Abstract
Merkel cell polyomavirus (MCPyV) is a DNA virus whose pathogenic mechanisms in Merkel cell carcinoma (MCC) are still being unraveled. Emerging reports of an association between Merkel cell polyomavirus and chronic lymphocytic lymphoma have begun to broaden our understanding of the oncogenic mechanisms of this virus, and the known association between these two malignancies. Herein, we report a case Merkel cell carcinoma demonstrating a B-cell immunophenotype arising in a patient with chronic lymphocytic lymphoma (CLL) being treated with rituximab. In this context, we discuss the differential diagnostic considerations, especially with cutaneous Richter transformation (diffuse large B-cell lymphoma). We also assessed for the presence of MCPyV in both the patient’s MCC and the CLL. Finally, we provide a large meta-analysis of patients with CLL and MCC. Patients with both MCC and CLL have a dismal prognosis, with greater than 50% overall mortality within the first year and a half after MCC diagnosis.
Keywords: Merkel cell polyomavirus, Merkel cell carcinoma, chronic lymphocytic lymphoma, Richter transformation
Introduction
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine tumor occurring most often in the skin of elderly patients, some of which are immunocompromised.(1) Merkel cell polyomavirus (MCPyV) is a non-enveloped double stranded human DNA virus detected and implicated in the pathogenesis of MCC.(2–7) Because patients with chronic lymphocytic lymphoma (CLL) have altered immunologic status related to their disease burden, they are at higher risk for developing a range of secondary malignancies, with MCC being one of the more potentially aggressive.(8, 9). In fact, a possible pathogenic link between MCC and CLL is suggested by the respective increased incidence of either cancer (MCC or CLL) occurring in patients with one or the other cancer types.(10–12) We present a case of primary cutaneous MCC mimicking a large B-cell transformation in a patient with CLL, assess for the presence of MCPyV, and perform a metanalysis of similar reported cases.
Case Report
A 65 year old male with a 7 year history of CLL presented with a single 2.0 cm subcutaneous nodule near the medial epicondyle. At this time, he was being evaluated for treatment of his CLL as he had developed thrombocytopenia, splenomegaly and fatigue related to his disease. The initial impression was that the lesion was felt to be most likely adenopathy related to progressive CLL. He underwent 2 cycles of treatment with fludarabine/cyclophosphamide/rituximab (FCR) at which point the upper extremity lesion was noted to progress rapidly in size without progression of CLL elsewhere. Due to the location of the lesion, clinical progression, and lack of overlying epidermal change, the differential diagnosis was expanded to include an enlarged trochlear lymph node, a deeply infiltrative tumor, or an abscess. An excisional biopsy demonstrated a deep atypical homogeneous infiltrate of medium to large cells with regular round nuclear contours and vesicular to granular chromatin (fig. 1A). Overt nuclear molding was not readily identified. While no superficial dermal or epidermal involvement was noted, no definitive capsular nodal tissue was identified either. Due to the clinical picture and lack of more definitive epidermal involvement, the differential diagnosis included a transformed CLL (ie Richter transformation in the form of deep dermal/subcutaneous diffuse large B-cell lymphoma). The cells were negative for CD3/CD5/CD23 and CD20 by IHC. Because the patient was treated with rituximab (a humanized anti-CD20 antibody), the negative CD20 finding was not unexpected, and additional hematolymphoid and B-cell markers were employed. The tumor cells were PAX5 positive (fig. 1B) and TdT was also positive in 10% of the tumor nuclei (fig. 1C). Due to the granular chromatin pattern and lack of prominent nucleoli, tumor cells were stained with, and positive for, pancytokeratin, CK20 (fig. 2A), chromagranin (fig. 2B), and CK8/18. CK7 was negative. The diagnosis of a deep dermal MCC with subcutaneous involvement was made. The lesion recurred locally after 1 month. At five months, metastatic disease was noted in the skin, axillary lymph node and lung. The patient was dead of disease at 10 months.
Figure 1.
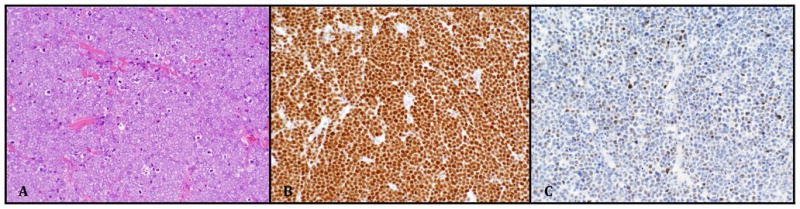
A) Merkel cell carcinoma with granular chromatin pattern (H&E 200x). B) Diffuse staining for PAX-5 (200x) and C) partial nuclear staining for TdT (200x).
Figure 2.
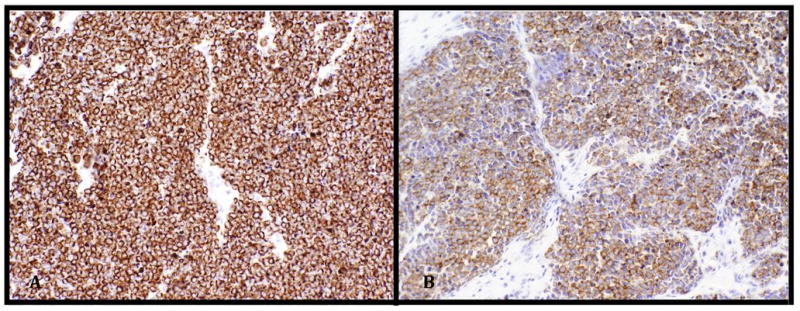
A) Merkel cell carcinoma cells staining diffusely positive for CK20 (with distinctive paranuclear dot-like pattern, characteristic of MCC) and B) chromogranin (both 200x)
In order to investigate for the presence of MCPyV, we performed PCR amplification of a ~350 bp segment of the MCPyV large-T antigen from the primary and relapse MCC specimens as well as CLL-involved bone marrow and normal fat tissue (Figure 3). This was accomplished by extracting total DNA and de-crosslinking followed by PCR and Sanger sequencing of each specimen by previously published methods (2). This revealed MCPyV positivity in both the primary and relapsed MCC tumors whereas the CLL-involved bone marrow and normal fat biopsy specimens were negative for MCPyV.
Figure 3.
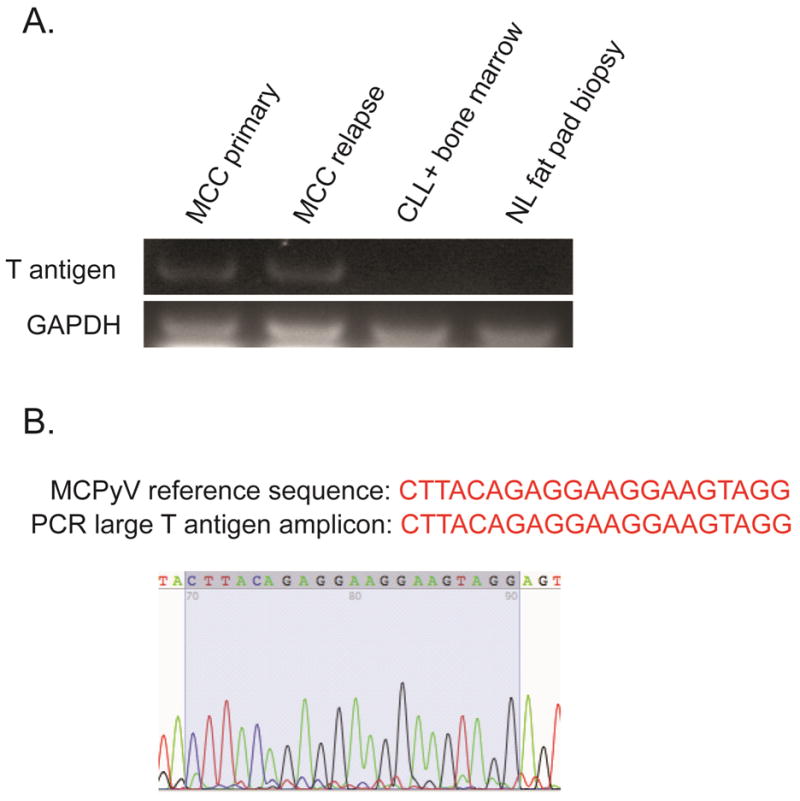
A) PCR amplicons using MCPyV large T antigen and GAPDH-specific PCR primers (20 ng of total genomic DNA per PCR reaction) and B) Sanger sequencing confirmation of MCPyV sequence in 356 bp large T antigen PCR amplicon. DNA extraction, de-crosslinking, and PCR sequencing of MCPyV is described elsewhere (2).
Discussion
The acknowledgement of the association of MCC arising in patients with CLL began in earnest in the mid-90’s with approximately 66 cases having been reported.(9, 11–31) Between 1986 and 2002, in a SEER review of 17,315 CLL patients, 14 patients would also develop MCC. (31) Heath et al found a 34–48-fold (depending on patient age) increased association of CLL in MCC patients when compared to the expected incidence in the US population.(30) Similar increased incidence of CLL in MCC patients has been described in Finnish and Dutch population studies as well.(10, 11)
The mechanisms underlying the association are still being elaborated. The oncogenic portions of the viral genome (lacking the viral replication constructs) are the portions of the MCPyV DNA detected in MCC.(32) While this finding is believed to underlie the role this virus plays in certain MCC, the significance between the association of MCPyV in CLL is currently unclear (33), with some arguing for (34) and others against (24) an oncogenic relationship. Furthermore, MCPyV DNA has also been detected in both basal cell and squamous cell carcinomas (with a higher frequency of detection in tumors from immunocomprimised individuals), supporting a more promiscuous link between the oncogenic virus and various malignancies.(35) We were able to detect MCPyV DNA in the primary and relapsed tumor, however we did not detect it in a bone marrow sample infiltrated by CLL, a finding that supports a less direct relationship between MCPyV and CLL, at least in this particular case. From a clinical and diagnostic perspective, we believe the current case deserves special attention because of the expression of a partial hematolymphoid (B-cell) immunophenotype and the deep location of the tumor with the resulting impression of adenopathy.
First, in the context of PAX-5 positive staining, the main differential diagnosis includes transformed CLL (diffuse large B-cell transformation, ie Richter’s transformation), progressive/refractory CLL, or other B cell malignancies. PAX-5 is a B-cell transcription factor that is expressed early on, and maintained throughout, pre-plasma cell B-cell development.(36) Since becoming commercially available, the antibody was found to be reactive against various non-hematopoietc tissues, including neuroendocrine carcinomas and MCC.(37–39) This association has not been widely reported outside the general surgical pathology or hematopathology literature. Some of the latest reports describing the immunohistochemical profile of MCC in dermatology/dermatopathology journals fail to discuss either PAX-5 or TdT, even after commenting on the association with CLL.(40–42) Furthermore, with 90% and 73% of MCC expressing PAX-5 and TdT respectively(39), a number of additional reports failed to employ these markers in differentiating MCC from basal cell carcinoma(42), cutaneous neuroendocrine carcinoma metastasis(43, 44), microcytic adnexal carcinoma(45), or MCC with squamous and/or sarcomatoid differentiation.(46, 47)
Another atypical feature of this case is the deep dermal and subcutaneous localization of the MCC without prominent epidermal changes. In a patient with CLL, this simulated nodal involvement and possible disease progression. Cutaneous lymphoid involvement in patients with CLL occurs in 4–20% of cases,(48) with up to 17% of patients with advanced disease presenting with skin lesions.(49) When there is cutaneous involvement by lymphoma in patients with CLL, the infiltrate almost always involves the deep dermis and subcutaneous tissues, similar to the distribution of MCC in the current case.(49) Cytologically however, MCC overlaps more with transformed (diffuse large B-cell lymphoma) CLL than with the conventional small cell morphology. Large cell (Richter’s) transformation in CLL develops in 2–8% of CLL patients.(50) Transformation with resulting cutaneous lesions is rare but is reported.(51–56) Patients with cutaneous transformation tend to be older than patients with extra-cutaneous transformation, further overlapping with the age of onset of Merkel cell carcinoma.(51, 57, 58) A small percentage (3%) of CLL patients with PET/CT findings considered highly suspicious for Richter’s transformation may in fact have MCC instead.(59) Conversely, nodal MCC and/or distant metastases may occur without detection of a primary cutaneous lesion in 10–20% of cases.(60)
The five-year overall and relative survivals for patients with MCC is 40% and 54% respectively.(61) Five-year disease specific survival has also been reported at 64%.(62) Some investigators have linked the presence of MCPyV DNA with better survival.(7) Although the confounding effect of CLL cannot be overstated, from our meta-analysis, cases of patients with CLL and MCC where survival was reported have an overall mortality rate of 54% by 17 months (table 1). This more aggressive clinical course corresponds with that reported by Khezri et al. who (in a series of 6 patients with MCC and CLL) found that low stage (MCC negative node biopsy) patients did worse than similar MCC patients without superimposed CLL.(25)
Table 1.
Clinical comparison of patients (including the current one) against series of patients with Merkel cell carcinoma only.
| From references below | Heath et al | Lemos et al | Allen et al | |
|---|---|---|---|---|
| Median age at MCC dx/(m/f) | 72.8 (25/12) | 69 (114/81) | 76 (61%/39%)* | 69 (150/101) |
| Years with CLL before MCC | 4.56 | 4.1% with CLL | n/a | n/a |
| largest dimension (cm) | 2.4 | 1.8 | n/a | 1.5 cm |
| % with metastatic dz at dx | 38% | 43% | 34% | 30% |
| % with local recurrence (time until) | 32% (3 mo) | n/a | n/a | 8% |
| Survival | 54% dead by 17 mo | n/a | 40% survival at 5 years | 5 yr DSS 64% |
Note: Only patients (n=37) with reported clinical information were included in this table. They are summarized from references 9, 12–27. MCC, Merkel cell carcinoma; CLL, chronic lymphocytic lymphoma; dx, diagnosis; m, male; f, female; dz, disease; DSS, disease specific survival; mo, months;
percentage of males and females.
In summary, we report a case of Merkel cell carcinoma with a partial B-cell immunophenotype and deep dermal/subcutaneous involvement in a patient with a history of CLL. The initial clinicopathologic consideration was transformed diffuse large B-cell lymphoma (Richter’s transformation in CLL). MCC tumor cells are often positive for PAX-5 and TdT, markers which are commonly seen in transformed CLL. Not surprisingly, patient with both MCC and CLL have a dismal prognosis, with greater than 50% overall mortality within the first year and a half after MCC diagnosis. Although we detected MCPyV in the patients MCC, we did not detect it in a bone marrow sample infiltrated by CLL. By highlighting both the increased frequency of the development of MCC in CLL patients and more importantly the partial immunophenotypic overlap, we hope to raise awareness of these clinicopathologically confounding issues.
Acknowledgments
Source of Funding:
Part of this work was supported by a training grant from the NHLBI (T32 HL 07057-37)
Footnotes
Conflicts of Interest:
All authors declare no conflicts of interest in the manuscript, including financial, consultant, institutional and/or other relationships that might lead to bias or a conflict of interest.
References
- 1.Kuwamoto S. Recent advances in the biology of Merkel cell carcinoma. Hum Pathol. 2011;42(8):1063–77. doi: 10.1016/j.humpath.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg Infect Dis. 2008;14(9):1491–3. doi: 10.3201/eid1409.080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68(13):5009–13. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 5.Becker JC, Houben R, Ugurel S, Trefzer U, Pfohler C, Schrama D. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol. 2009;129(1):248–50. doi: 10.1038/jid.2008.198. [DOI] [PubMed] [Google Scholar]
- 6.Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009;129(1):246–8. doi: 10.1038/jid.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101(13):938–45. doi: 10.1093/jnci/djp139. [DOI] [PubMed] [Google Scholar]
- 8.Quaglino D, Di Leonardo G, Lalli G, et al. Association between chronic lymphocytic leukaemia and secondary tumours: unusual occurrence of a neuroendocrine (Merkell cell) carcinoma. Eur Rev Med Pharmacol Sci. 1997;1(1–3):11–6. [PubMed] [Google Scholar]
- 9.Vlad R, Woodlock TJ. Merkel cell carcinoma after chronic lymphocytic leukemia: case report and literature review. Am J Clin Oncol. 2003;26(6):531–4. doi: 10.1097/01.coc.0000037108.86294.5E. [DOI] [PubMed] [Google Scholar]
- 10.Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102(11):793–801. doi: 10.1093/jnci/djq120. [DOI] [PubMed] [Google Scholar]
- 11.Koljonen V, Kukko H, Tukiainen E, et al. Second cancers following the diagnosis of Merkel cell carcinoma: a nationwide cohort study. Cancer Epidemiol. 2010;34(1):62–5. doi: 10.1016/j.canep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Koljonen V, Kukko H, Pukkala E, et al. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br J Cancer. 2009;101(8):1444–7. doi: 10.1038/sj.bjc.6605306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey U, Naraynan M, Karnik U, Sinha B. Carcinoma metastasis to unexpected synchronous lymphoproliferative disorder: report of three cases and review of literature. J Clin Pathol. 2003;56(12):970–1. doi: 10.1136/jcp.56.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinclair N, Mireskandari K, Forbes J, Crow J. Merkel cell carcinoma of the eyelid in association with chronic lymphocytic leukaemia. Br J Ophthalmol. 2003;87(2):240. doi: 10.1136/bjo.87.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agnew KL, Ruchlemer R, Catovsky D, Matutes E, Bunker CB. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150(6):1129–35. doi: 10.1111/j.1365-2133.2004.05982.x. [DOI] [PubMed] [Google Scholar]
- 16.Robak E, Biernat W, Krykowski E, Jeziorski A, Robak T. Merkel cell carcinoma in a patient with B-cell chronic lymphocytic leukemia treated with cladribine and rituximab. Leuk Lymphoma. 2005;46(6):909–14. doi: 10.1080/10428190500057759. [DOI] [PubMed] [Google Scholar]
- 17.Papageorgiou KI, Kaniorou-Larai MG. A case report of Merkel cell carcinoma on chronic lymphocytic leukemia: differential diagnosis of coexisting lymphadenopathy and indications for early aggressive treatment. BMC Cancer. 2005;5:106. doi: 10.1186/1471-2407-5-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-David A, Lazarov A, Lev S, Nussbaum B. Merkel cell tumor and chronic lymphocytic leukemia--coincidence or a possible association? Dermatol Online J. 2005;11(3):16. [PubMed] [Google Scholar]
- 19.Barroeta JE, Farkas T. Merkel cell carcinoma and chronic lymphocytic leukemia (collision tumor) of the arm: a diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 2007;35(5):293–5. doi: 10.1002/dc.20616. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Gupta G, Yang XY, Rosenbluth R, Bhattacharyya PK. A unique case of merkel cell carcinoma and chronic lymphocytic leukaemia presenting in a single cutaneous lesion (collision tumour) BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.09.2008.1016. doi:bcr09.2008.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig PJ, Calonje JE, Harries M, Stefanato CM. Incidental chronic lymphocytic leukaemia in a biopsy of Merkel cell carcinoma. J Cutan Pathol. 2009;36(6):706–10. doi: 10.1111/j.1600-0560.2008.01115.x. [DOI] [PubMed] [Google Scholar]
- 22.Turk T, Orlic ZC, Smoljan I, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;27(3):7270. doi: 10.1186/1752-1947-3-7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartley MA, Tao J, Baz R. Merkel cell carcinoma in the peripheral blood of a patient with concomitant chronic lymphocytic leukemia and multiple myeloma. J Clin Oncol. 2010;28(7):e113–4. doi: 10.1200/JCO.2009.23.4153. [DOI] [PubMed] [Google Scholar]
- 24.Tolstov YL, Arora R, Scudiere SC, et al. Lack of evidence for direct involvement of Merkel cell polyomavirus (MCV) in chronic lymphocytic leukemia (CLL) Blood. 2010;115(23):4973–4. doi: 10.1182/blood-2010-03-273177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khezri F, Brewer JD, Weaver AL. Merkel cell carcinoma in the setting of chronic lymphocytic leukemia. Dermatol Surg. 2011;37(8):1100–5. doi: 10.1111/j.1524-4725.2011.02045.x. [DOI] [PubMed] [Google Scholar]
- 26.Cottoni F, Montesu MA, Lissia A, et al. Merkel cell carcinoma, Kaposi’s sarcoma, basal cell carcinoma and keratoacanthoma: multiple association in a patient with chronic lymphatic leukaemia. Br J Dermatol. 2002;147(5):1029–31. doi: 10.1046/j.1365-2133.2002.49859.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuhajda FP, Olson JL, Mann RB. Merkel cell (small cell) carcinoma of the skin: immunohistochemical and ultrastructural demonstration of distinctive perinuclear cytokeratin aggregates and a possible association with B cell neoplasms. Histochem J. 1986;18(5):239–44. doi: 10.1007/BF01676233. [DOI] [PubMed] [Google Scholar]
- 28.Silva EG, Mackay B, Goepfert H, Burgess MA, Fields RS. Endocrine carcinoma of the skin (Merkel cell carcinoma) Pathol Annu. 1984;19(Pt 2):1–30. [PubMed] [Google Scholar]
- 29.Brenner B, Sulkes A, Rakowsky E, et al. Second neoplasms in patients with Merkel cell carcinoma. Cancer. 2001;91(7):1358–62. doi: 10.1002/1097-0142(20010401)91:7<1358::aid-cncr1139>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1545–9. doi: 10.1158/1055-9965.EPI-05-0895. [DOI] [PubMed] [Google Scholar]
- 32.Shuda M, Feng H, Kwun HJ, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105(42):16272–7. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teman CJ, Tripp SR, Perkins SL, Duncavage EJ. Merkel cell polyomavirus (MCPyV) in chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Res. 2011;35(5):689–92. doi: 10.1016/j.leukres.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 34.Pantulu ND, Pallasch CP, Kurz AK, et al. Detection of a novel truncating Merkel cell polyomavirus large T antigen deletion in chronic lymphocytic leukemia cells. Blood. 2010;116(24):5280–4. doi: 10.1182/blood-2010-02-269829. [DOI] [PubMed] [Google Scholar]
- 35.Kassem A, Technau K, Kurz AK, et al. Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer. 2009;125(2):356–61. doi: 10.1002/ijc.24323. [DOI] [PubMed] [Google Scholar]
- 36.Torlakovic E, Torlakovic G, Nguyen PL, Brunning RD, Delabie J. The value of anti-pax-5 immunostaining in routinely fixed and paraffin-embedded sections: a novel pan pre-B and B-cell marker. Am J Surg Pathol. 2002;26(10):1343–50. doi: 10.1097/00000478-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Dong HY, Liu W, Cohen P, Mahle CE, Zhang W. B-cell specific activation protein encoded by the PAX-5 gene is commonly expressed in merkel cell carcinoma and small cell carcinomas. Am J Surg Pathol. 2005;29(5):687–92. doi: 10.1097/01.pas.0000155162.33044.4f. [DOI] [PubMed] [Google Scholar]
- 38.Torlakovic E, Slipicevic A, Robinson C, et al. Pax-5 expression in nonhematopoietic tissues. Am J Clin Pathol. 2006;126(5):798–804. doi: 10.1309/XEC7-JMW9-YRM7-4RNO. [DOI] [PubMed] [Google Scholar]
- 39.Buresh CJ, Oliai BR, Miller RT. Reactivity with TdT in Merkel cell carcinoma: a potential diagnostic pitfall. Am J Clin Pathol. 2008;129(6):894–8. doi: 10.1309/R494HQ9VRDJWDY30. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger T, Ring J, Andres C. Histological, immunohistological, and clinical features of merkel cell carcinoma in correlation to merkel cell polyomavirus status. J Skin Cancer. 2012;2012:983421. doi: 10.1155/2012/983421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lien MH, Baldwin BT, Thareja SK, Fenske NA. Merkel cell carcinoma: clinical characteristics, markers, staging and treatment. J Drugs Dermatol. 2010;9(7):779–84. [PubMed] [Google Scholar]
- 42.Ball NJ, Tanhuanco-Kho G. Merkel cell carcinoma frequently shows histologic features of basal cell carcinoma: a study of 30 cases. J Cutan Pathol. 2007;34(8):612–9. doi: 10.1111/j.1600-0560.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee WJ, Kim CH, Chang SE, et al. Cutaneous metastasis from large-cell neuroendocrine carcinoma of the urinary bladder expressing CK20 and TTF-1. Am J Dermatopathol. 2009;31(2):166–9. doi: 10.1097/DAD.0b013e31818eba4c. [DOI] [PubMed] [Google Scholar]
- 44.Boyd AS, Hayes BB. Metastatic small cell neuroendocrine carcinoma of the breast. J Cutan Pathol. 2012;39(11):1042–6. doi: 10.1111/j.1600-0560.2012.01970.x. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Wolgamot G, Argenyi Z. Primary cutaneous neuroendocrine cell carcinoma (Merkel cell carcinoma) with prominent microcystic features, mimicking eccrine carcinoma. J Cutan Pathol. 2007;34(5):410–4. doi: 10.1111/j.1600-0560.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 46.Hwang JH, Alanen K, Dabbs KD, Danyluk J, Silverman S. Merkel cell carcinoma with squamous and sarcomatous differentiation. J Cutan Pathol. 2008;35(10):955–9. doi: 10.1111/j.1600-0560.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 47.Covello R, Licci S, Ferrari A, Morelli L, Catricala C. Merkel cell carcinoma of the thumb with squamous and leiomyosarcomatous differentiation. Eur J Dermatol. 2010;20(4):529–30. doi: 10.1684/ejd.2010.0983. [DOI] [PubMed] [Google Scholar]
- 48.Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol. 2008;129(1):130–42. doi: 10.1309/WYACYWF6NGM3WBRT. [DOI] [PubMed] [Google Scholar]
- 49.Cerroni L, Zenahlik P, Hofler G, Kaddu S, Smolle J, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20(8):1000–10. doi: 10.1097/00000478-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Swerdlow SH International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon, France: International Agency for Research on Cancer; 2008. p. 439. [Google Scholar]
- 51.Duong T, Grange F, Auffret N, et al. Cutaneous Richter’s syndrome, prognosis, and clinical, histological and immunohistological patterns: report of four cases and review of the literature. Dermatology. 2010;220(3):226–33. doi: 10.1159/000269737. [DOI] [PubMed] [Google Scholar]
- 52.Robak E, Gora-Tybor J, Kordek R, et al. Richter syndrome first manifesting as cutaneous B-cell lymphoma clonally distinct from primary B-cell chronic lymphocytic leukaemia. Br J Dermatol. 2005;153(4):833–7. doi: 10.1111/j.1365-2133.2005.06805.x. [DOI] [PubMed] [Google Scholar]
- 53.Ratnavel RC, Dunn-Walters DK, Boursier L, et al. B-cell lymphoma associated with chronic lymphatic leukaemia: two cases with contrasting aggressive and indolent behaviour. Br J Dermatol. 1999;140(4):708–14. doi: 10.1046/j.1365-2133.1999.02776.x. [DOI] [PubMed] [Google Scholar]
- 54.Novice FM, Mikhail GR, Maeda K, Hawley RG. Richter’s syndrome presenting as a solitary cutaneous nodule. Int J Dermatol. 1989;28(1):36–7. doi: 10.1111/j.1365-4362.1989.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 55.Zarco C, Lahuerta-Palacios JJ, Borrego L, Toscano R, Gil R, Iglesias L. Centroblastic transformation of chronic lymphocytic leukaemia with primary skin involvement--cutaneous presentation of Richter’s syndrome. Clin Exp Dermatol. 1993;18(3):263–7. doi: 10.1111/j.1365-2230.1993.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 56.Yamazaki ML, Lum CA, Izumi AK. Primary cutaneous Richter syndrome: prognostic implications and review of the literature. J Am Acad Dermatol. 2009;60(1):157–61. doi: 10.1016/j.jaad.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Harousseau JL, Flandrin G, Tricot G, Brouet JC, Seligmann M, Bernard J. Malignant lymphoma supervening in chronic lymphocytic leukemia and related disorders. Richter’s syndrome: a study of 25 cases. Cancer. 1981;48(6):1302–8. doi: 10.1002/1097-0142(19810915)48:6<1302::aid-cncr2820480609>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 58.Tsimberidou AM, O’Brien S, Khouri I, et al. Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24(15):2343–51. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]
- 59.Bruzzi JF, Macapinlac H, Tsimberidou AM, et al. Detection of Richter’s transformation of chronic lymphocytic leukemia by PET/CT. J Nucl Med. 2006;47(8):1267–73. [PubMed] [Google Scholar]
- 60.Pectasides D, Pectasides M, Economopoulos T. Merkel cell cancer of the skin. Ann Oncol. 2006;17(10):1489–95. doi: 10.1093/annonc/mdl050. [DOI] [PubMed] [Google Scholar]
- 61.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63(5):751–61. doi: 10.1016/j.jaad.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–9. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]


