mode of intervention are the cornerstones of modern perinatal medicine [1]. Clinical estimation of amniotic fluid volume (AFV) is an important part of fetal assessment as variation in its amount has been related to a variety of pregnancy complications. Amniotic fluid provides a protective milieu for the growing fetus, cushioning it against mechanical and biological injury [2, 3]. Quantification of amniotic fluid is an important component of the biophysical profile in ultrasound evaluation of fetal well-being, especially in the third trimester [4]. Antenatal tests use amniotic fluid volume as a fundamental assessment of chronic in utero stress. Ultrasound being a non-invasive test is ideal for application on a large scale and can be used frequently for repeat AFV determination in the case of suspected abnormalities [3]. Links have been found between decreased amniotic fluid volume and stillbirths, fetal anomaly, abnormal FHR tracings in labor, increase in cesarean section for fetal distress, and possibly fetal acidosis [2]. In the present study, amniotic fluid quantification was done by the four-quadrant technique as described by Phelan et al. [5] to determine AFI and we sought to determine if an antepartum AFI of 5 cm or less is a predictor of adverse perinatal outcome in terms of meconium staining, cesarean section for fetal distress, birth weight, low Apgar scores, and cord pH [3].
Materials and Methods
The present study was a prospective study carried out at the Dr. RML Hospital, New Delhi. The study participants included 200 booked antenatal women registered at RML Hospital with gestational age between 34 and 41 weeks, admitted for delivery over a 2-year duration from 2009 to 2010. Inclusion criteria were women with a singleton, nonanomalous fetus with intact membranes at the time of antepartum testing. Women with premature rupture of membranes, with known fetal or chromosomal anomalies, gestational diabetes, Rh incompatibility, placental anomalies, and multiple pregnancy were excluded from the study. On admission, a detailed history was taken, and a clinical exam was performed and gestational age assessed. Amniotic fluid index was determined using the Phelan’s technique [5] within 7 days of delivery or at the onset of labor after informed written consent. Non stress test (NST) was performed for all patients. Women were divided into two groups based on their AFI (done within 7 days of delivery): Group 1—AFI < 5; Group 2—AFI > 5. A note was made of meconium staining of amniotic fluid, the ultimate mode of delivery, birth weight, Apgar score at 1 and 5 min, and cord pH measured at the time of birth. Chi square (χ2) test was carried out at 5 % (α = 0.05) level of significance to test the homogeneity of the groups with respect to the distribution of patients over different classes of a characteristic of interest (Tables 1, 2, 3).
Table 1.
Maternal demographic and obstetric characteristics
| AFI < 5 (n = 25) | AFI > 5 (n = 175) | p value | |
|---|---|---|---|
| Maternal age (mean) | 27.04 | 27.95 | 0.34 |
| Nulliparity | 17 (68 %) | 103 (58.9 %) | 0.22 |
| Gestational age <37 weeks at delivery | 14 (56 %) | 60 (34.3 %) | 0.035 |
| Weight gain <10 kg | 9 (36 %) | 15 (8.6 %) | 0.001 |
| Induction of labor | 18 (72 %) | 89 (50.9 %) | 0.043 |
Table 2.
Obstetric and perinatal outcome
| AFI < 5 (n = 25) | AFI > 5 (n = 175) | p value | |
|---|---|---|---|
| Meconium-stained liquor | 4 (16 %) | 26 (14.9 %) | 0.881 |
| Total cesarean delivery | 14 (56 %) | 62 (35.4 %) | 0.047 |
| Cesarean for non-reassuring fetal status | 8 (57.1 %) | 24 (38.7 %) | 0.048 |
| Birth weight <2.5 kg | 14 (56 %) | 38 (21.7 %) | 0.001 |
| Apgar score | |||
| 1 min <7 | 9 (36 %) | 19 (10.9 %) | 0.001 |
| 5 min <7 | 1 (4 %) | 6 (3.4 %) | 0.884 |
| Cord pH <7.1 | 1 (4 %) | 5 (2.9 %) | 0.764 |
Table 3.
Secondary outcome measures
| AFI < 5 (n = 25) | AFI > 5 (n = 175) | p value | |
|---|---|---|---|
| Non-Reactive NST | 8 (32 %) | 17 (9.7 %) | 0.002 |
| Admission to NICU | 23 (92 %) | 125 (71.4 %) | 0.028 |
| NICU stay >2 days | 9 (36 %) | 42 (24 %) | 0.198 |
Results
Out of the 200 women, the mean maternal age was 27.04 in Group 1 and 27.95 in Group 2, out of which, 17 (68 %) women were nulliparous in Group 1 and 103 (58.9 %) in Group 2. Gestational age was <37 weeks in 14 (56 %) in Group 1 as compared to 60 (34.3 %) in Group 2. Maternal weight gain during pregnancy was <10 kg in 9 (36 %) in Group 1 as compared to 15 (8.6 %) in Group 2. 18 (72 %) patients were induced in Group 1 as compared to 89 (50.9 %) in Group 2. Obstetric and perinatal outcomes were studied in both the groups.
4 (16 %) women in Group 1 and 26 (14.9 %) women in Group 2 had meconium-stained liquor. The difference was not statistically significant (p = 0.881). Cesarean section was performed in 14 (56 %) women in Group 1 as compared to 62 (35.4 %) in Group 2 (p = 0.047). Cesarean section for fetal distress was higher in women with oligohydramnios (57.1 %) as compared to women with AFI > 5 (38.7 %) (p = 0.048). Birth weight <2.5 kg was found in 14 (56 %) patients in Group 1 as compared to 38 (21.7 %) in Group 2. In Group 1, the Apgar score at 1 min was <7 in nine women (36 %) as compared to 19 (10.9 %) in Group 2 (p = 0.001). An Apgar score <7 at 5 min was noted in 1 (4 %) woman in Group 1 and 6 (3.4 %) women in Group 2 (p = 0.884). Cord pH < 7.1 was found in 1 (4 %) woman in Group 1 as compared to 5 (2.9 %) in Group 2 and the difference was not statistically significant (p = 0.764).
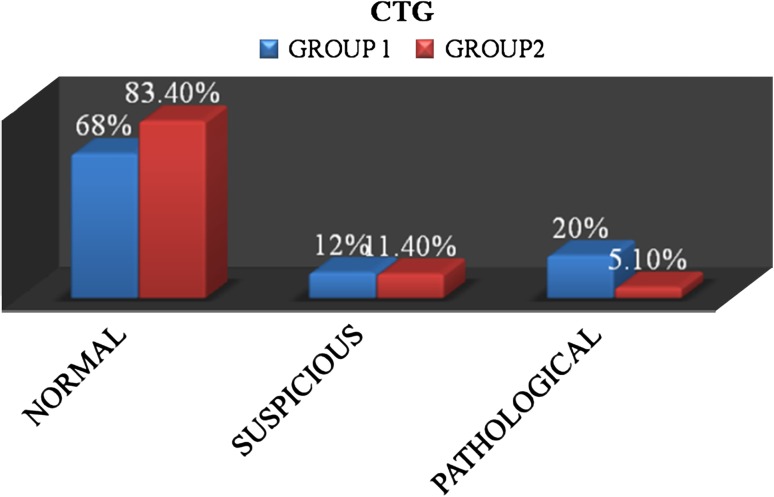
In Group 1, out of 25 women, 17 (68 %) had normal cardiotocography (CTG) and 5 (20 %) had pathological CTG. In Group 2, out of 175 patients, 146 (83.4 %) had a normal CTG and 9 (5.1 %) had pathological CTG. The rate of Pathological CTG in Group 1 was statistically significant (Fig. 1).
Fig. 1.
Intrapartum CTG
Non-reactive NST was present in a significant number of patients in Group 1 (32 %) as compared to Group 2 (9.7 %) (p = 0.002). Most of the babies in Group 1, that is 23 (92 %), were admitted to the neonatal intensive care unit (NICU). However, in Group 2, 125 (71.4 %) babies were admitted to the NICU. Thus, in Group 1, there was significant correlation to NICU admission. Duration of NICU stay of more than 2 days was found in 9 (36 %) in Group 1 and 42 (24 %) in Group 2 (p = 0.198). Therefore, the two groups were comparable with regard to the NICU stay.
Discussion
In the present study, meconium-stained liquor was present in 4 (16 %) of the patients in Group 1 and 26 (14.9 %) in Group 2, and the difference was not significant (p = 0.881). The cesarean section rate was higher in Group 1 with AFI ≤ 5, i.e., 56 % as compared to 35.4 % for Group 2, and the difference was statistically significant (p = 0.047). Cesarean section for fetal distress was also higher in patients with oligohydramnios as compared to the group with normal AFI (57.4 vs. 38.7 %) (p = 0.048). A study conducted by Baron et al. [6] showed that meconium-stained amniotic fluid occurred significantly less often in the oligohydramnios group as compared to the normal AFI group. A study by Voxman et al. [7] concluded that there was no difference between the groups with regard to meconium-stained liquor, which was comparable to our study. Chauhan et al. [8] in their meta-analysis (1999) found that intrapartum AFI ≤ 5 was associated with increased risk of cesarean section for fetal distress (pooled RR = 1.7), which was similar to our study. Rutherford et al. [9] found an inverse relationship between amniotic fluid index and cesarean section for fetal distress.
In the current study, birth weight <2.5 kg was found in 14 (56 %) of the patients in Group 1 versus 38 (21.7 %) in Group 2, and the difference was statistically significant (p = 0.001). Locatelli et al. [10] reported that in uncomplicated term pregnancies with oligohydramnios, the presence of an AFI < 5 independently increased the risk for a SGA infant. Morris et al. [11] found that 60 % of babies were of LBW in the group with AFI < 5, indicating that oligohydramnios had an association with growth restriction. A study by Rutherford et al. [9] showed that when the AFI was <5 (36 %), pregnancies resulted in infants with intra uterine growth restriction (IUGR).
In the present study, the 1-min Apgar score was <7 in 9 out 25 (36 %) babies in Group 1, whereas only 10.9 % babies in Group 2 had a 1-min Apgar score <7, and this difference was statistically significant (p = 0.001). However, the 5-min Apgar score <7 was almost equal in both the groups (4 vs. 3.4 %) (p = 0.884). Chauhan et al. [8] reported in their meta-analysis that antepartum AFI of ≤5 cm was associated with a 5-min Apgar score <7 (pooled RR −1.8, 95 % CI 1.1–2.6). A study by Driggers et al. [12] reported a 5-min Apgar score <7 in 3.8 % patients in an oligohydramnios group versus 4.6 % in a normal AFI group, and concluded that there was no significant difference. A study by Grubb et al. [13] found the 1-min Apgar score <7 in 84 % patients with AFI ≤ 5 as compared to 14 % in the normal AFI group, which was highly significant (p = 0.01). In the same study, the 5-min score <7 was seen in 13 % patients with AFI ≤ 5 versus 5 % in the normal AFI group.
In the present study, the cord pH at the time of birth, which is an objective marker of fetal distress, was <7.1 in one baby (4 %) in Group 1, whereas five babies out of 175 (2.9 %) in Group 2 had cord pH < 7.1, which was not statistically significant. A study by Chauhan et al. [8] also found no clear correlation between AFI and neonatal acidosis and it was stated that a multicentric study of sufficient power should be undertaken to demonstrate that low AFI is associated with umbilical artery pH < 7. A study by Morris et al. [11] found a significant association between the number of babies with cord pH < 7 and AFI ≤ as 5.1 % versus 1.3 % for AFI > 5 (RR −3.3 and p value 0.01).
Conclusions
In the present study, antepartum oligohydramnios (AFI < 5) was associated with increased cesarean delivery, particularly for fetal distress. A significant correlation was found between oligohydramnios and low birth weight babies. However, there was no difference in perinatal outcome in terms of meconium staining, 5-min Apgar score, and cord pH between the two groups. When the secondary outcome was measured, significant correlation was found in terms of Non-reactive NST and admission to the NICU. Therefore, patients with severe oligohydramnios with AFI < 5 should undergo antepartum management in the form of induction of labor in order to improve their perinatal outcome.
References
- 1.Manning FA. Antepartum fetal testing: a critical appraisal. Curr Opin Obstet Gynecol. 2009;21(4):348–352. doi: 10.1097/GCO.0b013e32832ae0b3. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain PF, Manning FA, Morrison I, et al. The relationship of marginal and decreased amniotic fluid volumes to perinatal outcome. Am J Obstet Gynecol. 1984;150(3):245–249. doi: 10.1016/S0002-9378(84)90359-4. [DOI] [PubMed] [Google Scholar]
- 3.Nageotte MP, Towers CV, Asrat T, et al. Perinatal outcome with the modified biophysical profile. Am J Obstet Gynecol. 1994;170(6):1672–1676. doi: 10.1016/S0002-9378(94)70339-6. [DOI] [PubMed] [Google Scholar]
- 4.Kofinas A, Kofinas G. Differences in amniotic fluid patterns and fetal biometric parameters in third trimester pregnancies with and without diabetes. J Matern Fetal Neonatal Med. 2006;19(10):633–638. doi: 10.1080/14767050600822547. [DOI] [PubMed] [Google Scholar]
- 5.Phelan JP, Ahn MO, Smith CV, et al. Amniotic fluid index measurements during pregnancy. J Reprod Med. 1987;32:601–604. [PubMed] [Google Scholar]
- 6.Baron C, Morgan MA, Garite TJ. The impact of amniotic fluid volume assessed intrapartum on perinatal outcome. Am J Obstet Gynecol. 1995;173(1):167–174. doi: 10.1016/0002-9378(95)90185-X. [DOI] [PubMed] [Google Scholar]
- 7.Voxman EG, Tran S, Wing DA. Low amniotic fluid index as a predictor of adverse perinatal outcome. J Perinatol. 2002;22(4):282–285. doi: 10.1038/sj.jp.7210697. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan SP, Sanderson M, Hendrix NW, et al. Perinatal outcome and amniotic fluid index in the antepartum and intrapartum periods: a meta-analysis. Am J Obstet Gynecol. 1999;181(6):1473–1478. doi: 10.1016/S0002-9378(99)70393-5. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford SE, Phelan JP, Smith CV, et al. The four quadrant assessment of amniotic fluid volume: an adjunct to antepartum fetal heart rate testing. Obstet Gynecol. 1987;70(3):353–356. [PubMed] [Google Scholar]
- 10.Locatelli A, Vergani P, Toso L, et al. Perinatal outcome associated with oligohydramnios in uncomplicated term pregnancies. Arch Gynecol Obstet. 2004;269(2):130–133. doi: 10.1007/s00404-003-0525-6. [DOI] [PubMed] [Google Scholar]
- 11.Morris JM, Thompson K, Smithey J, et al. The usefulness of ultrasound assessment of amniotic fluid in predicting adverse outcome in prolonged pregnancy: a prospective blinded observational study. Br J Obstet Gynaecol. 2003;110(11):989–994. doi: 10.1111/j.1471-0528.2003.02417.x. [DOI] [PubMed] [Google Scholar]
- 12.Driggers RW, Holcroft CJ, Blakemore KJ, et al. An amniotic fluid index ≤5 cm within 7 days of delivery in the third trimester is not associated with decreasing umbilical arterial pH and base excess. J Perinatol. 2004;24(2):72–76. doi: 10.1038/sj.jp.7211034. [DOI] [PubMed] [Google Scholar]
- 13.Grubb DK, Paul RH. Amniotic fluid index and prolonged antepartum fetal heart rate decelerations. Obstet Gynecol. 1992;79(4):558–560. [PubMed] [Google Scholar]