Abstract
Advanced basal cell carcinomas are a subset of basal cell carcinomas that can be difficult to treat either due to their local invasiveness, proximity to vital structures, or metastasis. The incidence of all basal cell carcinoma is increasing in the United States, although it is not known whether advanced basal cell carcinomas (aBCCs) are also increasing. Recently, highly targeted therapy based on knowledge of the basal cell carcinoma pathogenesis has become available either commercially or through human clinical trials. These orally available drugs inhibit the Hedgehog signaling pathway, and lead to advanced basal cell carcinoma shrinkage that can enable preservation of adjacent vital organs. In this review, we outline the role of Hedgehog pathway inhibitors as well as other treatment modalities such as excision, radiotherapy and more traditional chemotherapy in treating advanced basal cell carcinomas. We also highlight current gaps in knowledge regarding the use and side effects of this targeted therapy.
Keywords: Basal cell carcinoma, Advanced basal cell carcinoma, Metastatic basal cell carcinoma, Smoothened inhibitors, Hedgehog pathway inhibitors, Vismodegib, Targeted therapy, Basal cell nevus syndrome, Gorlin’s syndrome
Introduction
The recent introduction of targeted therapy for advanced basal cell carcinomas (aBCCs) in the form of Hedgehog signaling pathway inhibitors represents one of the greatest triumphs of translational medicine, bridging basic science of a conserved developmental pathway with clinical application in patients with difficult-to-treat skin cancers [1, 2, 3, 4, 5]. In the coming years, the number of agents in this drug class is expected to increase. These drugs will be a powerful tool to complement, or in some cases substitute, for traditional treatment modalities for aBCCS, such as excision or radiotherapy.
Epidemiology
BCC comprises the majority of non-melanoma skin cancers (NMSCs) and is more common than all other human malignancies combined. Several lines of evidence suggest that the worldwide incidence of BCCs is increasing. In the United States, the diagnosis and treatment of NMSCs has increased dramatically with a growth rate of 77 % over the past two decades. The fastest growing group is in women under the age of 40 years [6, 7]. In other countries such as Singapore where incidence of BCCs is monitored, the rate of BCCs has been rising over the past several decades as well [8]. Overall, the reasons for this dramatic growth have been postulated to include the aging population, changes in sun exposure habits, environmental changes, migration patterns, and to a lesser extent, increased prevalence of immunosuppressant use [9, 10].
More than 2.8 million new cases of BCC are diagnosed each year in the United States alone, and are estimated to result in over 3,000 deaths [1]. Fortunately, BCCs are usually diagnosed early and treated [11]. Nevertheless, a recent large, retrospective analysis from a major academic center reported 5-year recurrence rates at 2–3 % [12]. Given the high incidence of BCCs, this recurrence rate results in a large number of BCCs that are not cured by surgical excision. Furthermore, BCCs that are extensive and infiltrate structures below the skin or abut vital structures such as the brain or eyes may be difficult to surgically clear without significant morbidity. Many of these may become locally advanced or metastasize. BCCs that metastasize to either local or distant lymph nodes or distant organs would best be addressed through systemic therapy. Together, these locally advanced or metastatic BCCs comprise a disease group termed “advanced BCCs” (aBCCs).
While accurate estimates of the incidence of aBCCs are difficult to obtain, in part due to the lack of widespread use of a staging system by dermatologists and lack of uniform reporting requirements for NMSCs, aBCCs are thought to represent roughly 1–10 % of all BCCs, with metastatic BCCs accounting for 0.0028–0.5 % [13–15]. From our clinical experience, patients presenting with aBCCs appear to fall into two categories, (1) those who present with aBCC due to delay in accessing medical attention; or (2) those who have BCCs that are intrinsically aggressive and are refractory or recur after treatment.
Economic, Physical and Psychosocial Impact of Advanced BCCs
NMSCs including BCCs account for 4.5 % of Medicare cancer expenditures, making it the fifth most costly cancer to treat [16, 17]. From 2005 to 2008, NMSCs accounted for $2.9 billion in average annual expenditures on cancer conditions among adults in the U.S. While aBCCs are uncommon, their size and location may require excision in the operating room with specialized surgical teams and postoperative hospital stays, likely leading to far greater medical costs than operable BCCs that can be managed in the office setting. When non-surgical modalities are utilized in aBCCs, such as radiation or chemotherapy, the cost of treating aBCCs rises dramatically, although there are no studies to date that have formally quantitated this. Therapeutic innovations such as Hedgehog pathway inhibitors may improve quality of life and extend the length of productive lifespan in some patients; however, this treatment has a significant financial cost, as a one-month supply of vismodegib currently exceeds $7,500. Patients who respond to the drug and tolerate side effects may be on this drug indefinitely, as currently there is insufficient data as to when this drug might be discontinued, heightening long-term costs.
Exploratory studies into the physical and psychosocial impact of advanced BCCs are underway; however, the disease can be challenging to characterize, in part due to variable progression and rarity of the disease [18, 19]. Anecdotal evidence from our clinical experience suggests that aBCC patients are subject to significant physical and psychological burden. Physical burdens including pain, blood loss with anemia and fatigue, infection risk with open wounds, limitations in movement or function due to location of aBCCs and side effects of surgery, radiation or chemotherapy. Psychosocial burdens from aBCC disease include depression, anxiety, social isolation, depleted financial resources from treatments, inability to find or maintain employment or inability to provide for or care for dependent family members. Even patients whose disease is stable or successfully treated can have significant limitation in functional ability due to scarring, disfigurement, and/or chronic pain.
Life expectancy in aBCC patients also contributes to the economic impact of this disease. Historically, reports of survival after diagnosis of distant metastasis in BCC patients were grim, estimated at 8–14 months [20]. A more recent retrospective case series from 1997–2011 suggested markedly improved survival in patients followed at a tertiary care center, with median survival time of 7 years [13]. Future analysis will be needed to identify factors that contribute to survival time, and the financial cost of any treatments associated with survival prolongation.
Therapeutic Innovation of Advanced BCC Treatment
Historically, treatment options available to patient with aBCCs were not necessarily based on an understanding of the molecular characteristics of BCC pathogenesis. Treatment options included surgery, radiation, and traditional chemotherapies such as cisplatin-based treatment, but without systematic trials to follow for efficacy and safety, in part due to the rarity and heterogeneity of the condition (Table 1). In essence, there was no clearly established or superior treatment modality for aBCCs.
Table 1.
Treatment modalities for advanced basal cell carcinoma (aBCC). Summary of therapeutic options, estimated response and recurrence rate and types of evidence
| Treatment modality | Type of aBCC | Response Rate* | Recurrence Rate | Type of Evidence in aBCCs | Possible Side Effects or Limitations | References | |
|---|---|---|---|---|---|---|---|
| Surgical | Excision with margin evaluation | Locally advanced | NA | 0.7 % after 4 years in a study including high risk facial lesions | No high quality studies comparing excision with Moh’s surgery in locally advanced BCCs alone due to rarity of disease; one randomized study included periocular BCCs with no difference in recurrence rate in excision (n = 5) versus Moh’s (n = 6) | incomplete removal, scarring, poor functional or cosmetic outcome | [12, 38–41] |
| Moh’s micrographic surgery | Locally advanced | NA | NA but likely less than excision | incomplete removal, skip areas | |||
| Field Therapy | Radiotherapy | Locally advanced | NA | 7.5 % after 4 years in study including high risk facial lesions | Randomized trial comparing radiotherapy with surgery including high risk lesions | Less cosmesis than surgical treatment, scarring and dyspigmentation, risk of secondary cancer | [41, 42] |
| Targeted chemotherapy | Smoothened inhibitor (vismodegib) | Locally advanced; metastatic | 30–43 % | 20 % | Cohort studies for response rate; small retrospective study for recurrence rate | Muscle spasms, dysgeusia, alopecia, fatigue, nausea | [30••, 43, 44] |
| Non-targeted chemotherapy | Cisplatin-based chemotherapy | Locally advanced; metastatic | 71 % | - | Case series of seven BCC patients with locally advanced disease using cisplatin and doxorubicin; case reports of cisplatin leading to complete response in a few metastatic patients | Severe nausea and vomiting, diarrhea, alopecia, joint pain, loss of balance, tinnitus, edema, fatigue | [45–49] |
* Estimated response rate based on range derived from cited references. Please see original articles for specific details and explanation for basis of estimate
In the 1990s, the connection between aberrations of the Hedgehog signaling pathway and BCCs in mice was made [21•]. Around the same time, multiple studies connected this pathway in humans with both sporadic BCCs and an autosomal dominant genetic syndrome predisposing to multiple BCCs, basal cell nevus syndrome (BCNS) or Gorlin-Goltz syndrome [22]. The majority of mutations in sporadic BCCs and BCNS patients occur in PTCH1 [23, 24], a protein that inhibits Smoothened. BCNS patients experience early onset and numerous BCCs, due to loss-of-heterozygosity in the PTCH1 gene. The second most common mutation in sporadic BCCs and BCNS patients are gain-of-function mutations of the Smoothened gene [25, 26]. Loss of PTCH1 results in the lack of Smoothened inhibition, leading to increases in GLI1 levels, changes in transcription, and subsequent tumorigenesis. Gain-of-function Smoothened mutations also leads to increased GLI1 levels and tumorigenesis [27]. A simplified schematic of the Hedgehog (Hh) pathway is presented in Fig. 1a.
Fig. 1.
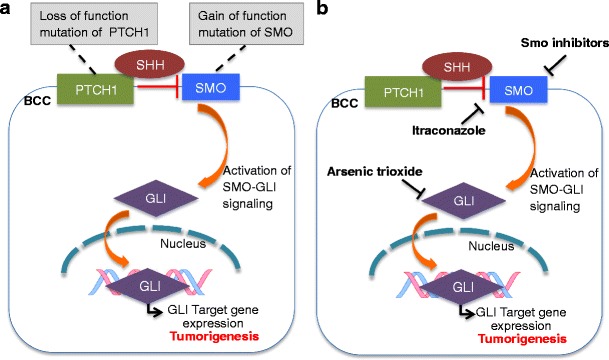
Simplified schematic of common mutations in the Hedgehog signaling pathway leading to basal cell carcinoma from pathway activation (a), and therapeutic targets of this pathway (b)
The story of how the first inhibitor of the Hh pathway, cyclopamine, was discovered is one of the most fascinating in biomedicine. In the 1960s, pregnant ewes ingesting the California corn lily were found to produce one-eyed offspring, or extreme holoprosencephaly [28]. In the 1970s, the active agent inducing these changes, cyclopamine, was isolated and its structural formula identified. Subsequent studies in the 1990s in chick embryos demonstrated cyclopamine’s ability to induce holoprosencephaly and to bind the transmembrane protein, Smoothened [29]. Subsequently, a number of analogues were developed by modifications to cyclopamine to improve solubility, and oral bioavailability. Collectively, these analogues are called Smoothened inhibitors (SIs), due to their targeting of the Smoothened protein (Fig. 1b).
Multiple orally available SIs are currently in human clinical trials against BCCs. The first and only U.S. Food and Drug Administration (FDA) approved SI for aBCCs to date is vismodegib, which became commercially available in 2012. The phase II study with 96 aBCC patients leading to FDA approval demonstrated an independently assessed response rate of 30 % in patients with metastatic BCC and 43 % response rate in locally advanced BCC [30••]. A subsequent study of 119 aBCC patients showed similar findings [31]. The availability of vismodegib as highly targeted therapy for aBCCs is one of the greatest success stories in translational medicine.
While most SIs in human trials come from the research pipelines of pharmaceutical companies, two existing FDA approved drugs have shown activity against the Hh pathway. These are oral ketoconazole and intravenous arsenic trioxide, both of which are being been tested in mouse models [32•] and a small number of BCC patients in the research setting, with results currently unpublished.
Role of Smoothened inhibitors in Advanced BCC Treatment
Along with innovation in therapy come many questions that remain to be answered. First, which aBCC patients are appropriate for SI treatment? An example clinical decision tree is shown in Fig. 2. However, given the heterogeneity of aBCC patients as far as tumor location and extent and comorbidities, each patient should be considered on a case-by-case basis, in conjunction with multidisciplinary consultation such as medical oncology, radiation oncology and surgical specialties. In addition, patients may have differential tolerance for side effects of SIs. As a class, these side effects include muscle spasms, taste disturbance, alopecia, nausea, and fatigue [33]. Anecdotally, muscle spasms have been ameliorated with muscle relaxants such as cyclobenzaprine. Nausea and poor oral intake have been addressed with megestrol acetate or dronabinol. SIs are also potent teratogens and two forms of medically reliable birth control should be used in patients (and their partners) with reproductive potential. Patients on SI treatment need to follow closely with their treating physicians to monitor for tumor response and side effects. In addition to regular skin checks, patients may need regular radiologic imaging to monitor for disease recurrence in cases where the aBCC has a deep component or is metastatic to non-skin organs. If the aBCC is refractory or recurrent, timely intervention with other treatment modalities is critical.
Fig. 2.
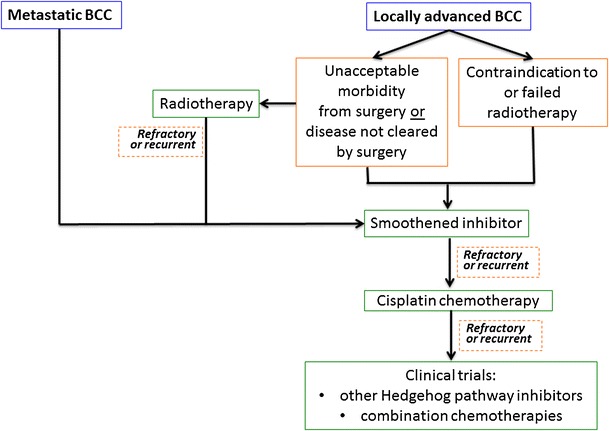
Example treatment algorithm for advanced basal cell carcinoma. Due to disease heterogeneity in advanced basal cell carcinomas, actual treatment depends on tumor location, prior treatments and patient comorbidities
Second, can the response rate for aBCCs be increased when combined with other treatment modalities, such as other chemotherapies or radiation treatment? Can recurrence rates be decreased when combined with other treatment modalities? Clinical trials are underway to explore these questions.
Third, what is the impact of SI treatment on progression free survival or overall survival in aBCCs? Given the rarity of this disease, multicenter registry studies are in progress to assess these.
Fourth, what is the impact of SI treatment on quality of life and psychosocial outcomes? The impact of SI treatment relates to the efficacy of the drug against the aBCC, as well as the tolerability of the side effects. Compared to many other forms of chemotherapy, the side effects of SI may be more tolerable. Alternatively, muscle spasms or other side effects may limit the ability of patients to take the drug in the long term, and/or maintain a good quality of life despite disease stabilization. Patient-based instruments recently have been developed to better assess these endpoints [18, 19].
Fifth, are there clinical predictors for response to SI treatment? One analysis from an expanded access study in patients for vismodegib identified prior treatment with systemic therapy as negatively correlated with laBCC response in 56 patients [34]. There was no association with age, prior radiotherapy or number of sites affected with aBCCs. In this same study, analysis of 39 metastatic BCCs showed that neither age, prior radiotherapy, prior systemic therapy or number of sites affected with aBCCs were associated with tumor response. Larger prospective studies are underway to assess clinical predictors for SI response.
Sixth, are there tumor markers from biopsies to predict response to SI treatment? Given the aggressiveness of many aBCCs, the decision of which treatment modality to undertake could be life-saving. Current studies are underway to assess for mutations, gene expression changes or protein levels within the Hh signaling pathway [35–37] that could correlate with response or lack of response, with the ultimate goal of assisting with clinical decision-making. As the current cost of SI drugs is quite high, a diagnostic test that could indicate the likelihood of response to these drugs may be cost effective.
Many additional issues remaining to be clarified regarding the use of SIs, including: effective management of common side effects, long-term side effects such as the potential for secondary cancers, whether there is differential response based on BCC subtype, when SI treatment may be safely discontinued after apparent complete clinical response, and whether SIs should be used to treat microscopic disease in surgical cases where margins are positive.
Conclusion
Advanced BCCs are often difficult to treat and life-threatening. SI drugs can be life-saving in many cases, though disease progression or recurrence is a major concern that requires regular monitoring through skin examinations and/or radiologic imaging. Successful management of side effects is critical to continued treatment with these drugs. When aBCCs are refractory, achieve a partial response, or are recurrent after SI therapy, other treatment modalities such as radiation, traditional chemotherapy or surgical excision need to be considered for optimum patient outcomes.
Compliance with Ethics Guidelines
Conflict of Interest
SV Mohan declares no conflicts of interest.
ALS Chang has received research support from Genentech, Novartis, Infinity, and Lilly.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Shalini V. Mohan, Email: shalinim@stanford.edu
Anne Lynn S. Chang, Phone: +1-650-7217151, FAX: +1-650-7213464, Email: alschang@stanford.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Gould A, Missailidis S. Targeting the hedgehog pathway: the development of cyclopamine and the development of anti-cancer drugs targeting the hedgehog pathway. Mini Rev Med Chem. 2011;11(3):200–13. doi: 10.2174/138955711795049871. [DOI] [PubMed] [Google Scholar]
- 2.Sekulic A, Mangold AR, Northfelt DW, LoRusso PM. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25(3):218–223. doi: 10.1097/CCO.0b013e32835ff438. [DOI] [PubMed] [Google Scholar]
- 3.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, Epstein EH., Jr Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;386(23):2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr, Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 5.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–54. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 6."Skin Cancer Foundation." Basal Cell Carcinoma (BCC). Skin Cancer Foundation, 2013. Web. 09 June 2013.
- 7.Wu TP, Stein JA. Nonmelanoma skin cancer in young women. J Drugs Dermatol. 2013;12:568–572. [PubMed] [Google Scholar]
- 8.Koh D, Wang H, Lee J, Chia KS, Lee HP, Goh CL. Basal cell carcinoma, squamous cell carcinoma and melanoma of the skin: analysis of the Singapore Cancer Registry. Br J Dermatol. 2003;148:1161–1166. doi: 10.1046/j.1365-2133.2003.05223.x. [DOI] [PubMed] [Google Scholar]
- 9.Weinstock MA, Fisher DE. Indoor ultraviolet tanning: what the data do and do not show regarding risk of melanoma and keratinocyte malignancies. J Natl Compr Canc Netw. 2010;8(8):867–72. doi: 10.6004/jnccn.2010.0063. [DOI] [PubMed] [Google Scholar]
- 10.Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer. 2009;125(4):909–17. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam M, Goldberg LH, Silapunt S, Gardner ES, Strom SS, Rademaker AW, Margolis DJ. Delayed treatment and continued growth of nonmelanoma skin cancer. J Am Acad Dermatol. 2011;64(5):839–48. doi: 10.1016/j.jaad.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Chren MM, Linos E, Torres JS, Stuart SE, Parvataeni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133:1188–96. doi: 10.1038/jid.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danial C, Lingala B, Balise R, Oro AE, Reddy S, Colevas A, Chang AL. Markedly improved overall survival in 10 consecutive metastatic basal cell carcinoma patients. Br J Dermatol 2013: 1365–2133. [DOI] [PMC free article] [PubMed]
- 14.Soleymani AD, Scheinfeld N, Vasil K, Bechtel MA. Metastatic basal cell carcinoma presenting with unilateral upper extremity edema and lymphatic spread. J Am Acad Dermatol. 2008;59(2 suppl 1):s1–s3. doi: 10.1016/j.jaad.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Moser S, Borm J, Mihic-Probst D, Jacobsen C, Kruse Gujer AL. Metastatic basal cell carcinoma: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2013 [DOI] [PubMed]
- 16.Machlin S, Carper K, Kashihara D. Health Care Expenditures for Non-Melanoma Skin Cancer among Adults, 2005–2008 (Average Annual) Rockville: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 17.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–87. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 18.Haves AW, Schaffer PR, Carucci JA. The Impact of Inoperable Advanced Basal Cell Carcinoma: the Economic, Physical and Psychological Burden of the Disease. J Drugs Dermatol. 2013;12(10):s151–53. [PubMed] [Google Scholar]
- 19.Shingler SL, Garside J, Samanta K, Lear JT, Keohane S, Lloyd AJ. Utilities for advanced basal cell carcinoma. J Med Econ. 2013;16(6):777–83. doi: 10.3111/13696998.2013.800822. [DOI] [PubMed] [Google Scholar]
- 20.Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149(5):615–6. doi: 10.1001/jamadermatol.2013.3064. [DOI] [PubMed] [Google Scholar]
- 21.•.Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276(5313):817–21. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 22.Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Derm. 1998;110(6):885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 23.Wollina U, Tchernev G. Advanced basal cell carcinoma. Wien Med Wochenschr 2013 [DOI] [PubMed]
- 24.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8(10):743–54. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391(6662):90–2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 26.Skvara H, Kalthoff F, Meingassner JG, Wolff-Winiski B, Aschauer H, Kelleher JF, Wu X, Pan S, Mickel L, Schuster C, Stary G, Jalili A, David OJ, Emotte C, Antunes AM, Rose K, Decker J, Carlson I, Gardner H, Steutz A, Bertolino AP, Stingl G, De Rie MA. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131(8):1735–44. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 27.Atwood SX, Chang AS, Oro AE. Hedgehog pathway inhibition and the race against tumor evolution. J Cell Biol. 2012;199(2):193–7. doi: 10.1083/jcb.201207140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeler RF, Binns W. Teratogenic compounds of Veratrum Californicum Coparison of Cyclopian effects of steroidal alkaloids. Teratology. 1968;1:5–10. doi: 10.1002/tera.1420010103. [DOI] [PubMed] [Google Scholar]
- 29.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smothened. Gene Dev. 2002;16:2743–48. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.••.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Soloman JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin CM, Chang AL, Low JA, Mackey HM, Yauch RL, Graham RA, Reddy JC, Hauschild A. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang AL, Atwood SX, Tartar DM, Oro AE. Surgical excision after neoadjuvant therapy with vismodegib for a locally advanced basal cell carcinoma and resistant basal carcinomas in Gorlin syndrome. JAMA Dermatol. 2013;149(5):639–41. doi: 10.1001/jamadermatol.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.•.Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, Riggins GJ, Epstein EH, Jr, Beachy PA, Rudin CM. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23(1):23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimeno A, Weiss GJ, Miller WH, Gettinger S, Eigl BJ, Chang AL, Dunbar J, Devens S, Faia K, Skliris G, Kutok J, Lewis KD, Tibes R, Sharfman WH, Ross RW, Rudin CM. Phase 1 study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19(10):2766–74. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang ALS, Soloman JA, Hainsworth JD, Goldberg L, McKenna E, Day B, Chen DM, Weiss GJ. Expanded access study of advanced basal cell carcinoma patients treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2013. [DOI] [PubMed]
- 35.Li ZJ, Mack SC, Mak TH, Angers S, Taylor MD, Hui CC. Evasion of p53 and G2/M checkpoints are characteristic of Hh-driven basal cell carcinoma. Oncogene 2013. [DOI] [PubMed]
- 36.Heller ER, Gor A, Wang D, Hu Q, Lucchese A, Kanduc D, Katdare M, Liu S, Sinha AA. Molecular signatures of basal cell carcinoma susceptibility and pathogenesis: a genomic approach. Int J Oncol. 2013;42(2):583–96. doi: 10.3892/ijo.2012.1725. [DOI] [PubMed] [Google Scholar]
- 37.Almquist LM, Karagas MR, Christensen BC, Welsh MM, Perry AE, Storm CA, Nelson HH. The role of TP53 and MDM2 polymorphisms in TP53 mutagenesis and risk of non-melanoma skin cancer. Carcinogenesis. 2011;32(3):327–30. doi: 10.1093/carcin/bgq256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuijpers DI, Thissen MR, Berretty PJ, Ideler FH, Nelemans PJ, Neumann MH. Surgical excision versus curettage plus cryosurgery in the treatment of basal cell carcinoma. Dermatol Surg. 2007;33(5):579–87. doi: 10.1111/j.1524-4725.2007.33117.x. [DOI] [PubMed] [Google Scholar]
- 39.Smeets NW, Kuijpers DI, Nelemans P, Ostertag JU, Verhaegh ME, Krekels GA, Neumann HA. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face—results of a retrospective study and review of the literature. Br J Dermatol. 2004;151(1):141–7. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JR, Parvataneni R, Stuart SE, Chrem MM. Recurrence 5 years after treatment of recurrent cutaneous basal cell and squamous cell carcinoma. JAMA Dermatol. 2013;149(5):616–8. doi: 10.1001/jamadermatol.2013.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avril MF, Auperin A, Margulis A, Gerbaulet A, Duvillard P, Benhamou E, Guillaume JC, Chalon R, Petit JY, Sancho-Garnier H, Prade M, Bouzy J, Chassagne D. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. Br J Cancer. 1997;76(1):100–6. doi: 10.1038/bjc.1997.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall VL, Leppard BJ, McGill J, Kesseler ME, White JE, Goodwin P. Treatment of basal-cell carcinoma: comparison of radiotherapy and cryotherapy. Clin Radiol. 1986;37(1):33–4. doi: 10.1016/S0009-9260(86)80161-1. [DOI] [PubMed] [Google Scholar]
- 43.Amin SH, Tibes R, Kim JE, Hybarger CP. Hedgehog antagonist GDC-0449 is effective in the treatment of advanced basal cell carcinoma. Laryngoscope. 2010;120(12):2456–9. doi: 10.1002/lary.21145. [DOI] [PubMed] [Google Scholar]
- 44.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM, Lum BL, Darbonne WC, Marsters JC, Jr, de Sauvage FJ, Low JA. Inhibition of hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 45.Moeholt K, Aagaard H, Pfeiffer P, Hansen O. Platinum-based cytotoxic therapy in basal cell carcinoma a review of the literature. Acta Oncol. 1996;35(6):677–82. doi: 10.3109/02841869609083998. [DOI] [PubMed] [Google Scholar]
- 46.Guthrie TH, Porubsky ES, Luxenberg MN, Shah KJ, Wurtz KL, Watson PR. Cisplatin-based chemotherapy in advanced basal cell carcinoma and squamous cell carcinoma of the skin: results in 28 aptients including 13 patients receiving multimodality therapy. J Clin Oncol. 1990;8(2):342–6. doi: 10.1200/JCO.1990.8.2.342. [DOI] [PubMed] [Google Scholar]
- 47.Denic S. Preoperative treatment of advanced skin carcinoma with cisplatin and bleomycin. Am J Clin Oncol. 1999;22(1):32–4. doi: 10.1097/00000421-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Carneiro BA, Watkin WG, Mehta UK, Brockstein BE. Metastatic basal cell carcinoma: complete response to chemotherapy and associated pure red cell aplasia. Cancer Invest. 2006;24(4):396–400. doi: 10.1080/07357900600705474. [DOI] [PubMed] [Google Scholar]
- 49.Khandekar JD. Complete response of metastatic basal cell carcinoma to cisplatin chemotherapy: a report of two patients. Arch Dermatol. 1990;126(12):1,660. doi: 10.1001/archderm.1990.01670360128037. [DOI] [PubMed] [Google Scholar]


