Abstract
A 69-year-old woman with a history of Hodgkin's lymphoma at age 17 was admitted to our hospital with 3 weeks of progressive dyspnoea on exertion. Her medical history and physical exam were concerning for superior vena cava (SVC) syndrome and while her workup did indeed reveal such, its aetiology was not due to a compressive mass associated with malignancy. Rather, she developed chronic thromboses in the setting of radiation-induced venous fibrosis. This case report details her case and explains the key clinical concerns, pathophysiology and incidence of radiation-induced SVC syndrome.
Background
Malignancy accounts for approximately 90% of cases of superior vena cava (SVC) syndrome with intravascular catheters having more of a recent role. In this case, we present a rare case of SVC syndrome in a patient who was initially suspected to have a malignant mass. Although arterial injury from radiation therapy is well-documented, venous injury is not as well-described. Radiation-induced SVC syndrome needs to be included in the differential diagnosis in patients with a history of prior radiation exposure as workup and treatment for this condition differs from that of SVC syndrome due to other causes.
Case presentation
A 69-year-old woman presented to our emergency department with 3 weeks of progressive dyspnoea on exertion, paroxysmal nocturnal dyspnoea, chest tightness, and upper and lower extremity swelling. The patient had a history of Hodgkin's lymphoma diagnosed at age 17 with right neck lymph node dissection followed by right neck radiation performed in her native Cuba. She later developed recurrent disease in her chest at age 24 and underwent a lymph node dissection followed by mediastinal radiation. At age 43, she developed a right clavicular sarcoma, which was resected. At age 59, she developed bilateral breast cancer and underwent bilateral mastectomy followed by tamoxifen therapy. At age 60 she developed right subclavian artery stenosis and, due to failed stenting, underwent subsequent subclavian artery bypass which became occluded. She was then placed on chronic anticoagulation with warfarin. At age 66, she was diagnosed with pericardial constriction, as well as severe tricuspid regurgitation and underwent pericardiectomy and tricuspid valve replacement. She was born in Cuba and immigrated to New York City. She did not smoke, drink alcohol or use illicit drugs. Her family history was unremarkable. On review of systems, she denied fever, chills, cough, haemoptysis, sick contacts, trauma or recent travel. She did complain of worsening sinus congestion with facial and upper extremity swelling. The patient also had a history of iron-deficiency anaemia, believed to be secondary to chronic epistaxis in the setting of warfarin use. She had been on and off warfarin given her epistaxis. Her other medications included carvedilol, losartan and furosemide.
On presentation she was afebrile and haemodynamically stable but noted to have a respiratory rate of 22 bpm and oxygen saturation was 97% while breathing 2 L of supplemental oxygen. Physical exam was notable for an ill-appearing woman with accessory respiratory muscle use, decreased breath sounds in the base of the right lung field, and 2+ lower extremity oedema as well as 2+ right upper extremity oedema. She had facial plethora as well as distention and increased tortuosity of external neck veins (figure 1).
Figure 1.
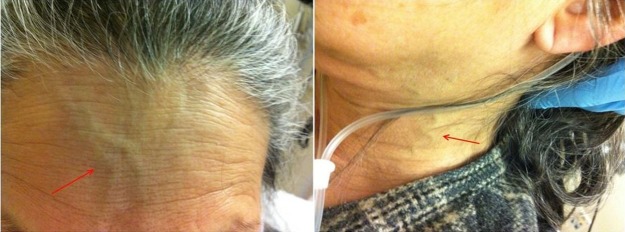
Facial plethora, venous distention and increased tortuosity of the external neck veins in our patient.
Investigations
Initial lab work was significant for an International Normalised Ratio 2.62 and brain natriuretic peptide level=263 pg/mL. Her ECG demonstrated normal sinus rhythm with a right bundle branch block, a left anterior fascicular block, and left ventricular hypertrophy with QRS widening, which when compared to a prior ECG demonstrated a new bifascicular block. A chest X-ray showed new right-sided opacities and new small bilateral pleural effusions. Based on these findings, the impression of her admitting physician was that of an exacerbation of congestive heart failure and a diuretic was given. On receiving furosemide intravenously, her blood pressure dropped to 80/50 which subsequently increased with a 500 cc bolus of normal saline. Her respiratory status remained unchanged. An echocardiogram was performed which showed normal left ventricular size and function with ejection fraction >55%, paradoxical septal motion, moderate mitral regurgitation, bioprosthetic tricuspid valve, mild/moderate left atrial enlargement, mild right atrial enlargement, mild global right ventricular dysfunction, with no significant pericardial effusion. These findings were similar to an echocardiogram obtained at another hospital 3 months prior. Pulmonary function test performed 1 year prior were obtained which showed forced expiratory volume in 1 s, FEV1=24% of predicted and forced vital capacity, FVC=25% of predicted as well as reduced total lung capacity.
A CT scan of the chest and neck was performed which showed a dense right lower lobe consolidation, bilateral pleural thickening with small bilateral pleural effusions, right paramediastinal fibrosis, non-visualisation of the right internal jugular vein and non-filling of the left brachiocephalic vein with prominent left chest wall venous collateral and filling of the SVC via the azygous system, consistent with SVC syndrome.1
Differential diagnosis
It was difficult to discern a single aetiology of our patient's progressive dyspnoea in light of her multiple cardiopulmonary comorbidities. From a cardiac function standpoint, her echocardiogram did not reveal a decline in ventricular or valvular function. From a pulmonary perspective, she was ruled out for a pulmonary embolism although her CT scan of the chest did show a dense right lower lobe consolidation which could have represented pneumonia, but her symptoms failed to improve after two courses of antibiotics. Given that the new finding of SVC syndrome most closely coincided with the development of her symptoms, we concluded that the primary aetiology of her dyspnoea was related to SVC syndrome.
Treatment
The patient underwent a right upper extremity venogram via the right upper extremity cephalic vein which demonstrated bilateral brachiocephalic vein and distal superior vein occlusion with venous return primarily via the azygous system. Several attempts were made to cross the supra-azygous SVC with a guidewire without success. She was deemed a high-risk operative candidate for an open thrombectomy and decision was made for medical management.
Outcome and follow-up
Further workup included a CT scan of the chest, pelvis and lower extremities which did not show pulmonary embolism or deep venous thrombosis. She completed a course of antibiotics for pneumonia without improvement in her symptoms. Her oxygen saturation remained around 80% breathing ambient air with minimal ambulation and accessory muscle use. CT positron emission tomography did not show the right lower lobe consolidation or pleural effusions to be fluorodeoxy-glucose-avid.
Discussion
Over 90% of cases of SVC syndrome are caused by malignancy, mostly lung cancer. In our patient's case the cause of the obstruction was not a mass, but rather venous fibrosis likely a consequence of her prior radiation therapy. Prior cases of radiation-induced SVC syndrome are reported in the literature.2 Radiation fibrosis, along with central venous catheters, arteriovenous fistulas and haemodialysis catheters account for the remaining 5–10% of causes of SVC syndrome.3 Finally, an important cause of SVC syndrome in the past was infection, but this has been substantially decreased in the antibiotic era.4
Reports of radiation-induced SVC syndrome are rare in the medical literature.2 4 A total of four reported cases were identified in the literature. Van Putten et al2 reported two cases, which occurred in a patient with lung cancer receiving external beam radiation for isolated mediastinal recurrence and another receiving radiation therapy to cervical lymph nodes. Lee et al5 report on another case which occurred 20 years after radiation therapy for anterior mediastinal germ cell tumour. Dhaliwal et al6 report the case of a man who developed SVC syndrome 4 years after receiving radiation for an unresectable malignant thymoma.
While the pathogenesis of radiation-induced injury to large vessels following radiation is not completely understood, it is hypothesised that these changes are caused by the effects of radiation on the vasa vasorum of the large vessel wall and the cells in the tunica intima layer. Radiation stimulates the proliferation of the intimal cells and the formation of generalised thickening in the tunica intima. Furthermore, since the vasa vasorum is the microvasculature that supplies oxygen to the large blood vessels, radiation-induced loss of the vasa vasorum may cause hypoxic conditions leading to intimal proliferation. The chronic hypoxic conditions may increase the late vascular damage of these large arteries. The obstruction of these large vessels may also be caused by direct fibrosis of the muscles and surrounding tissue that entrap the vessel contributing to the obstruction.2 7
Subclavian artery disease, present in our patient, has also been reported as a long-term consequence of radiation therapy.8 Of 415 patients, 10.4% developed coronary artery disease, 7.4% developed carotid and/or subclavian artery disease and 6.2% developed clinically significant valvular dysfunction. The most common valve lesion was aortic stenosis, which occurred in 14 valves.9 Veins are apparently less prone to developing radiation-induced fibrosis, probably because their walls are less cellular.2
Perhaps one of the most important lessons learned from this patient is the long-term consequences and sequelae of not only radiation therapy, but medical therapy in general. In 1958, when this patient first received radiation therapy, cure rates previously unattained were being seen for Hodgkin's lymphoma. This new and exciting field was pushed ahead, but the long-term consequences were unknown. Much of our patient's complex medical history can almost certainly be drawn back to her early exposure to radiation. New treatments are often given to our patients with excitement for positive results. But, it is important to remember that we lack long-term outcome data for many of these treatments. Our case demonstrates that understanding the implications of the therapies we prescribe our patients is of paramount importance.
Learning points.
Over 90% of cases of superior vena cava (SVC) syndrome are caused by malignancy, mostly lung cancer.
Radiation-induced SVC syndrome needs to be included in the differential diagnosis in patients with a history of prior radiation exposure.
Arterial injury due to radiation is well-documented but venous fibrosis should be considered as well and may share the same pathogenesis.
Footnotes
Contributors: SVM and DJK were responsible for identifying the case, acquisition and analysis of data and preparation and submission of manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Eren S, Karaman A, Okur A. The superior vena cava syndrome caused by malignant disease: imaging with multi-detector row CT. Eur J Radiol 2006;59:93–103 [DOI] [PubMed] [Google Scholar]
- 2.Van Putten JW, Schlosser NJ, Vujaskovic Z, et al. Superior vena cava obstruction caused by radiation induced venous fibrosis. Thorax 2000;55:245–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornak A, Wicky S, Ris HB, et al. Endovascular treatment of stenoses in the superior vena cava syndrome caused by non-tumoral lesions. Eur Radiol 2003;13:950–6 [DOI] [PubMed] [Google Scholar]
- 4.Lanciego C, Chacon JL, Julian A, et al. Stenting as first option for endovascular treatment of malignant superior vena cava syndrome. Am J Roentgenol 2001;177:585–93 [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Doering R, Jihayel A. Radiation-induced superior vena cava syndrome. Tex Heart Inst J 1995;22:103–4 [PMC free article] [PubMed] [Google Scholar]
- 6.Dhaliwal RS, Das D, Luthra S, et al. Management of superior vena cava syndrome by internal jugular to femoral vein bypass. Ann Thorac Surg 2006;82:310–12 [DOI] [PubMed] [Google Scholar]
- 7.Knighton DR, Hunt TK, Scheuenstuhl H, et al. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science 1983;221:1283–5 [DOI] [PubMed] [Google Scholar]
- 8.Wilson CB, Lambert HE, Scott RD. Subclavian and axillary vein thrombosis following radiotherapy for carcinoma of the breast. Clin Radiol 1987;38:95–6 [DOI] [PubMed] [Google Scholar]
- 9.Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA 2003;290:2831–7 [DOI] [PubMed] [Google Scholar]


