1. INTRODUCTION
Retinoids bind to six distinct nuclear receptors in mammals, altering the transcriptional activity of these transcription factors from repressors to activators of gene expression. The Retinoic Acid Receptors (RARs) and Retinoid X Receptors are among the most intensely studied nuclear hormone receptors. These proteins mediate both the organismal and cellular effects of intracellular retinoic acids and their synthetic analogs. A broad landscape of experimental and clinical studies have now revealed how retinoids regulate essential biological processes, acting as potent gene expression modulators during embryogenesis, organogenesis, cell growth, differentiation and apoptosis. The six mammalian nuclear receptors for retinoids are encoded by distinct genes, but share many architectural and mechanistic features. More than two decades of exciting structure-function studies on these receptors together with the characterization of retinoid signaling pathways have led to innovative strategies and developments in the therapeutics for cancer, dermatology, metabolic disease and other human diseases.
1.1. The Retinoid Path to Nuclear Receptors
Retinol, the lipid-soluble vitamin A, is an absolute requirement for normal growth, vision and differentiation of epithelial tissues in mammals.1–2 Retinol must be obtained directly through dietary intake, but may also be derived in its provitamin A forms obtained through dietary carotenoids.3–4 Enzymes within mammalian tissues cleave β-carotene, either symmetrically at its central 15,15′ carbon-carbon double bond, forming two molecules of retinaldehyde, or alternatively by making an asymmetrical cleavage at other carbon-carbon double bonds to form two products of unequal chain length.5 Vitamin A and other retinoids are distinguished in their molecular scaffold by the presence of relatively long chain conjugated polyene frame. Due to their general lack of solubility in aqueous solutions, retinol is required to move in cells in association with a number of carrier proteins. The distinguishing chemical structures of natural retinoids, as well as their carotenoid precursors, render them highly sensitive to light, heat and acid induced oxidation and isomerization.6
The concentration of retinoids in the body is maintained within a very narrow range. This balance is required to avoid their deficiency and equally their potential toxicity, including their teratogenic effects.7–8 The balance of these molecules in cells is maintained through a variety of regulatory proteins that control both the uptake of retinol from intestinal cells and the storage in the liver.5,9 Dietary retinol is taken up directly from the lumen of the intestine into the enterocyte. In adults, a two-step enzymatic pathway oxidizes retinol first to retinaldehyde and then to RA. Retinol oxidization to retinaldehyde occurs through enzymes in two classes: the cytosolic alcohol dehydrogenases (ADHs) and the microsomal short-chain dehydrogenases/reductases (retinol dehydrogenases, RDHs).10 The subsequent oxidation of retinaldehyde to retinoic acid (RA) takes place through additional enzymes, the retinaldehyde dehydrogenases.11 The production of RA is balanced by its degradation, the latter process being carried out by three cytochrome P450 (CYP) enzymes.12–14
There are various forms of retinoid-binding proteins in cells, some of which are in intracellular compartments while others carry the retinoids in the extracellular environment.15 All of these proteins allow the transport of retinoids in a stable form, preventing their toxicity to cells. Vitamin A circulates in blood plasma in association with retinol-binding protein (RBP). The supply of RBP in the liver is regulated by the concentrations of available retinol.15–16 Inside cells, all-trans retinol and all-trans retinal associate with cellular retinol-binding proteins (CRBP).17 All-trans RA is bound by cellular retinoic acid-binding protein isoforms (CRABP-I and CRABP-II). In adults, CRABP-I is widely distributed across cell types, but CRABP-II is specifically expressed in the skin, uterus, ovary and choroid plexus. 15 Both of these proteins are also abundantly expressed in the embryo. Disruption of either protein results in defects in organogenesis, suggesting these proteins may be responsible for the generation of RA concentrations in cells in a morphogenic manner.15,18
Maternal vitamin A deficiency severely impacts the development and survival of embryos, causing developmental abnormalities. As the importance of vitamin A was recognized in mediating a broad program of biological functions in development and homeostasis, it became increasingly clear that many of these activities could be ascribed more specifically to retinoic acid (RA). RA has profound influence on embryonic developmental patterning responsible for the formation of limb buds 19–21, hindbrain 22–23, spinal cord 24–25, and eye 26–28.
The segmental patterning associated with vertebrate body axis extension and somite formation during embryogenesis is highly dependent on RA signaling.29–30 Somite formation relies on a “clock and wavefront” mechanism in which a molecular oscillator that depends on Notch and Wnt signaling controls genes along the developing mesoderm.31 A moving wavefront of Fgf8 expression in the primitive streak regresses posteriorly as the body axis is extended, giving rise to a somite determination front.28,32 A critical role for RA during somite development was uncovered by studies showing that RA regulates the expression of the transcription factor Cdx1, a factor crucial for the somatic expression of multiple Hox genes during development of the axial skeleton.28,33 RA also has a crucial role in ensuring the suppression of left-right asymmetries during developmental pattern formation in embryos.32,34–35 Accordingly, RA is not produced by all cells of the body at all stages of development, but is rather produced in a unique spatiotemporal pattern.28
RA signaling specifies cell identities during development and potently controls gene expression in adults. The mechanistic basis for these remarkable activities of RA remained largely unknown until it was revealed that retinoids were the ligands for several members of the nuclear receptor (NR) superfamily (see Figure 1). The NRs are DNA binding transcription regulators that bear distinct pockets, and a subset of NRs have pockets specifically evolved for high affinity RA binding.36–37 Ligand binding results in a conformational change in the NR polypeptides, switching their transcriptional activities on genes controlling embryonic development, organogenesis, tissue homeostasis, cell proliferation, differentiation and apoptosis.38–39
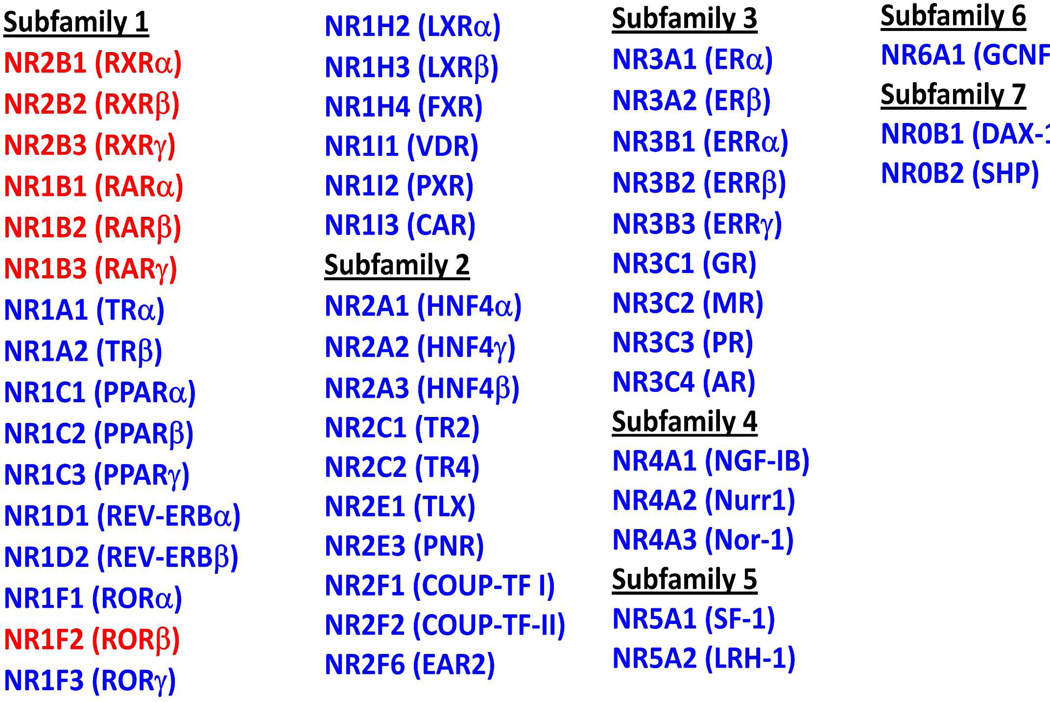
Figure 1.
The NR family. Shown are the human NRs divided into multiple sub-families based on sequence alignments. Receptors in red are the high-affinity retinoic acid binding receptors.
1.2. The RAR and RXR Members of the NR Superfamily
In the nucleus, RA binds to RARs and RXRs, members of the nuclear receptor (NR) superfamily. Other NRs also bind to lipophilic molecules, including diverse steroids, thyroid hormone, retinoids, a variety of dietary lipids and their metabolic derivatives, and also heme.36–37,40–41 The set of human NRs are shown in Figure 1, divided into their multiple subgroups. Some of these receptors display high affinity for endocrine ligands, with equilibrium Kd values for receptor-ligand interactions in the single digit nanomolar range or better. This is true for the glucocorticoid receptor (GR), estrogen receptor (ER), progesterone receptor (PR), and androgen receptor AR.
Other NRs have three to five orders of magnitude weaker affinity for their endogenous ligand. These receptors are generally referred to as metabolic sensors, given their ability to bind and transcriptionally control their target genes with the lower ligand affinity. The metabolic sensor group includes the liver-X receptor (LXR), and the farnesoid x receptor (FXR).37 LXR and FXR bind to oxysterols and bile acids, respectively, with affinities that are appropriately tuned to the abundant concentrations of these molecules in cells. Each NR controls the transcriptional activities of hundreds, and sometimes thousands of genes.
The genetic responses that NRs mediate allow living organisms to adapt and respond to their nutritional intakes and states, as well as their energetic and metabolic conditions. Some NRs have been show to bind avidly to phospholipids and fatty acids, and in these cases there has always been some controversy and doubt as to whether fatty acids that are cell abundant are the bona fide endogenous ligands, or simply caught filling the LBD pockets as surrogate ligands.42–44 A still significant subset of NRs still have unknown ligands and are “orphan receptors”.
With a ligand binding domain (LBD) contained in their polypeptides together with a nearby positioned DNA binding domain (DBD), the NR family functions as ligand-activated transcription factors.45–46 Because of the numerous important endocrine and metabolic genetic pathways regulated by these receptors, they have been exploited widely in small-molecule screens for the discovery of synthetic ligands that can be used to alter their normal gene regulatory functions. The LBD pockets are particularly attractive, because they are both solvent shielded and adaptable.45–46 The endogenous ligands, whether they are RA molecules or other signals, are normally capable of diffusing or being carried into the nucleus. The hydrophobic pockets for these endogenous molecules, together with the molecular sizes of these ligands, match the properties that pharmaceutical companies expect from their drug targets.
Yet, a major limitation in exploiting these receptors for therapeutic benefits has been their overly broad ability to control diverse genetic pathways aside from those seen to be most appropriate for therapeutic intervention. Small molecule modulation of NR activities typically leads to the unwanted effects associated with “off-target” genetic pathways. A long-standing goal in the field has been to discover selective gene modulators, molecules that do not impact every gene under the control of a NR, but only those genes and pathways deemed to be of therapeutic importance. This goal has met some limited success in the case of estrogen receptors.45–46
The RARs form heterodimers with the retinoid X receptors (RXRs), as shown in Figure 2b. The RARs are able to bind both all-trans RA and 9-cis RA and, but the RXRs have the ability to only bind to the 9-cis RA isomer. There are three separate RAR genes encoding nuclear receptor (RARα, RARβ and RARγ) that are conserved throughout vertebrates. RARα was the first to be cloned, and described in 1987.47 Its discovery was followed by the identifications of RARβ and RARγ.48–51
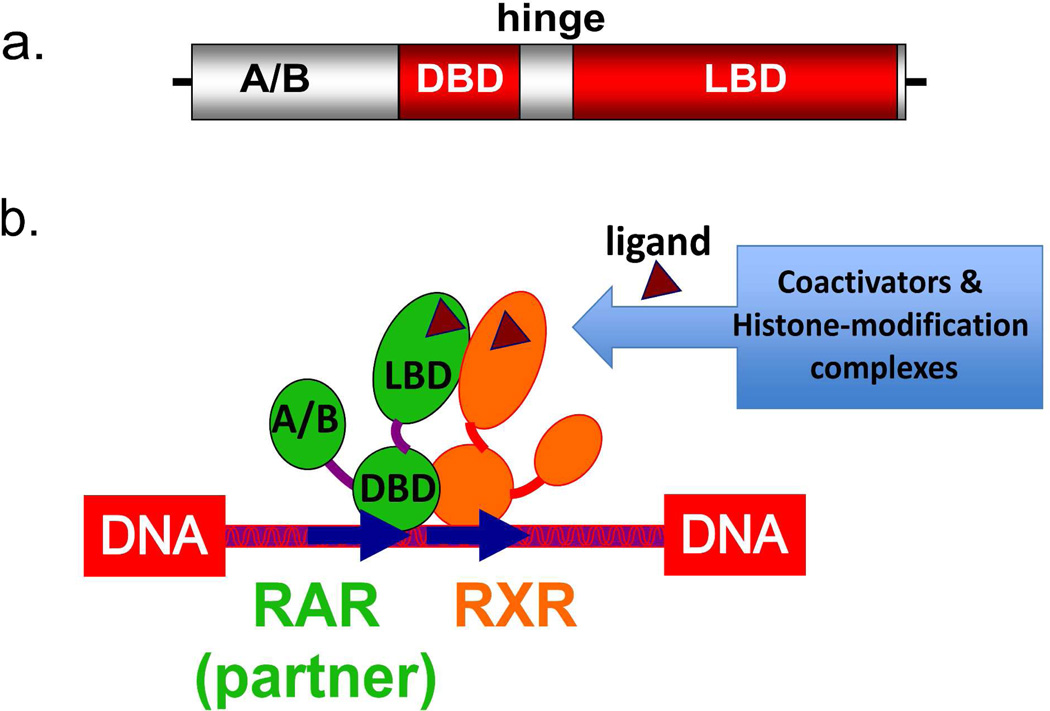
Figure 2.
The domains of NRs. (A) The protein architecture of NRs consist of a N-terminal A/B domain, a DNA binding domain (DBD), a hinge region, and a ligand-binding domain (LBD). (B) Illustration of how different portions of NRs interact in an RXR heterodimer, how the ligand, DNA and coactivator portions must further interact in a functional receptor complex.
Multiple receptor isoforms for each of these RARs have since also been identified, these arising due to splicing variations or the use of distinct promoters which can be found upstream of specific exons within gene coding sequences.15,52 There are two predominant isoforms for RARα (α1 and α2) and RARγ (γ1 and γ2). There are five isoforms for RARβ (β1–β4 and β1'). RAR isoforms can be classified as those that are transcribed from either P1 promoter (class I: RARα1, β1, and β3, γ1) or P2 promoter (class II: RARα2, β2 and β4, γ2). Class I promoters contain TATA boxes whereas class II promoters do not. Studies on the tissue distribution of the RARs have revealed many differences in their spatial patterns of expression.53 Many of the RAR promoters are RA inducible. RARα is expressed in the hindbrain, spinal cord, and eye. RARβ is expressed broadly in brain, CNS, intestine, liver, kidney and limbs. RARγ is expressed most strongly in the skin.52–53
There are also three RXRs (RXRα, RXRβ and RXRγ) in the NR family. As with the RARs, all three of these proteins are encoded by separate genes. Their genes were cloned between the late 1980s and early 1990s.48–49,54–60 There are two major isoforms for RXRα (α1 and α2), RXRβ (β1 and β2), and RXRγ (γ1 and γ2).52 RXRβ is the ubiquitously expressed; RXRα is mainly expressed in liver, lung, muscle, kidney, epidermis, and intestine and is the major RXR form seen in skin; and RXRγ is predominantly found in brain, cardiac and skeletal muscle.40,61 The splicing isoforms typically produce amino-acid distinctions within their N-terminal A/B regions.61–63
With their cloning, all of these retinoic acid receptors were recognized to be potential responders to nutrients and their derivatives. This distinguished them early on from the classic steroid hormone receptors, which bound to steroids and other endocrine ligands. The superfamily of NRs remains one of the most intensely pursued group of targets for the development of therapeutic drugs in the pharmaceutical sector.
1.3. The RXR and RAR Protein Domains
NR proteins have a highly variable sequence in their N-terminal regions, known as the A/B region, a DNA binding domain (DBD), a hinge region of variable size, and a C-terminal positioned ligand binding domain (LBD), as illustrated in Figure 2a. The A/B domains are functionally important in these receptors, but fail to show structural order when examined in the context of the nuclear receptors alone. It is likely that they contain segments that fold upon interactions with specific binding partners that assemble as part of their active or repressive transcriptional complexes. No three-dimensional structure has been determined for any A/B segment of NRs. The crystal structure of full-length RXRα-PPARγ complex, together with a hydrogen-deuterium mass spectrometry study, showed that the RXR A/B segments is disordered.64 It is possible that the disordered nature of the NR A/B segments facilitate their interactions with kinases or ubiquitin-ligases, and indeed most NRs contain many post-translational modification sites in these A/B segments.65
The DBDs are the most conserved domains of NRs, and have the same overall three-dimensional structure.46,66 The DBD consists of two loops that are each zinc-nucleated, together with a pair of alpha helices positioned perpendicularly to each another. One of these helices is responsible for most of the DNA specific contacts, and lies across the DNA major groove of the DNA. There are several crystal structures available for RXR and RAR DBDs (including the RXR ortholog in Drosophila known as ultraspiracle).67–70
The LBDs in the retinoic acid receptors show more modest levels of sequence conservation, but still have a similar architectural fold.45,71 NR LBDs fold into a three-layered α-helical sandwich, giving rise to an internal hydrophobic ligand-binding pocket. The LBD contains twelve alpha-helices a single beta turn, and short connecting loops.71 The LBD is responsible for forming all the protein contacts with retinoic acids. It is also the site for binding of synthetic analogs of RA.72–78
2. FUNCTIONAL INSIGHTS
2.1. Early Insights about RXR and RAR Functions from Knockout Studies
The role of RA in embryogenesis was initially suggested by its teratogenic effects; seeing how the administration of high doses of RA interfered with developmental processes in animals. These studies used a number of species including amphibians, zebrafish, and chick.79–81 Gene knockout studies in mice were later conducted to confirm the importance of RXR and RAR receptors for RA signaling.82 Here, we briefly review the key findings from those knockout studies conducted on the retinoic acid receptors.
Mouse knockouts were constructed for all three RXRs, to address whether they account for the morphogenic properties associated with RA signaling. Loss of RXRα function proved lethal in embryogenesis.82 This lethality was caused by the hypoplastic development of the heart ventricular chamber, although there were other developmental defects noted too. These findings confirmed that retinoid signaling through RXRα was required for the appropriate developmental programs of vertebrates.83 The loss of RXRβ caused embryonic lethality before birth in about half of the mice examined.84 The surviving males, however, were sterile. 84 By contrast, RXRγ deficient mice showed no lethality or obvious phenotypic changes on the whole animal basis.85 This could be explained by the notion that in most, but not all, tissues, RXRα has the capacity to functionally compensate for any of loss of RXRγ function.85
The cloning of the three RARs also was followed by comprehensive knockout experiments to address the importance of retinoic acid signaling through the RAR.47,54 RARα, RARβ, and RARγ null mutant mice are all viable to different extents.82 Null mice for RARα were discovered to exhibit 50% decreased viability. They showed a deficiency in overall growth, sterility in the case of male mice due to developmental defects, and other malformations, including deformed paws. RARβ has five distinct splicing variants.48,50,86–87 The RARβ knockout phenotype includes a loss of striosomal compartmentalization in the rostral striatum, locomotor abnormalities, and defects in the brain’s dopamine regulated pathways.88–89 Single isoform knockouts of RARβ had more specific phenotypic characteristics, such as defective eye development in the case of RARβ2.82 RARγ also has multiple splicing variants.50,90 When all the RARγ variants are simultaneously lost, the mouse mutants exhibit growth deficiency, early lethality, and male sterility due to squamous metaplasia of the seminal vesicles and prostate.90
2.2. Controversy About 9-cis RA as the RXR Ligand
9-cis RA binding to RXR was discovered by several groups.91–93 Yet, the question of whether physiological retinoid signaling requires binding of 9-cis RA to RXRs has not been fully resolved for the field.94–96 The role of the 9-cis RA isomer as the bona fide endogenous RXR ligand remains controversial, because this molecule is difficult to detect endogenously in embryos or in most adult tissues.95,97 By contrast, all-trans RA is easily detectable in many tissues.98–101 It has been shown that all-trans RA can undergo isomerization to 9-cis RA in bovine liver membranes.102–103 When all-trans RA and 9-cis RA were tested for their ability to rescue the embryonic lethal phenotype associated with the knockout of a cytosolic retinaldehyde dehydrogenase gene (Raldh2), it was concluded that 9-cis RA was not required.95
Whether 9-cis RA is the endogenous physiological ligand of RXRs or not, it does have the ability to physically bind the pockets of these receptors in a complementary fashion and with very tight (single digit nanomolar) affinity. This binding also potentiates the activation of transcription from genes under RXR control. The difficulty in validating the existence of 9-cis RA in tissues has prompted the search for possible other physiological ligands, among which the unsaturated fatty acid docosahexanoic acid (DHA) was identified.104 DHA is a major constituent of nutrients rich is w2 polyunsaturated fatty acids, and also becomes enriched in mammalian brain during late gestation and early postnatal periods. Deficiencies in this molecule lead to impaired spatial learning abnormalities.104–105
Linoleic and linolenic acids have also been described as potential binders and activators of RXR.96 An asymmetric cleavage product of b-carotene was also shown to switch RXR transcriptional activity.106 This product (apo14), but not other closely related apocarotenals, was shown to interact with RXR, PPARγ, and PPARα, opposing the known effects of both synthetic and natural ligands on these receptors, including target gene induction and cellular responses.106 Phytol metabolites have been shown to bind RXR and act as potentially endogenous ligands.107 Yet none of these studies have fully confirmed the physiological relationship of the ligand with the receptor.
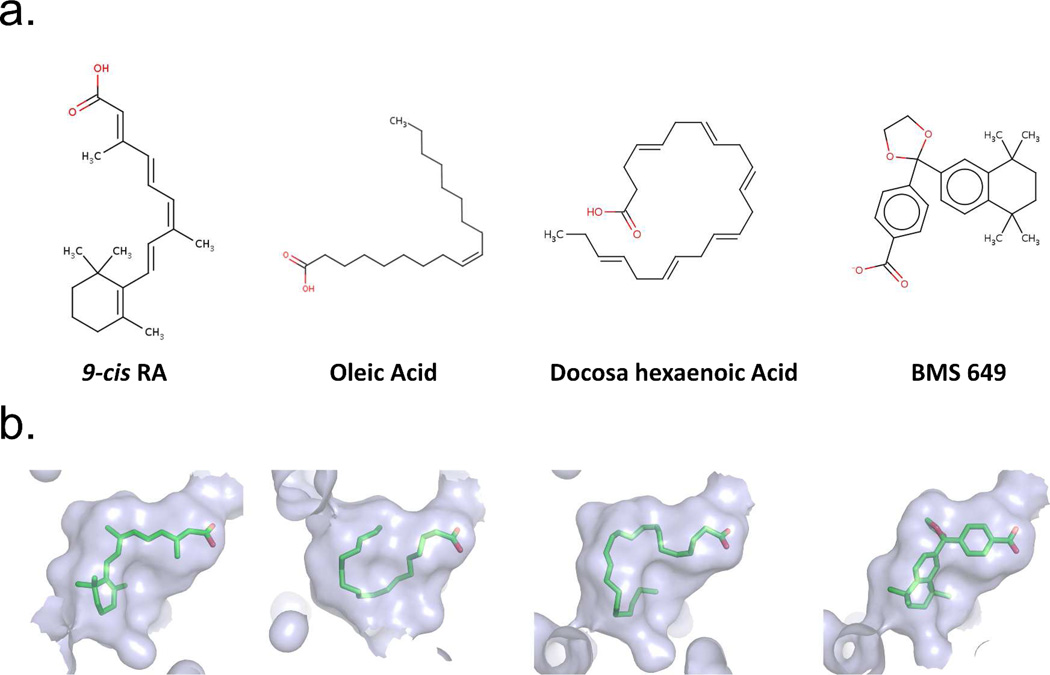
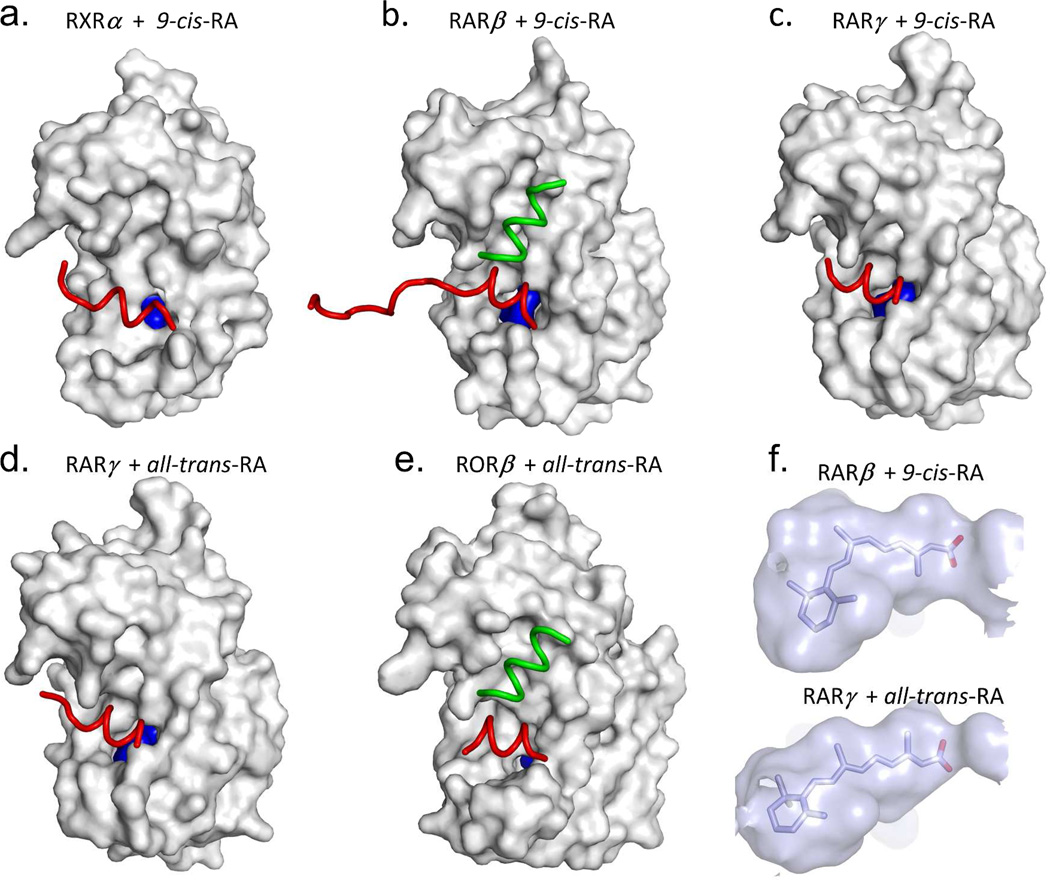
Figure 3a–b show three naturally occurring RXR binding molecules, together with a synthetic agonist.73 It is clear from these pictures that the pocket of RXR must be highly adaptable for binding to molecules of such diverse chemical structures and sizes. These molecules have little in common, beside their general hydrophobic nature and the positioning of carboxylates on one extreme end. However, all of these molecules bind to RXR avidly, as confirmed by crystallography.73 Figure 3B further shows the shape of the pocket that is created in each case around these molecules. It is reasonable to expect that RXR may use a broad range of endogenous ligands, depending on the tissue type and the spatial-temporal abundance of these molecules during the development and homeostasis of an organism.
Figure 3.
Examples of some chemically diverse molecules that can act as RXR ligands. (A) The chemical structures of the molecules. (B) The solventaccessible molecular surfaces from the crystallographically observed structures of RXR/ligand complexes with each molecule.73 Coordinates are from PDB IDs: 1FBY, 1KDF (chain A), 1MV9, and 1MVC.
2.3. The Heterodimeric Partners of RXR
RXR has a special role in the NR family, because it acts as a common heterodimeric partner to a RAR and many other nuclear receptors, such as the thyroid hormone receptor (TR), vitamin D receptor (VDR), liver X receptor (LXR), peroxisome-proliferator-activated receptors (PPARs), farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor (CAR), among others (see Figure 1). Therefore, the pleiotropic effects of RA could involve the repertoire of all these NRs with which RXR is a combinatorial partner. Here we describe the important physiological roles of each of these RXR partners. RXR heterodimers with the thyroid hormone receptor (TR) are responsible for a wide variety of physiological processes that include embryonic development, the regulation of metabolic rate, and the regulation of heart and digestive functions.108–110 There are two types of TRs in vertebrates, TRα and TRβ, both of which bind to thyroid hormone with very high affinities.111–113 TR, without its bound natural ligands T3 (3,3′,5-triiodo-L-thyronine) or T4 (3,3′,5,5′-tetraiodothyronie) is a strong repressor of many target genes, due to the association of TR with corepressor.114–115 The endogenous TR ligands are the only known iodine containing molecules in vertebrate biology.
Tissue targets of thyroid hormone include the pituitary, the developing brain, liver, bone, heart, and skeletal muscle.116 Thyroid hormone, when given to animals or humans, rapidly increases energy expenditure, while lowering cholesterol and triglycerides. However, serious side effects also result, including cardiac arrhythmia, bone loss, and anxiety. It has been postulated that TRβ selective agonists could be useful in avoiding the unwanted side effects, while capturing the benefits of increased energy expenditure and fat loss.117–118 The crystal structures of the TR LBD with its endogenous ligand, as well as with synthetic molecules, have been described. 119–121 In addition, the crystal structures of the heterodimeric complexes involving RXRα and TRα LBDs, with 9-cis RA and T3 hormones bound have been reported.122 Transcriptional activation in response to thyroid hormone involves an exchange of corepressors with coactivators.123–124 Interestingly, it has been shown that the presence ofT3 together with the absence of 9-cis RA, activates the transcriptional activity of the TR-RXR. By contrast, the presence of both ligands produces diminished transcriptional activation.122
RXR also forms a functional heterodimer with the vitamin D receptor (VDR), the receptor responsible for the genomic actions of 1,25(OH) vitamin D3, the biologically active metabolite of vitamin D.125 Binding of its cognate ligand to VDR occurs with a Kd of better than 1 nM.126 VDR expression has been noted in enterocytes, osteoblasts, skin keratinocytes, lymphocytes, colon, pituitary and ovary.127 The functions of VDR include its ability to regulate calcium and phosphate uptake, transport, and homeostasis for bone mineralization.125 VDR also has critical functions in cellular differentiation and in the immune system.128 Multiple analogs of 1,25(OH) vitamin D3 have been designed over the years, carefully examined for their ability to bind to VDR, and further tested against specific diseases.125,129 High resolution crystal structures of the VDR with a number of such molecules have been described.129 Moreover, the low-resolution structural organization of the full-length RXR-VDR complex has been studied using electron microscopy, and through solution-based biophysical studies.130–131
RXR also partners with both LXRα and LXRβ, which function as oxysterol sensors.132–136 LXRα is seen at high levels in macrophages, adipose tissues, kidney, lung, and spleen, these being tissues with generally high metabolic demands.137 In contrast, LXRβ is ubiquitously expressed. Together, these receptors are key regulators of lipid and cholesterol metabolism.135 They regulate genetic programs associated with hepatic lipogenesis and plasma triglyceride levels.37 Both LXRs are also important in the function of the immune system, and appear in macrophages, dendritic cells and lymphocytes.138–139 The genome-wide profiling of LXR has been reported both in a macrophage cell line and in the liver.140–141 The two LXRs function to integrate macrophage gene regulation with the levels of cellular cholesterol and oxysterols.142
The association of RXR with the PPARs allows the cells to sense and genetically respond to fatty acid molecules.143 There are three distinct PPAR proteins, encoded for by separate genes: PPARα, PPARβ/δ, and PPARγ,143–144 Each of these receptors forms a heterodimer with RXR, and binds to direct-repeat response element. Fatty acid binding causes the activation of target gene transcription.37,145–146 PPARα, regulates the lipid metabolism program by regulating genes involved in fatty acid uptake and degradation, and for reverse cholesterol transport.143 It is abundantly expressed in liver, heart, muscle, and kidney. Activating PPARα with a synthetic ligand lowers serum lipid levels, and this receptor is viewed as a potential target for the treatment of hyperlipidemia.147–148
PPARγ is expressed predominantly in macrophages and in adipocytes. It is considered the master regulator of adipocyte differentiation, but also has important roles in lipid homeostasis, and in inflammation.147,149 Thiazolidinediones (TZDs), a class of drugs that specifically bind to and activate PPARγ, have been used to treat type II diabetes by acting as insulin sensitizers.147 PPARβ/δ, the third member of this subfamily functions in regulating energy balance. The gene regulatory targets of PPARβ/δ include programs in lipid and glucose metabolism. The activation of this PPARβ/δ increases lipid catabolism in skeletal muscle and adipose tissue, prevents obesity, and enhances insulin sensitivity and exercise endurance.150–153 For these reasons, this receptor has been viewed as a potential drug target for metabolic syndrome.154
RXR also forms functional heterodimers with FXR, the nuclear receptor that acts as the metabolic sensor for bile acids.155–156 FXR is highest expressed in liver, intestine, kidney and the adrenal gland.157 Studies have shown that chenodeoxycholic acid is the most potent of the naturally occurring bile acids in terms of its ability to activate FXR.156 Our laboratory has described the crystal structure of the FXR LBD bound to this bile acid.158 FXR upregulates genes involved in the transport and metabolism of cholesterol and bile acids, promoting their clearance form the enterohepatic system. FXR counteracts some of the functions of the LXRs, in terms of cholesterol and triglyceride metabolism.135 FXR knockout mouse have been shown to display high serum bile acids, cholesterol and triglycerides.159
The pregnane X receptor (PXR, also known as SXR) is another partner of RXR for heterodimerization. PXR was initially described as a sensor for protecting the liver against injury from toxic molecules.160–164 This receptor is now more recognized to be activated by a broad group of structurally unrelated drugs, as well as steroids and xenobiotics, including rifampicin and hyperforin derived from St. John’s Wort (Hypericum perforatum). With its expression highest in liver, small intestine and colon, it is believed that PXR may be responsible for upregulating the expression of detoxifying enzymes upon binding to xenobiotics.165 The crystal structure of the PXR has been described, showing the pocket to be unusually large and flexible, allowing the receptor to capture diverse molecules.166
RXR also forms heterodimers with the constitutive androstane receptor (CAR), a receptor closely related to PXR in its LBD amino-acid sequence, and which binds to many of the same molecules.167–168 CAR and PXR have overlapping target gene patterns.167–168 These similarities suggest that CAR is another cellular responder for protection against potentially harmful molecules, upregulating genes involved in detoxification and elimination of toxic organic molecules. The detoxification gene program controlled by CAR consists of enzymes responsible for the oxidation, conjugation and transport of xenobiotics.168 A number of structures have been determined for CAR with different ligands, using X-ray crystallography.169–171 In addition, the crystal structure of the heterodimeric RXRα-CAR LBD complex has been determined, with the CAR ligand 1,4-bis[2-(3,5-dichloro- pyridyloxy)]benzene (TCPOBOP) and the RXR ligand 9-cis RA.172
2.4 RXR as a Permissive or Non-Permissive Partner
Since RXR forms physical and functional partners with other NRs, this raises an important question as to whether each subunit requires its own ligand for the heterodimer to activate transcription.36,173–174 Studies looking into this important question have shown that RXR can be a silent or an active transcription partner in its heterodimers. In the case of RAR-RXR and VDR-RXR heterodimers, there is no transcriptional activity seen in the cellular environment in the absence of an RXR ligand, even when RXR’s partner has an agonist ligand bound.173 This lack of transcriptional activity may be caused by the inability of corepressors to be released from the apo-RXR subunit of the heterodimer. The associated corepressor has been suggested to sterically block the binding of coactivator to the partner receptor, RAR.173 Such RXR-heterodimers are referred to as nonpermissive. Agonist ligands bound to both of the RXR-RAR subunits lead to cooperative coactivator binding. The cooperative binding involves a single coactivator (p160) polypeptide that can span both subunits of the heterodimer simultaneously, using adjacent recognition motifs within the p160 protein to form the interaction.173
Other complexes of RXR can be significantly activated by RXR partner’s ligands alone, and these complexes are referred to as “permissive”.173 The PPAR-RXR, LXR-RXR and FXR-RXR heterodimers fall into the permissive category. A plausible explanation for the permissivity may be that apo-RXR subunit in these complexes is disposed in a conformation that does not allow it to eassociate with corepressors. In the case of the PPAR-RXR heterodimeric complex, RXR agonists have been shown to exhibit some of same biological effects of PPARγ ligands. RXR in these complexes could, in principle, be targeted by drugs that act effectively act as insulin sensitizers and treatments of type 2 diabetes mellitus.175
2.5. The Role of Transcriptional Coregulators
The control of gene regulation by RXRs and RARs is a highly coordinated process that involves protein players from outside the NR family. The transcriptional activities of RXR and RAR are initiated by the ligand interactions in the LBD pocket. This binding manifests as a conformational switch whereby co-repressors are displaced and coactivators are recruited to the LBD.176–178 Without its ligand, RARs physically associate with corepressor proteins. The corepressor proteins further recruit complexes that have histone deacetylase (HDAC) activity.178–179 The HDACs modify histone tails to remove acetylation marks associated with active transcription. The two related corepressors best associated with NRs are nuclear receptor corepressor (NCOR) and silencing mediator of retinoic acid and thyroid hormone receptor (SMRT).180–182 In addition to their constitutive interaction with unliganded RARs, corepressors can be recruited in an antagonist-dependent manner to the RARs.183
The binding of the endogenous ligands causes NRs to alter the surface conformation of their LBD.184 The resulting active conformation allows the binding of coactivators. The coactivator binding further recruits proteins that harbor histone acetyl transferase (HAT) activity, histone methyl transferase activity (HMT), and DNA dependent ATPase activity.176,178,185 These complexes subsequently modify the histone tails in chromatin to promote active transcription. The coactivators used by the RARs belong to the p160 family of steroid receptor coactivators (SRCs).177,186–187
A third pathway has been described based on the finding that RAs can also repress certain genes through the RARs. This pathway may involve the recruitment of protein such as RIP140, LCoR, and PRAME that interact specifically with the agonist bound RARs, but repress transcription of target genes.188–190 These coregulators appear to share some common features with the p160 coactivators, such as the use of their LLxxLL motifs (where L is leucine and x is any amino-acid). Yet, unlike the p160 coactivators, these coregulators subsequently recruit repressive complexes (with HDAC function) for gene repression. Associated with this diversity of cofactors, the RARs/RXRs heterodimeric complexes can use distinct combinations of cofactors, in their cell types, promoters, and actions to mediate a variety of signaling pathways.
3. STRUCTURAL INSIGHTS
3.1. The Ligand-Inducible Helix-12 Switch
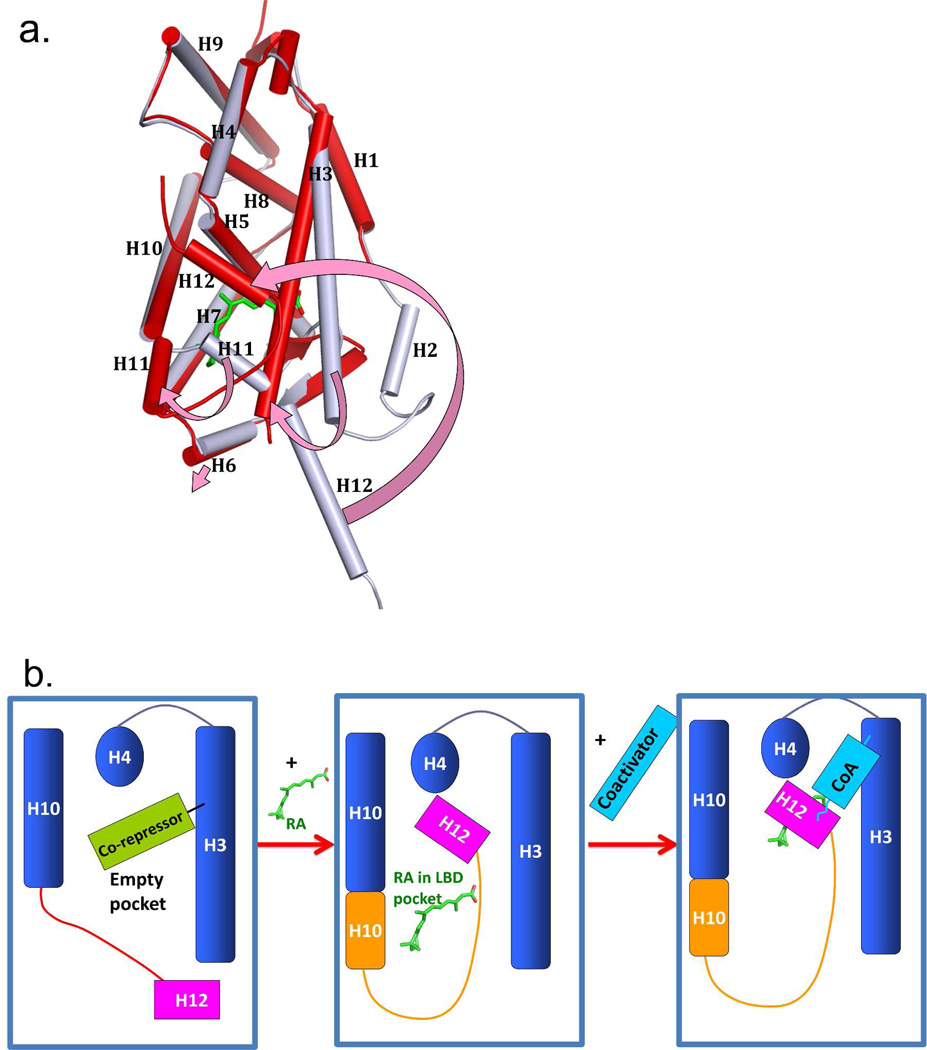
Figure 4a–b shows the ligand-induced conformation switch on the surface of the LBD that was first understood from X-ray crystallographic studies on RXR LBD and related LBDs.71 Agonist ligands (molecules causing receptors to activate transcription of target genes) such as 9-cis RA induce the folding and/or rearrangement of the helix 12 (H12) in the LBD.191 In the apo-form, H12 is positioned away from the rest of the LBD. But in the holo-form, this helix moves significantly in position to close on top of the ligand and trap it, in what was elegantly described as mouse-trap mechanism.71
Figure 4.
The ligand induced positioning of helix-12 into the “active conformation”. (A) the superposition of RXRα LBD in the apo-state (grey) and the same receptor in the holo-state (red) with 9-cis RA (green) in the pocket. The four arrows (pink) show the helical movements induced by ligand binding. (B) Illustration of how corepressors, ligand, and coactivators interact with a NR LBD. The blue box represents the LBD portion of NRs. The binding of RA molecules or other endogenous ligands or synthetic agonists results in a major repositioning of helix-12. Ligand binding releases corepressor binding, and causes helix-12 to trap the ligand, by closing on top of its pocket. The resulting active conformation also creates a groove for the binding of coactivator LLxxLL motifs on the surface of the LBD.74 The apo-RXRα is from PBD ID 1LBD, and the 9-cis RA complex is from PDB ID 1FBY.
Since the first visualization and understanding of the helix-12 rearrangement made possible by the comparison of the unliganded RXRα with the liganded RARγ, an expansion in our understanding of helix-12 induced movements by ligands has come from crystallographic studies on other NR LBDs.45,71,119,158,191–196 The agonist positioning of helix-12 into its mouse-trapped position is referred to as the “active conformation” of the receptor. This conformation is essential for the docking of coactivator proteins as the latter use LLxxLL motifs to dock to the surface of the LBD. Figure 4b shows in diagrammatic fashion the physical changes that a ligand induces in order to recruit a coactivator.
NRs can bind and respond to synthetic antagonists; molecules that cause receptors to repress transcription from target genes. The antagonism is associated with yet another position of helix-12 that allows corepressor proteins to dock to the surface of the LBD, instead of coactivators. A structural study of PPARα LBD with the synthetic antagonist GW6471,showed how helix-12 can be move into this so-called antagonist conformation. This conformation does not foster the recruitment of coactivator motifs, but instead the recruitment of corepressor segments with LxxxIxxxL sequences.45,197
3.2. The Sequence and Geometry of DNA Response Elements
The DNA targets of nuclear receptors, known as response elements, are the sequences through which NRs mediate their gene-regulatory effects. RXR and its partner receptors recognize the consensus sequence 5′-AGGTCA-3′ within their response elements. This hexameric “half-site” is typically arranged as direct-repeats (DRs), with different inter-half-site spacings that vary from 1–5 base-pairs (forming DR1 – DR5 response elements).198–199 DRs are used by RXR heterodimers with RAR, the thyroid hormone receptor (TR), the vitamin D receptor (VDR), the peroxisome-proliferator-activated receptors (PPARs) and other RXR partners (see Figure 1c).46,66 By contrast, the steroid receptors such as those for glucocorticoids, mineralocorticoids, androgens and progesterone, require half-site sequences of AGAACA-3′, with the additional requirement that they are oriented in inverted repeat fashion. 46, 200–203 Other NRs can bind to DNA as monomers.46,204–208
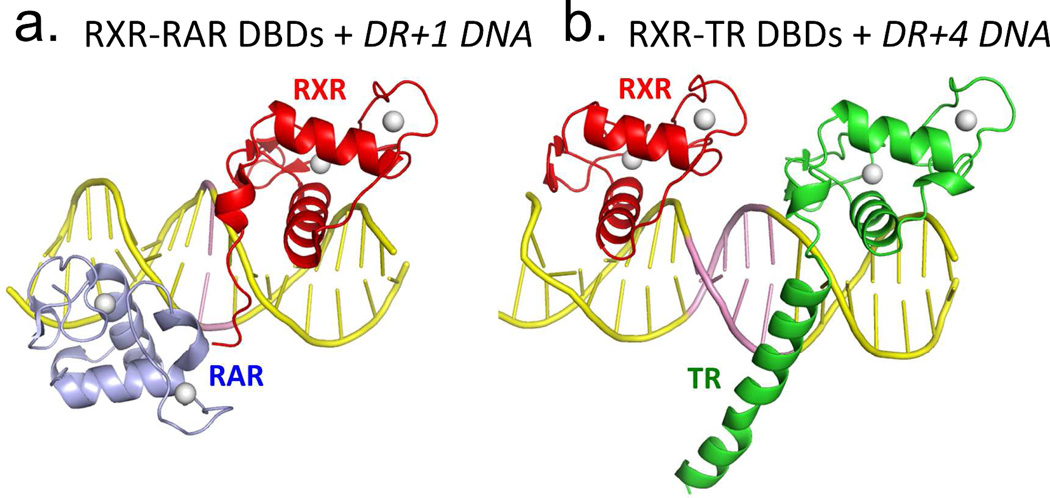
Co-crystal structures of DNA-binding complexes involving RXR have allowed us to visualize how the retinoid receptor DBDs cooperate in homodimeric or heterodimeric forms.46,67–68,209 The first glimpse came from the structure of the RXR–TR DNA-binding complex on DR4.67 The asymmetrical head-to-tail placement of the subunits fosters productive interactions between subunits only when RXR sits upstream of TR. The DNA minor groove of the four-base-pair spacer engages different parts of each DBD and brings them together for their interactions. Spacing selectivity is established by the ability of these receptors to form stabilizing contacts with each other only when their relative distance and geometries are correct on the DNA. As each extra base-pair in the spacer, varying from 1–5 in size, alters the relative distance and orientation of the two half-sites, it also acts as the DNA selectivity feature required for promoting or preventing DBD-DBD interactions between RXR and its partners.66–67
In the case of RAR/RXR heterodimers, the DR1 and DR5 elements were the first ones described, and it was demonstrated that negative or positive gene regulation could be attributed to these different spacings in these two direct repeat elements.210 Moreover, the RXR homodimer and the RXR–RAR heterodimer can both cooperatively bind to DR1 elements. But the heterodimer has an advantage in terms of the higher affinity for this site and may preclude the possibility of RXR homodimers functioning in cells.
A detailed examination of the protein–DNA contacts has been possible from the crystal structures of both of these DBD complexes. As with the RXR-TR, in the RXR–RAR heterodimer and RXR-RXR homodimer, each DBD makes a series of base-specific contacts along the AGGTCA elements.68,209 These structural studies revealed the mode of base-specific interactions by NRs, and the basis for DNA binding cooperation between their two subunits. All the structural studies with RXRs and RARs used “consensus” response element, and not the natural sequence variations of DR1-DR5 seen in the genome.
3.3. The Genomic DNA Binding Sites of RAR and RXR
Hundreds of retinoic acid regulated genes have been described date, many of which are also evolutionarily conserved across metazoans.81,211 Such conserved genes include the three encoding the RARs themselves, the gene encoding the orphan NR TR2, and genes encoding multiple homeodomain proteins. The homeodomain genes prominently include the Hox family members, important developmental regulators of the embryonic body plan. Other RA regulated genes encoded for transcription factors that include C/EBP, Stat1, Oct4, Pit-1, HNF1a, ETs1, EGr1 and Foxa1. The genes further include a set responsible for the metabolism, degradation and movements of retinoids in the body, such as Adh7, RALDH1, CRABPII, and RBP1.81 Other gene clusters belonging to adhesion/extracellular matrix proteins, secreted proteins/signaling factors, membrane receptors, neurotransmitter receptors and enzymes have also been noted to be RA regulated.81
The recent application of deep genomic sequencing techniques has led to the genome-wide identification of cis-acting DNA binding sites (cistromes), used by a variety of RXR-heterodimers, including RXR-LXR, RXR-VDR, and RXR-PPARs. 141,212–215 A surprising, but general finding of these studies has been that NR response elements are typically not upstream of the transcriptional start sites or near TATA elements. Instead, they are more often seen within introns, or at a significant distance from the transcription start sites. Another observation is that the vast majority of these heterodimer binding sites are pre-bound by RXR, and that ligand treatment, as well as the increased expression of the binding partner, enhances the association of the heterodimer on the response element. These findings suggest a model in which liganded RXR serves as the nucleating recruitment factor for its partners.
The genome-wide identification of RAR binding sites using ChIP-seq (chromatin immunoprecipitation coupled with deep sequencing) also revealed a highly complex and expanded repertoire of DNA binding sites/216 Besides the previously known DR1 and DR5 element used by the RXR-RAR complex, RAR was observed to associates with other sites that include IR0, DR0, DR2, and DR8.216 Although RXR and RAR may be binding to these sites, the data do not preclude the possibility of these sites being shared with other transcription factors. Indeed, NRs are capable of displaying complex DNA binding modes, including tethering to other transcription factors.217
3.4. The Dimerization Interfaces of RXR and RAR
Figure 5a–d show two dimerization interfaces used by RXR for its partners: An LBD-LBD interface that can be formed in solution, and a DBD-DBD interface that forms only on DNA.66,218–220 The DNA response element is a direct participant in aligning the DBD-DBD interface for dimerization. The understanding of how heterodimerization surfaces of NRs are formed via their DBDs was largely through crystallographic studies conducted in our laboratory.46,64,67–70,209,221 The RXR DBD uses its second zinc module to forms a surface that interacts with a different portion of RAR and its other partners bound on their target DNA elements. These dimerization domains are illustrated in Figure 5a–b. We developed a set of basic rules about DNA recognition and how the inter-half-site spacing acts as a selectivity feature for NRs.46,66,220
Figure 5.
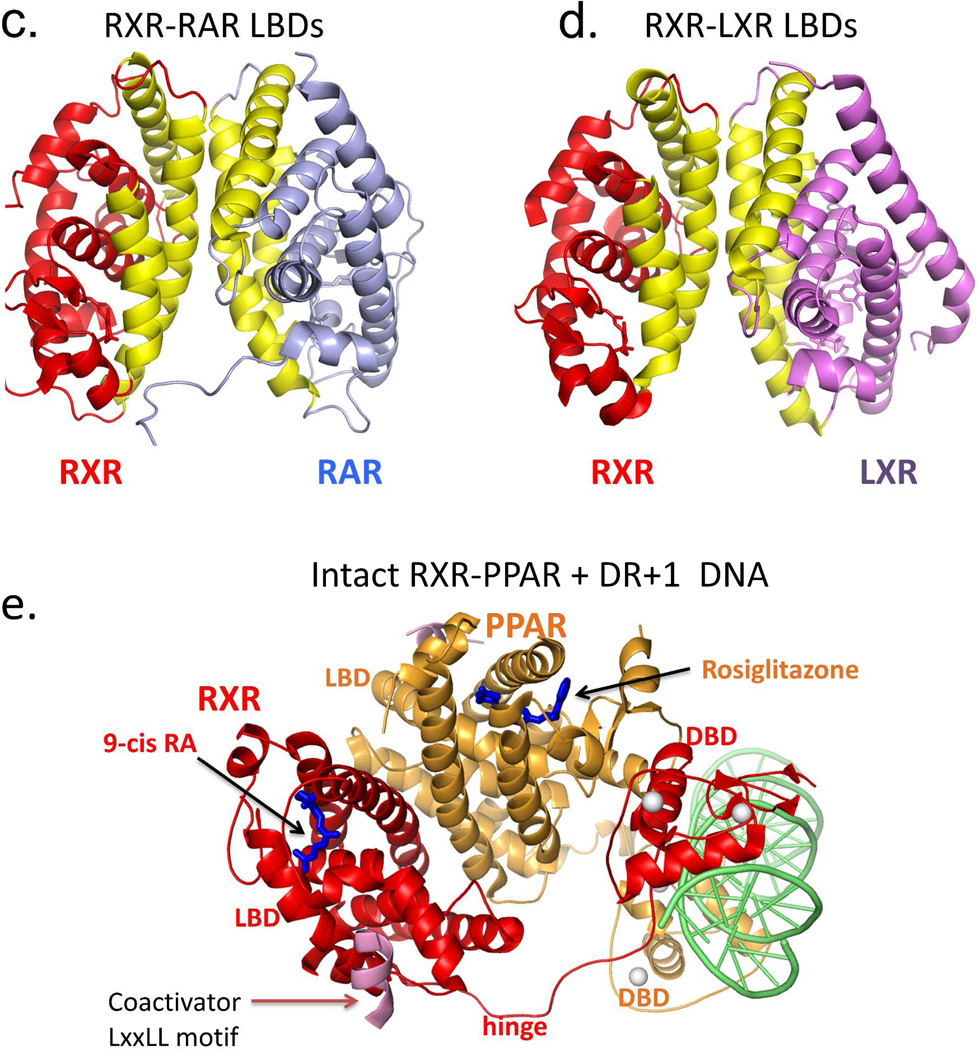
RXR heterodimerization and DNA binding. (A-B) The interactions of RXR DBD and its partners (RAR or TR) on direct-repeat DNA response elements. RXR-RAR DBDs are shown on DR1 DNA, and RXR-TR DBDs are shown on DR4 DNA. Note that RXR occupies the upstream half-site in its complex with RAR, and the downstream half-site in its complex with TR. The RXR-RAR DBD complex is from PDB ID 1DSZ, and the RXR-PPAR DBD complex is from PDB ID 2NLL. B: The dimer interfaces that form between RXR LBD and other NR LBDs. Shown are the RXR-RAR (PDB ID 1XDK) and RXRLXR interfaces (PDB ID 3FAL). The regions in yellow indicate the protein-protein interfaces in these heterodimers. E. Structure of the intact RXRα-PPARγ complex on DR1 DNA (PDB ID 3E00). RXRα and PPARγ are in red and gold, respectively, with the DNA in green. The domains, ligand and coactivator peptide of RXR are labeled. In addition to the dimerization interfaces shown above, this complex also shows domain interactions involving the PPAR LBD and the RXR DBD.64
The dimerization interface that joins two LBD is much larger in size, in terms of buried solvent exposed surfaces.66,220 The LBD-LBD interface of RXR-RAR, RXR-LXR, and other RXR heterodimers including RXR-PPAR are generally of a similar nature, as shown in Figure 5c–d. Helices H7, H9, H10 and loops L8–9 and L9–10 of each subunit face each other to form this interface.72,222–223 A sequence alignment of RXR partners suggests that a common heterodimerization motif involving many hydrophobic aromatic residues and a few acidic residues allow multiple LBD dimerizations with RXR-LBD.222
The LBD-LBD interactions of RXR-RAR show some differences with those of the RXR-LXR and RXR-PPAR. In the case of the RXR-RAR, the interface closely resembles that of the homodimers of RXR and estrogen receptor (ER), in being quite symmetrical.72 But for RXR-LXR, the interface shows significant asymmetry, involving residues from H7 of RXR, but not from H7 of LXR.223 Moreover, the loop in LXR connecting H8 and H9 helices participates in the binding, while the equivalent loop of RXR does not. In the PPAR-RXR interface, the PPAR-LBD is rotated approximately ten degrees from the C2 symmetry axis.222
Our laboratory has solved the only two crystal structures of nearly complete NR complexes (a heterodimer and a homodimer) on DNA.64,224 The first complex is the RXR-PPAR heterodimer shown in figure 5e, and the second is that of the HNF-4α homodimer. Both complexes contain the DR1 DNA response element. The PPAR-RXR complex shows a much more complex pattern of subunit dimerization than had been expected.64 A new DNA-dependent interface is seen between the LBD of PPAR and the DBD of RXR, for example (Figure 5e). The intact complex has a great degree of quaternary organization between its domains, lacks symmetry, and two receptors are not evenly positioned over their DR1 element.64 This study showed that the long held concepts of conserved domains (DBD and LBD) acting independently in NRs is not correct. Moreover, the molecular architecture of full-length PPAR-RXR revealed using X-ray crystallography is quite distinct form the picture obtained using low-resolution solution studies.131
We recently expanded our crystallographic visualization to the HNF-4α multidomain receptor homodimer complex.224 This structure again shows that the quaternary organization of the HNF-4α homodimer is far more complex and interconnected than had been expected from previous studies. The homodimer is unique in its quaternary organization, and not similar to the RXR-PPAR heterodimer, even though both complexes are bound to DR1. As the full quaternary structures of the NRs becomes available, our understanding of the complexities of receptor domain organization and the allosteric communications within their polypeptides will expand. Neither the RXR-PPAR nor the HNF-4α crystal structures are supportive or consistent with the generalized concept of “common nuclear receptor architecture” suggested by a study based on low-resolution analysis of the RXR-RAR, PPAR-RXR and RXR-VDR complexes.131
3.5. Tetramer Formation by RXR
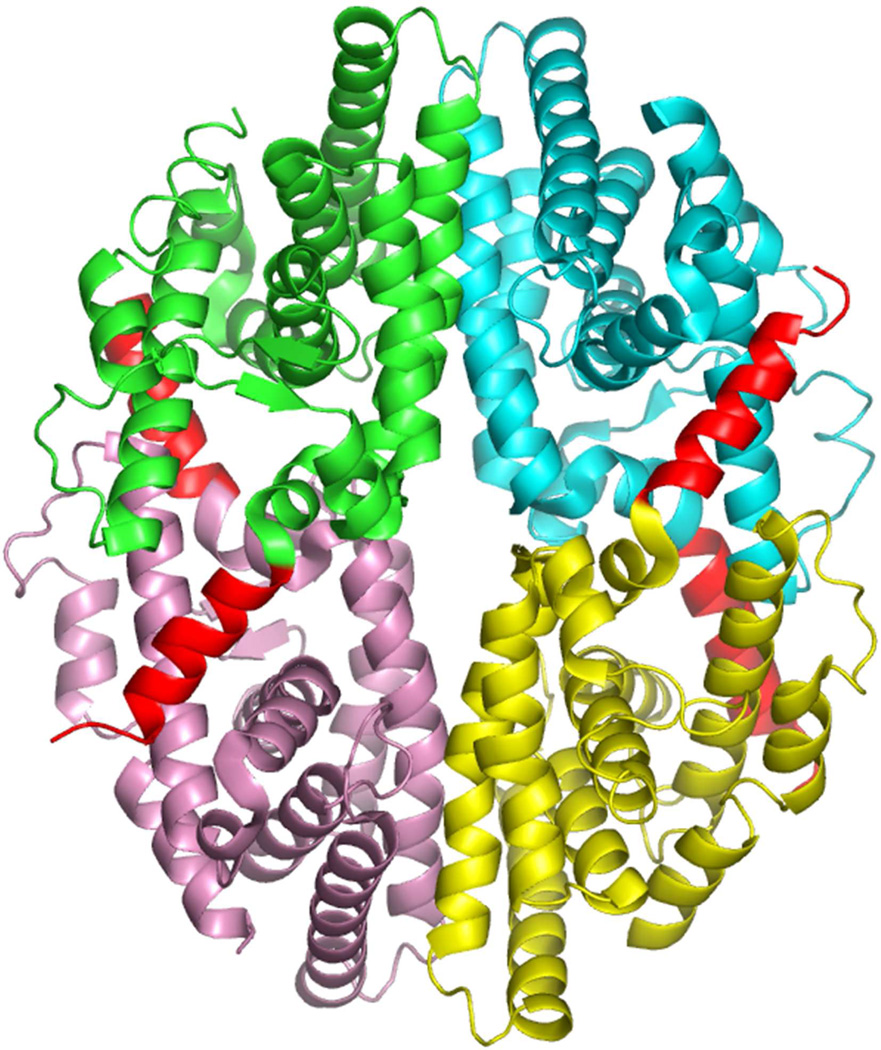
In addition to its dimerization patterns, RXR also has the unusual capacity to form tetramers (Figure 6). Studies have shown that the RXR LBD can self-associate into tetramers with a high affinity (Kd of 3–5 nM for dimer-dimer associations).225 RXR dissociates into monomers and dimers upon binding of 9-cis RA.226–227 Since ligand binding can dissociate the tetramer, it has been suggested that the RXR tetramer represents the inactive receptor form.228
Figure 6.
Structure of the RXRα LBD tetramer in the absence of ligand.233 The four subunits are shown in different colors, but in each case helix-12 is in red. Helix-12 from each subunit traverses to the adjacent subunit to occupy the groove normally reserved for coactivator LLxxLL motif binding. Coordinates are from PDB ID 1G1U.
That expectation was further confirmed through studies that used specific RXR protein mutations. A mutation in RXRα that fails to dissociate the tetramer upon ligand binding also prevents the activation of transcription.229 Conversely, another point mutation that blocks tetramer formations induces high levels of transcriptional activity even without ligand.229 Without an activating ligand, RXR fails to convert its tetrameric form into productive RXR heterodimers with RAR or VDR.230 A direct physiological role for RXR tetramers has been suggested in the regulation of the CRBP-II gene, a known target of RXR.231 The CRBP-II promoter contains two imperfect DR1 sites that prefer the binding of RXRα or RXRγ tetramers over dimers of RXR.232
To understand the structural basis for tetramer formation, a crystallographic study was used to visualize the RXRα LBD tetramer both in the absence of ligand, and in the presence of added 9-cis RA.233 A disc shaped tetramer was observed showing a compact arrangement of the LBDs, where each monomer buries a substantial 2600 Å2 of surface-accessible area in the complex (Figure 6). The four subunits each contribute three distinct areas to the tetramer interface (helix-3, helix-11, including a provocative interface between Helix-12 and the coactivator binding site). As a result, the tetramer can no longer accommodate a coactivator LLxxLL peptide, since it is auto-repressed structurally by this domain-sharing arrangement.233
Nevertheless, the ligand-binding pocket of the tetramer was seen to remain open and accessible. When 9-cis RA was added, the new crystal structure showed essentially no change in the tetramer organization.233 This was unexpected, but a close look at the electron density in the pocket indicated that 9-cis RA had been converted to a trans-isomer and then bound the tetramer. The isomerization of 9-cis RA presumably was caused by the light or reducing conditions associated with the crystallization conditions. The ligand did not adopt the low-energy state of all-trans RA, but rather a trans-like configuration at the C9-C10 double bond. The overall electron density within the four pockets also suggested poor ligand occupancy in each subunit.233
Our understanding of the RXR LBD tetramer state was recently expanded by new crystallographic studies that included the analysis of SMRT corepressor derived peptide and an antagonist of RXR in the complex.234 While maintaining a tetrameric arrangement, the corepressor peptide induced a notable rearrangement in the quaternary organization of the four subunits. The antagonist Rhein, derived from rhubarb, was studied crystallographically in the tetramer. This molecule binds competitively with 9-cis RA, with an IC50 of 0.75 µM, and was observed to bind to only two of the four subunits of the tetramer, Rhein binding causes helix-12 from two of the subunits to move into their neighboring coactivator binding sites and displace two SMRT peptides, leaving the other two SMRT peptides of the tetramer in place. These crystallographic studies on the RXR tetramer have been exciting and also revealing previously unknown modes of repression and antagonist action in RXR. At the same time, it is interesting that no other NR has been show to undergo tetramerization aside from RXR.
3.6 Recognition of 9-cis RA by RXR
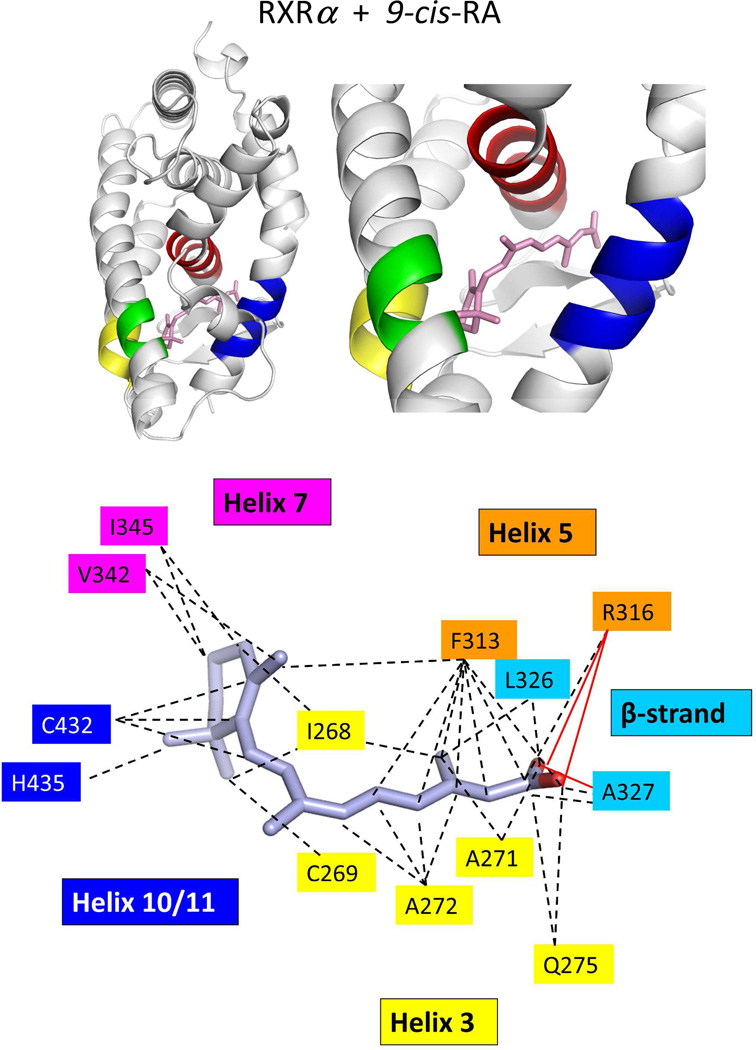
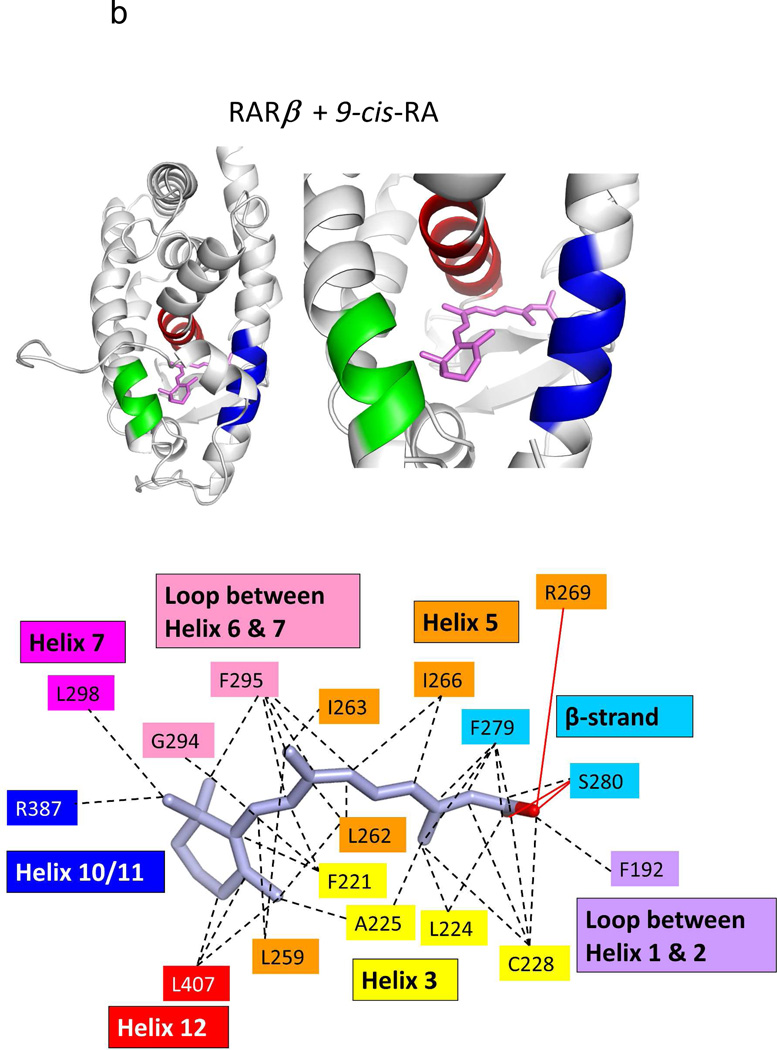
Two groups independently used X-ray crystallography to reveal how RXRα LBD recognizes 9-cis RA.74,222 The binding mode observed in these two studies was identical, and also consistent with our own visualization of the 9-cis RA binding mode to the full-length RXRα-PPARγ-DNA complex.64 9-cis RA penetrates into a hydrophobic pocket, formed by residues on helix-3, helix-5, helix-7, helilx-11, and the β-strand region of the LBD (see Figure 7a). The high affinity of 9-cis RA for the pocket (1.5 nM) is largely explained by the complimentary hydrophobic interactions of amino-acid side-chains with the isoprene chain of the ligand.
Figure 7.
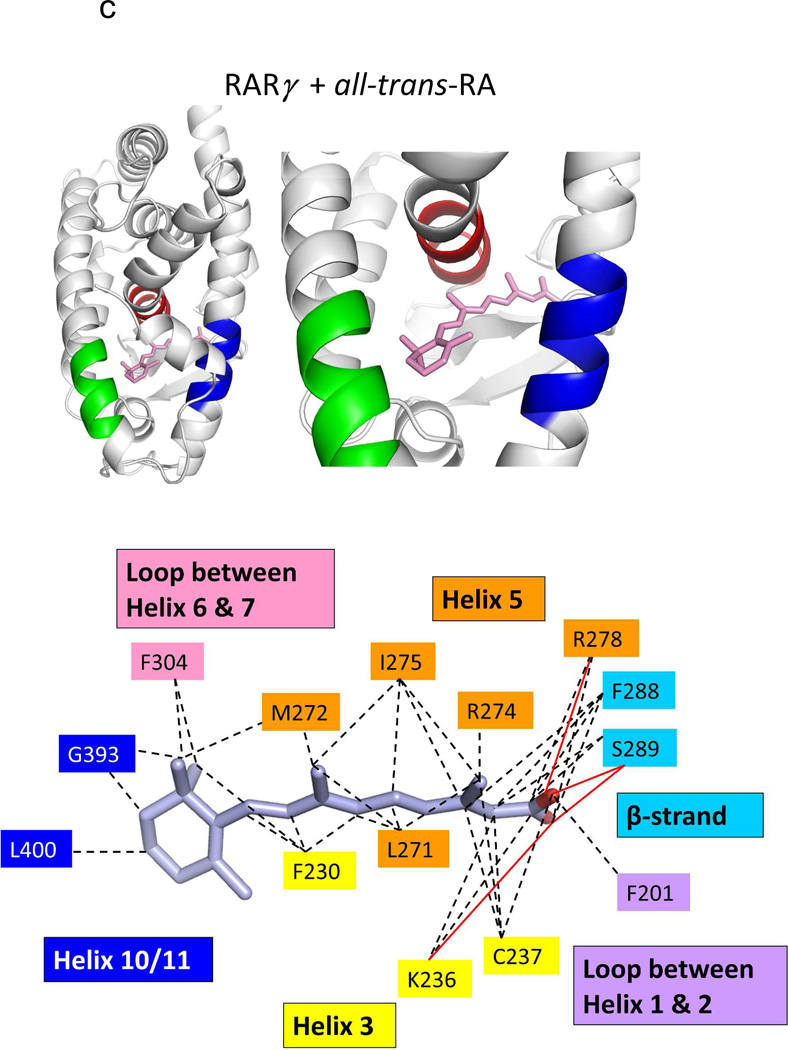
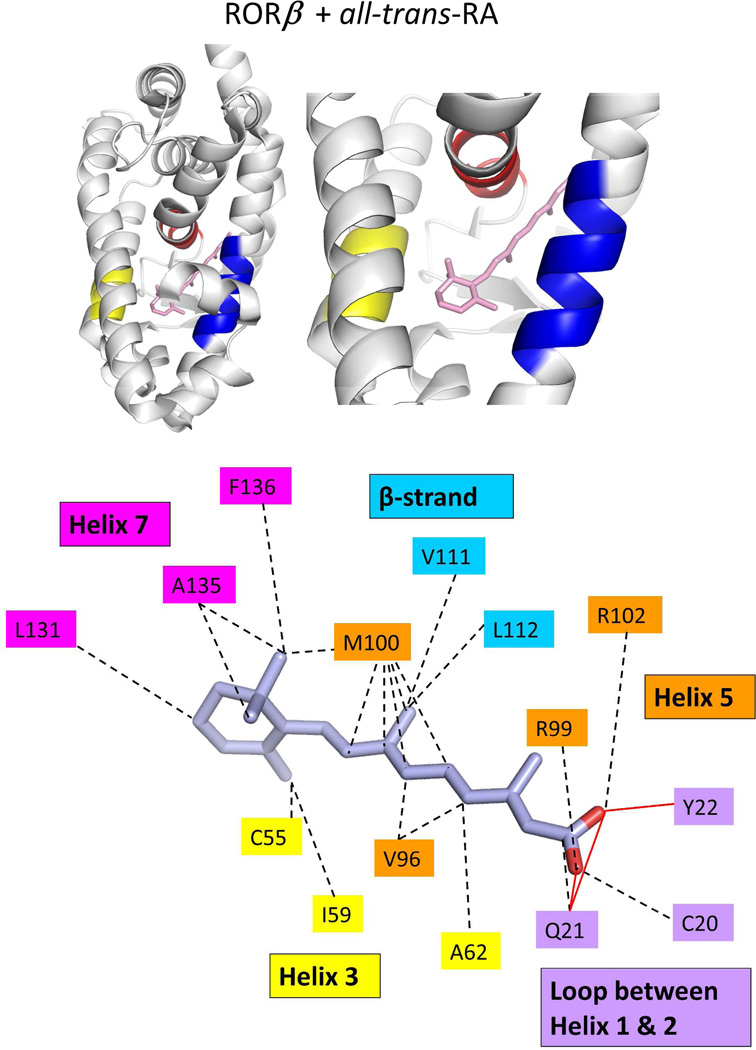
The binding location and specific interactions of 9-cis RA and all-trans RA inside (A) RXRα, (B) RARβ, (C) RARγ and (D) RORβ LBDs. Dotted lines indicate van der Walls contacts, and solid red lines indicated hydrogen bonding. Coordinates are from PDB ID 1FBY chain A, PDB ID 1XDK chain B, PDB ID 2LBD, and PDB ID 1N4H chain A.
The β ionone ring of the ligand is recognized by the interactions of I268 and C269 from helix-3, V342 and I345 from helix-7, and C432 and H435 from helix-10 (Figures 7b and 9a). The only hydrogen bonding observed involves the carboxylate groups of the ligand. Arg-316 is the most important pocket residue in some ways, because it caps the elongated cavity where the ligand is solvent shielded (Figure 8). The amino acids responsible for binding interactions visualized in the crystal structure were further confirmed through mutational studies.71,235
Figure 9.
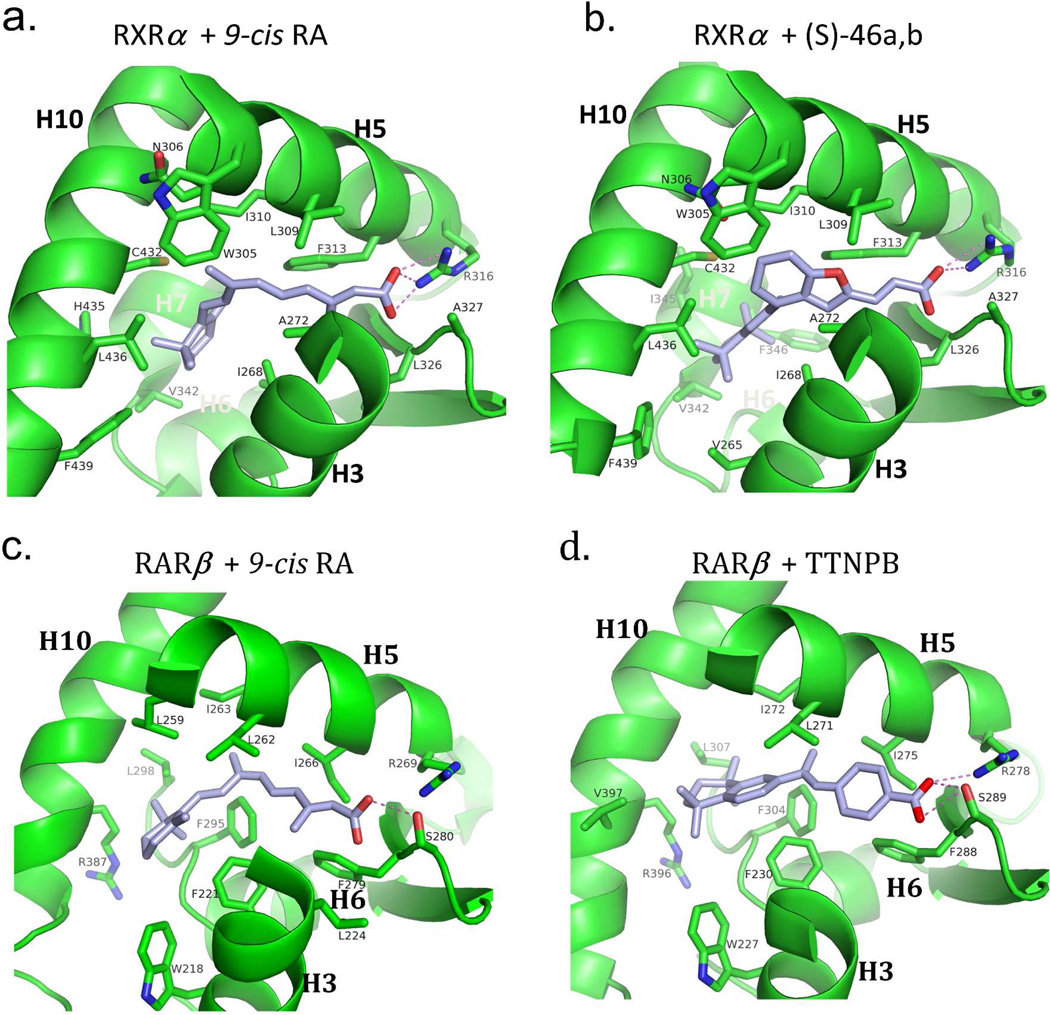
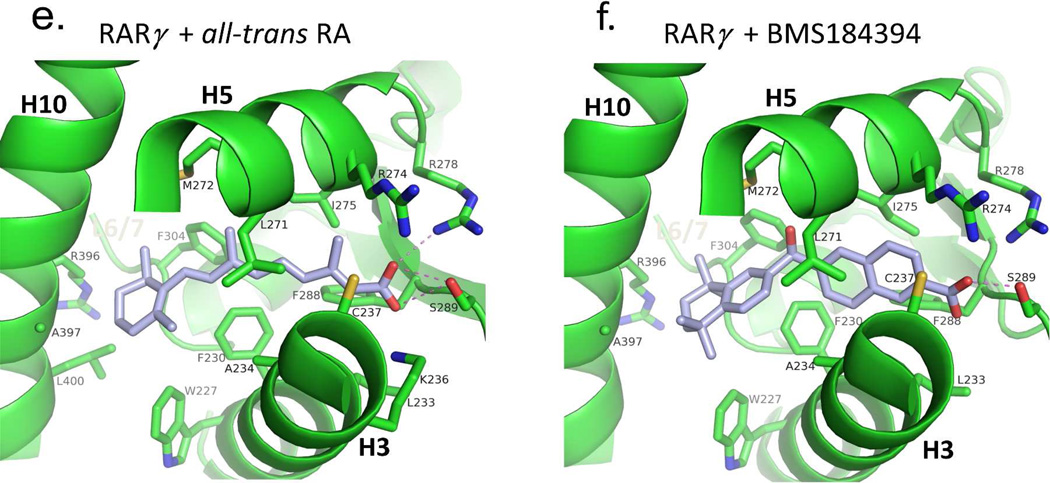
The adaptability of RXR and RAR LBDs to synthetic ligands. (A–B) The comparison of RXRα LBD bound to 9-cis RA and the synthetic ligand (S)- 46a,b.317 PDB ID 1FBY and 1RDT. (C-D) The comparison of RARβ bound to 9- cis RA and TTNBP.237 PDB ID 1XDK and 4DM6. (E-F) The comparison of RARβ bound to all-trans RA and BMS184394.76 PDB ID 2LBD and 1FCX.
Figure 8.
The degree of ligand burial inside the RAR, RXR and RORβ LBDs. (A–E): The LBDs are in white, the ligands in blue, helix 12 in each case is red, and the coactivator LLXXLL motifs is green. F. The solvent-accessible molecular surfaces from the LBD pockets surrounding 9-cis RA and all-trans RA in RARβ and RARγ, respectively. Coordinates for RARβ+9-cis RA are from PDB ID 1XDK chain B, for RARγ+9-cis RA are from PDB ID 3LBD human, for RARγ+ alltrans RA are from PDB ID 2LBD human, for RXRα+9-cis RA are from PDB ID 1FBY chain A, and for RORβ+ all-trans RA are from PDB ID 1N4H chain A.
The surface accessible volume of the pocket in the RXRα LBD is nearly 500 Å2. Yet, 9-cisRA, with its own volume of approximately 300 Å2, occupies only about 60% of this available space (see Figure 3b). Unoccupied volume is seen particularly in the vicinity of C19 and the C18 methyl groups of the ligand, suggesting that alternatively designed molecules that occupy these spaces may show better affinities. Indeed, synthetic molecules with these features have been described such as SR11237.74 All the residues that are responsible for 9-cis RA binding and recognition in the pocket are perfectly conserved in all three RXR isotypes. This impressive conservation suggested it would be difficult to identify synthetic RXR ligands that can bind in an isotype selective manner.
The selective binding of RXR only to 9-cis RA, and not all-trans RA, can be explained by a shape feature of the LBD pocket. The pocket contains a sharp bend that is selective for 9-cis RA (Figure 9a). It has been suggested that any ligand that binds to RXR LBD must physically adapt to this bend by twisting around single bonds appropriately. In the case of 9-cis RA, there is noted twist of the C8-C9 bond which orients the cyclohexenyl ring at an angle that is nearly perpendicular to the C9-C15 carbons, so as to be accommodated the pocket.236
A series of crystallographic studies later revealed that the RXR pocket is far more adaptable than was initially assumed, with the position of the carboxylic group being the most stable feature when multiple agonist structures of different chemical classes were compared (9-cis RA, DHA, BMS-649 and Oleate).73 Interestingly, interactions between the 9-cis RA molecule and helix-12 were not seen in any of these crystal structures. This raised the question of how the ligand activates the receptor by placing helix-12 in the agonist conformation. The answer lies in the indirect ability of hydrophobic residues in helix-10/11 and helix-3 to communicate the presence of ligand to helix-12. In particular, helix-12 becomes firmly positioned in its active conformation through a series of hydrophobic residues in helix-3 and helix-10/11 whose position is impacted by the binding of the ligand, This phenomenon of ligand communication with helix- 12 through a distance has been seen in other NR LBD structures as well.45 However, in some other NRs, the ligands do directly contact helix-12, forcing it into the active conformation.45
3.7. Recognition of RA Molecules by RAR
Crystal structures have now been described for all three isotypes of RAR in complex with different ligands.72,77–78,237–238 Sequence alignment has revealed that only three residues lining the pockets of the three RARs are variable.78 Unlike the case with RXR, no crystal structures of the apo-state of the RARs has become available. Figures 7b–c, 8b–f, and 9c–f together display multiple crystal structures of RAR LBDs with two RA molecules in detail.
It is informative to study the RAR LBD and its mode of binding to both 9-cis and all-trans RA (Figures 7b–c). The dihedral angle between the b-ionine ring (C5-C6) and the tetrene chain (C9, C12) is 43 degrees for all-trans RA, but 24 degrees for 9-cis RA, when their co-crystal structures of RAR are compared. Despite this marked differences in their bending angles, the pockets of RARs appear to accommodate both ligands with single nanomolar binding affinities.77 The pockets in all the RAR structures are essentially in the same position as the RXRα LBD pocket (see Figure 7a–d). These are all also similar to that of the RORβ LBD in its all-trans RA complex (Figure 7d, this receptor is discussed below). But these NRs pockets, as well as the general architecture of the LBDs, are altogether unrelated to those of other retinoid binding proteins, such as RABP, CRABP-I and CRABP-II.239–241
Figure 8 shows how the RA molecules are nearly completely buried in each of their NR LBDs. Moreover, Figure 9 shows how each of RXRα RARβ and RARγ pockets can accommodate the binding of synthetic ligands that are chemically distinct from RAs. One notable similarity in all the structures of RAR and RXR LBDs shown in these figures is the conserved arginine positioned to cap the pocket in each case, which also forms the critical salt bridge to the carboxylates end of each of these ligands.75 Despite the close sequence conservation of amino acids in the three RAR pockets, retinoids selective to each of the three RAR subtypes have been described.237,242–243
4. RETINOID RECEPTORS AS DRUG TARGETS
4.1 Cancer Therapeutics
Retinoids have a demonstrated capacity to act as chemopreventors.244 In many respects, the cancer benefits of retinoids can be directly linked to their activities as cellular differentiation promoters. The anti-cancer actions appear to be mediated largely through the RARs. In the mid-1980’s the only retinoid available for clinical use was 13-cis-retinoic acid (13-cis-RA). Retinoids are now known to induce differentiation in promyelocytic leukemia, myelodysplastic syndrome, cutaneous T-cell lymphoma (mycosis fungoides), and advanced squamous carcinoma of the skin.245
All-trans RA has became the treatment of choice for treating acute promyelocytic leukemia (APL), showing only minimal toxicity.246–248 APL is most often caused by a reciprocal chromosomal translocation of the RARα gene on chromosome 17 with the promyelocytic leukemia gene on chromosome 15, although other genetic translocations can also cause this disease. The gene fusion causes a hybrid protein to be produced in cells which is capable of RA binding.248 The application of all-trans RA to APL has rendered this disease the most curable subtype of all acute myeloid leukemias in adults.248
Besides their ability to target differentiation pathways, RA molecules can also block the growth of tumors through two other mechanisms: inhibiting cell proliferation and inducing apoptosis.249 Retinoids have been shown as strong inhibitors of epithelial cancer promotion and progression in a variety of experimental carcinogenesis models. These molecules have demonstrated their benefits in the treatment of both premalignant lesions and the reduction of second tumors in lung, liver, and head and neck patients.244,249 A wide range of studies, from experimental cell and animal models, to epidemiological data and clinical trials, have continuously supported the potential for retinoids in cancer prevention and therapies.244,250–254
Isotretinoin (13-cis RA) has been shown to reduce second aerodigestive tract tumor in patients with head and neck cancers.252 Retinol palmitate reduces the number of new primary tumors in patients with stage I non-small-cell lung cancer.255 It has been postulated that loss of retinoid signaling to maintain normal cellular functions is linked directly to the development of hepatocellular carcinoma. The retinoid polyprenoic acid [(2E,4E,6E, 10E)-3,7,11,15-tetramethyl-2,4,6,10,14-hexadecapenta- enoic acid], when orally administered, acts to delete malignant clones and inhibit secondary hepatocellular carcinomas after ablation of primary liver cancer.256 The mechanism of polyprenoic acid action appears to be derived from its induction of both cellular differentiation and apoptosis.256
RA is also believed to be one of the most potent differentiation inducers for human neuroblastoma cells, and treatment of these cells with RA arrests their proliferation.257 RA therapy improves the survival of children with neuroblastoma by suppressing the residual disease after chemotherapy.244,258 Other uses of RA include its ability to act as a chemopreventive in premalignant lesions such as oral leukoplakia, cervical dysplasia, and xeroderma pigmentosa.244,254,259
Treatment of osteosarcoma and chondrosarcoma cell lines with all-trans RA has shown reversible growth inhibition and simultaneously blocking of colony formation.260–261 Retinoids have recently entered different phases of clinical trials for the treatment of solid tumors.262–263 These molecules include TAC-101 (Taiho Pharmaceuticals, Japan, and Tazaroten (Allergen, USA).
4.2. Skin Indications
As a class of therapeutics, retinoid therapy has been long used for the treatment of severe acne and psoriasis.264–267 Severe acne can be associated with psychological and physical morbidity for millions of affected patients. Isotretinoin (13-c/s retinoic acid) is an oral retinoid that is effective against severe acne and used clinically for this disease. Isotretinoin targets the sebaceous gland and is potent and effective clinically. Yet, this drug is also a known teratogen whose use is now highly restricted within an FDA-mandated registry system. Incomplete understanding of 13-cis RA’s mechanism of action within the sebaceous gland has slowed progress to find alternative therapies.
Psoriasis is a chronic, inflammatory condition typically systemic in its profile and characterized by skin lesions and co-morbidities that affect approximately 7 million patients in the USA alone.268 Topical retinoids such as Tazarotene (belonging to the acetylenic class of retinoids) can be an effective treatment for chronic psoriasis clinically. As the case with isotretinoin in acne, the detailed mechanism of action for this molecule has not been described with respect to how its alters keratinocyte proliferation and inflammatory responses in the dermis and epidermis.269 More detailed studies are needed, for example, to understand whether this molecule induces apoptosis and/or cell cycle arrest, and which nuclear receptor mediates its functions. For both isotretinoin and Tazarotene, obtaining gene expression profiles in sebocytes in response to these molecules would be a forward step in generating new insights for further development of drugs for these conditions.
4.3 Metabolic Disease Targeting
On the basis of the anti-tumor properties activities of RA molecules acting on RAR, various RXR targeted molecules have also emerged or are being developed for other disease indications.270 As RXR is the combinatorial partner for a significant number of human NRs, many of which are permissive complexes, it is possible to activate multiple NR signaling pathways through the RXR receptor pocket, rather than its partner. Rexinoids, a term used to describe RXR selective modulators, have been undergoing active development in many laboratories over the past ten years.270–272 Yet, the ability of RXRs to coordinate the functional activities of so many different heterodimerization partners also raises the concern that rexinoids may not provide a good therapeutic window.
An avidly pursued area for rexinoid development appears to be as metabolic diseases interventions, where PPAR and LXR pathways are particularly important. Indeed, despite some beneficial effects of rexinoids already demonstrated on enhancing insulin sensitivity and reducing fat mass through their permissive complexes, the promiscuous nature of RXRs has made it difficult to develop this therapeutic area thoroughly. Rexinoid treatment has been linked to hypertriglyceridemia, a well-known risk factor for cardiovascular disease.273 Several rexinoids with no RXR isotype selectivity have been well-described.270 Some of these molecules do exhibit relatively select patterns of gene expression induction.272,274
Two well-described rexinoids, HX630 and LG268, have been shown to modulate RXR complexes through their RXR moiety. LG268 is able to activate both RXR– LXR and RXR–PPARγ heterodimers. LG268 dramatically reduced cholesterol absorption in mice. The mechanism may involve the regulation of ATP-binding cassette transporters ABCG5/G8.275 By contrast, HX630 preferentially activates RXR–PPARγ complexes.276–277 These two rexinoids appear to be relatively specific with respect to their gene-activation profiles, when examined using DNA microarray analyses.278
Another rexinoid, LG101506, has been shown to selectively activate RXR–PPARγ heterodimers, but not RXR–RAR or RXR–LXR heterodimers, making it potentially useful as an insulin sensitizer 279. This compound lowers blood glucose levels in genetically disposed type II diabetic mice.280 Antagonism of RXR, when in the RXR–PPARγ dimer, was also reported to normalize glycemia and fat mass in animal models, although the mechanism for this action is not clear.281 Taken together, these data support the concept and future promise of heterodimer-specific rexinoids, as therapeutic ligands for metabolic diseases.
5. OTHER RECEPTORS ASSOCIATED WITH RA SIGNALING
5.1. RORβ interactions with RA
The retinoid-related orphan receptor (RORs) form a subgroup of the NR family with three distinct members (RORα, RORβ, RORγ).282 The RORs are unable to form homodimers or heterodimers, instead functioning as monomers and binding to response elements with a single half-site.283 The nuclear receptors Rev-Erbα and Rev-Erbβ have similar DNA binding properties as the RORs, and compete for some sites in vivo, while displaying opposite effects on the transcription of target genes.284 Despite early suggestions that melatonin could be a ROR ligands, that finding was not validated, and the true endogenous ligands for this receptor subfamily has remained obscure.284–285
RORα is widely expressed in peripheral tissues, with highest levels seen in liver, muscle, and brain.284,286 Among its potential functions, it appears to have a role in the differentiation of the Th17-cell lineage, although this differentiation is more reliant on RORγ.287–288 Indeed, RORγ is currently being avidly pursued in industry as a drug target for its potential to control autoimmune diseases related to the Th17 cell lineage.288–290 Both RORα and RORγ are regulated in a circadian manner in many tissues, and appear to have metabolic regulatory functions that are somewhat redundant.288,291–292 Mice with deleted RORα genes display the so-called stagger phenotype, with cerebellar defects, abnormal inflammatory cytokine levels, and deficient intestinal apolipoprotein A-1 and apolipoprotein CIII levels.293–296
RORβ is distinct in many ways from its RORα and RORγ counterparts. RORβ has a restricted pattern of expression, localized to specific areas of the brain and retina.297 This receptor appears to regulate genes with essential roles in sensory input integration and the control of circadian timing.297 Mice deficient in RORβ develop retinal degeneration that results in blindness and show disrupted circadian behavior.298
X-ray crystallography first revealed information about the size of the ligand binding pockets of RORs.299–300 The ligand-binding pocket of RORβ was estimated at 770 A3, similar to that of RORα (720 A3). Cholesterol, 7-dehydrocholesterol, and cholesterol sulfate have been suggested as RORα agonists, but not ligands for RORβ.299,301 These molecules bind RORα reversibly, and cause a enhancement in its transcriptional activation. RORα is believed to function as a possible lipid sensor and indeed, this receptor has been implicated broadly in the regulation of lipid metabolism.292,299,302–303 A crystal structure of RORγ with 25-hydroxyl cholesterol, a surrogate ligand, has also been reported.304 RORγ has the highest expression in skeletal muscle, but a thymus specific isoform (RORγt) is particularly important for the maturation of naïve T-helper immune cells into Th-17 cells.289,305–306
The first structural study on the RORβ LBD showed stearic acid being fortuitously captured in the pocket, with the receptor helix-12 being positioned in the active conformation.300 While this fatty acid nicely fills the ligand-binding pocket, no strong evidence was presented to indicate it is a physiological ligand.300 Given that RORs are evolutionarily close to the RARs, a subsequent study checked by mass-spectrometry whether retinoids or their synthetic analogs could bind to RORs.307 Those studies suggested that all-trans retinoic acid and the synthetic retinoid ALRT-1550 could bind and act as potential ligands for RORβ.307 These two ligands were shown to bind RORβ LBD in a reversible manner, and with high affinity. Figure 7d shows the crystal structure of RORβ with all-trans RA.307 The ligand occupies less than 40% of the pocket volume, with numerous van der Waals interactions involving helix-3, helix-7, and the beta-strand region. The carboxylate groups are recognized by hydrophilic side-chains of three residues through hydrogen bonding.
The observed transcriptional effects of retinoids on RORβ suggest that they may be partial antagonists. These molecules can apparently also bind to RORγ and inhibit its transactivation, but not to RORα.307 Moreover, the repression of RORβ target genes appeared to be robust in neuronal cells, but not in other cell types tested. This latter finding suggested that the antagonism of these retinoids may require the physiological interactions of a neuronal cell-specific corepressor, which has yet to be identified.307 Clearly, additional studies are required at this time to validate whether all-trans RA is the endogenous physiological ligand of RORβ and/or RORγ. The identification of endogenous ligands for all three RORs remains an important goal, as they are clearly linked to important human pathologies, including inflammation, immunity, obesity, asthma, and circadian disorders.308
5.2 Orphan members of the NR family
Besides the RXRs, RARs and RORβ, several other NRs may have the ability to form direct physical interactions with RA molecules. The PPAR family member PPARγ/δ has been shown to bind RA with a relatively high affinity (Kd of 15 nM), which is an order of magnitude tighter than RA binding to the other PPAR subtypes.309 Still, the binding affinity of RA to RAR is approximately ten fold better than seen with PPARβ/δ.309
The further observations that RA can activate gene expression through PPARβ/δ, raise the possibility that some of the biological activities of this hormone may be mediated by an RAR-independent pathway. This interaction and signaling through PPARβ/δ is thought to be potentially responsible for RA molecules controlling insulin responsiveness and energy homeostasis.310 Administration of RA to obese mice leads to fat mass loss, as well as improved glucose tolerance in these animals.310 These effects have been linked to the activation of both PPARβ/δ and RARs in adipose tissue, muscle, and liver.310 Based on these finding, it seems plausible that RA can directly bind to and regulate PPARβ/δ, to impact insulin control and adiposity.310
Reports on several other orphan NRs have also suggested their possible involvement in RA binding and signaling. These receptors include the chicken ovalbumin upstream promoter-transcription factors COUP-TF1 and COUP-TFII and the testicular receptors TR2/4.311–312 Although the COUP-TFs have been well studied, the ligand for these receptors have remained unknown for the decades since their initial cloning. It was recently demonstrated that retinoic acids can promote the binding of COUP-TFII to its coactivators, leading to increased activity from a reporter construct.311 Retinoic acid binding appears to release COUP-TFII from a repressed state.311 The orphan NRs TR2/4 homodimerize as well as heterodimerize with each other. These receptors may also be able to bind to retinol, which activates them by converting the autorepressed LBD into an active conformation.312 But in the case of both COUPTFII, and TR2/4, the concentration of retinoids required to see a measureable change in their transcriptional activities is considerably higher than seen with RXRs and RARs, and outside the necessary range required to make these receptors RA responsive in vivo.
6. FUTURE DIRECTIONS
While there have been numerous crystallographic studies revealing the basis for natural and synthetic ligand binding to each of the RXR and RARs, attempts to obtain high-resolution structures of their complete NRs, with all of their domains, in a functionally revealing complex have failed. Consequently, we do not know to what extent the DBD, hinge regions, and LBD regions of the RXR and RAR communicate, both within their respective polypeptides and through their heterodimerization interfaces. As the crystal structure of the intact RXR-PPAR already revealed, there are many functionally meaningful connections in full-length NR complexes, and this is likely to be true also for the RXR-RAR heterodimer. Without knowing all the physical connections in these complexes, it is difficult to develop synthetic ligands that may act allosterically, i.e. binding to one domain in the protein complex, but inducing a functional change in another domain of the receptor
A better structural understanding of NR coactivators or corepressors is also required in the field, beyond the short 10–20 amino-acid peptide derivatives that have so far been visualized in crystallographic studies. The interactions of these proteins is of great significance for our understanding of retinoid actions, since RAR can activate or repress their target genes differentially in response to a single ligand, depending on the coregulator availabilities, and interactions in each cell type, and the cues provided by the response elements. Indeed, as described here, RARs and RXRs are highly responsive molecular devices through which the precise control of gene transcription is achieved during development, both via their coregulators and their ligands. The dynamic expression of these retinoid receptors throughout different stages of development considerably suggests that we should now move beyond ligand binding, to undertake new studies that visualize the specific interactions of coregulators complexes on these receptors.
While the functional interactions and mechanisms associated with gene activation have been relatively well studied, the mechanism underlying gene repression by the RXRs and RARs have not been equally thorough. Given the fundamental importance RAR-mediated gene repression signaling during skeletal development and in skin homeostasis, an expanded understanding of repression pathway and protein-interactions responsible for repression could advance medical developments in the treatment of disorders of the skeleton or skin.313–316 We suggest that understanding the mechanisms that involve the complete receptor polypeptides, as well as their partners and functional macromolecular complexes will fuel the next decade of intervention strategies into many human diseases, including cancer, dermatology, defects of development, diabetes and other metabolic disorders.
Acknowledgment
Work in the F.R. laboratory on nuclear receptors is currently supported by the National Institutes of Health grants R01 DK094147 and R01 DK097475. FR is grateful to all the past and present members of his laboratory for their important contributions to the nuclear receptor field.
Abbreviations
- RAR
retinoic acid receptor
- RXR
retinoid x receptor
- PPAR
peroxisome-proliferator activated receptor
- VDR
vitamin D receptor
- TR
thyroid hormone receptor
- all-trans RA
all-trans retinoic acid
- 9-cis RA
9-cis retinoic acid
- RA
retinoic acid
- 13-cis RA
13-cis retinoic acid
- DBD
DNA binding domain
- LBD
Ligand binding domain
- DHA
docosa hexaneoic acid
- DR
direct repeat
- ROR
retinoid-related orphan receptor
- CAR
constitutive androstane receptor
- PXR
pregnane x receptor
- FXR
farnesoid x receptor
- TR2/4
testicular receptors 2 and 4
- COUP-TF
chicken ovalbumin upstream promoter-transcription factor
- AR
Androgen receptor
- PR
progesterone receptor
- MR
mineralocorticoid receptor
- GR
glucocorticoid receptor
- LXR
liver x receptor
Biographies

Pengxiang Huang
Pengxiang Huang received his B.S. in biology at Tsinghua University in China (2002) and his Ph.D. in biophysics at the University of Virginia (2009) in Dr. Fraydoon Rastinejad’s laboratory. He was a recipient of Chinese Government Award for Outstanding Self-Financed Students Abroad (2009). Upon graduation, he continued as a postdoctoral associate in Dr. Rastinejad’s lab at the Sanford-Burnham Medical Research Institute in Lake Nona, Florida. His Ph.D. and postdoctoral work contributed to the discovery of heme as the natural ligand for Rev-Erb receptors and the crystal structure determination of intact nuclear receptor complexes with DNA. His research has focused in the areas of biochemical, cell biology and X-ray crystallographic understanding of the nuclear receptors.

Vikas Chandra
Vikas Chandra received his B.S. degree in Biology from Meerut University (1993), his M.S. degree in Biotechnology from Indian Institute of Technology (1995) and his Ph.D. degree in Biophysics from All India Institute of Medical Sciences (2001) India. He Dr. Fraydoon Rastinejad’s laboratory as a research associate, and then later became Instructor at Center for molecular design, at the University of Virginia (2008). In 2010, he joined Sanford-Burnham Medical Research Institute in Lake Nona, Florida as Staff Scientist, also being a senior scientist in Dr. Rastinejad’s laboratory. His research has been focused the on structure-function studies of intact nuclear receptor complexes with DNA.

Fraydoon Rastinejad
Fraydoon Rastinejad received his B.A. degrees in mathematics and in biochemistry (1987) from Northwestern University. and his Ph.D. degree in Biophysics (1992) from the University of Pennsylvania. He then continued to postdoctoral work at Yale University with the late Paul Sigler. After three years, he joined the University of Virginia, School of Medicine’s Pharmacology Department laboratory as Assistant Professor. In 2010, he left his position as Professor of Pharmacology and Biochemistry, and director of Center of Chemical Design to join the Sanford-Burnham Medical Research Institute in Lake Nona, Florida as Professor in the Metabolic Disease and Signaling Program. His laboratory works in the areas of structural and chemical biology of nuclear receptors, and his team is currently using novel high-throughput screens and cell-based studies that identify small molecule modulators for these proteins. Dr. Rastinejad is on the editorial boards of Molecular Cell Biology and Nuclear Receptor Signaling.
REFERENCES
- 1.Wolbach SB, Howe PR. J. Exp. Med. 1925;42:753. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCollum EV, Davis M. J. Biol. Chem. 1913;15:167. [Google Scholar]
- 3.Wald G. Nature. 1934;134:65. [Google Scholar]
- 4.Bendich A, Olson JA. FASEB J. 1989;3:1927. [PubMed] [Google Scholar]
- 5.D'Ambrosio DN, Clugston RD, Blaner WS. Nutrients. 2011;3:63. doi: 10.3390/nu3010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curley RW., Jr Biochim. Biophys. Acta. 2012;1821:3. doi: 10.1016/j.bbalip.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Guillonneau M, Jacqz-Aigrain E. Arch. Pediatr. 1997;4:867. doi: 10.1016/s0929-693x(97)88158-4. [DOI] [PubMed] [Google Scholar]
- 8.Collins MD, Mao GE. Annu. Rev. Pharmacol. Toxicol. 1999;39:399. doi: 10.1146/annurev.pharmtox.39.1.399. [DOI] [PubMed] [Google Scholar]
- 9.Blomhoff R, Green MH, Green JB, Berg T, Norum KR. Physiol. Rev. 1991;71:951. doi: 10.1152/physrev.1991.71.4.951. [DOI] [PubMed] [Google Scholar]
- 10.Pares X, Farres J, Kedishvili N, Duester G. Cell. Mol. Life Sci. 2008;65:3936. doi: 10.1007/s00018-008-8591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duester G. Eur. J. Biochem. 2000;267:4315. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. Genes. Dev. 2001;15:226. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Dev. Cell. 2004;6:411. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 14.Uehara M, Yashiro K, Mamiya S, Nishino J, Chambon P, Dolle P, Sakai Y. Dev. Biol. 2007;302:399. doi: 10.1016/j.ydbio.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 15.Bushue N, Wan YJ. Adv. Drug Deliv. Rev. 2010;62:1285. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf G. Nutr. Rev. 2007;65:385. doi: 10.1301/nr.2007.aug.385-388. [DOI] [PubMed] [Google Scholar]
- 17.Albalat R. Mol. Cell. Endocrinol. 2009;313:23. doi: 10.1016/j.mce.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Fawcett D, Pasceri P, Fraser R, Colbert M, Rossant J, Giguere V. Development. 1995;121:671. doi: 10.1242/dev.121.3.671. [DOI] [PubMed] [Google Scholar]
- 19.Stratford T, Logan C, Zile M, Maden M. Mech. Dev. 1999;81:115. doi: 10.1016/s0925-4773(98)00231-7. [DOI] [PubMed] [Google Scholar]
- 20.Tickle C, Alberts B, Wolpert L, Lee J. Nature. 1982;296:564. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- 21.Helms JA, Kim CH, Eichele G, Thaller C. Development. 1996;122:1385. doi: 10.1242/dev.122.5.1385. [DOI] [PubMed] [Google Scholar]
- 22.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Development. 2000;127:75. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Glover JC, Renaud JS, Rijli FM. J. Neurobiol. 2006;66:705. doi: 10.1002/neu.20272. [DOI] [PubMed] [Google Scholar]
- 24.Sockanathan S, Jessell TM. Cell. 1998;94:503. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- 25.Maden M. J. Neurobiol. 2006;66:726. doi: 10.1002/neu.20248. [DOI] [PubMed] [Google Scholar]
- 26.Hyatt GA, Schmitt EA, Marsh-Armstrong N, McCaffery P, Drager UC, Dowling JE. Development. 1996;122:195. doi: 10.1242/dev.122.1.195. [DOI] [PubMed] [Google Scholar]
- 27.Dickman ED, Thaller C, Smith SM. Development. 1997;124:3111. doi: 10.1242/dev.124.16.3111. [DOI] [PubMed] [Google Scholar]
- 28.Duester G. Cell. 2008;134:921. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Neuron. 2003;40:65. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 30.Sirbu IO, Duester G. Nat. Cell. Biol. 2006;8:271. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pourquie O. Science. 2003;301:328. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- 32.Duester G. Birth Defects Res. C. Embryo. Today. 2007;81:84. doi: 10.1002/bdrc.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houle M, Sylvestre JR, Lohnes D. Development. 2003;130:6555. doi: 10.1242/dev.00889. [DOI] [PubMed] [Google Scholar]
- 34.Moreno TA, Kintner C. Dev. Cell. 2004;6:205. doi: 10.1016/s1534-5807(04)00026-7. [DOI] [PubMed] [Google Scholar]
- 35.Vermot J, Pourquie O. Nature. 2005;435:215. doi: 10.1038/nature03488. [DOI] [PubMed] [Google Scholar]
- 36.Mangelsdorf DJ, Evans RM. Cell. 1995;83:841. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 37.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Science. 2001;294:1866. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 38.Morriss-Kay GM, Ward SJ. Int. Rev. Cytol. 1999;188:73. doi: 10.1016/s0074-7696(08)61566-1. [DOI] [PubMed] [Google Scholar]
- 39.Leid M, Kastner P, Chambon P. Trends Biochem. Sci. 1992;17:427. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 40.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Cell. 2006;126:789. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Nat. Struct. Mol. Biol. 2007;14:1207. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, Williams JD, Fletterick RJ, Ingraham HA. Mol. Endocrinol. 2009;23:25. doi: 10.1210/me.2007-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortlund EA, Lee Y, Solomon IH, Hager JM, Safi R, Choi Y, Guan Z, Tripathy A, Raetz CR, McDonnell DP, Moore DD, Redinbo MR. Nat. Struct. Mol. Biol. 2005;12:357. doi: 10.1038/nsmb910. [DOI] [PubMed] [Google Scholar]
- 44.Forman BM. Cell Metab. 2005;1:153. doi: 10.1016/j.cmet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Huang P, Chandra V, Rastinejad F. Annu. Rev. Physiol. 2010;72:247. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rastinejad F, Huang P, Chandra V, Khorasanizadeh S. J. Mol. Endocrinol. 2013;51:T1. doi: 10.1530/JME-13-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giguere V, Ong ES, Segui P, Evans RM. Nature. 1987;330:624. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 48.Brand N, Petkovich M, Krust A, Chambon P, de The H, Marchio A, Tiollais P, Dejean A. Nature. 1988;332:850. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- 49.Benbrook D, Lernhardt E, Pfahl M. Nature. 1988;333:669. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- 50.Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Nature. 1989;339:714. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- 51.Giguere V, Shago M, Zirngibl R, Tate P, Rossant J, Varmuza S. Mol. Cell. Biol. 1990;10:2335. doi: 10.1128/mcb.10.5.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolle P. Nucl. Recept. Signal. 2009;7:e006. doi: 10.1621/nrs.07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giguere V, Yang N, Segui P, Evans RM. Nature. 1988;331:91. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 54.Petkovich M, Brand NJ, Krust A, Chambon P. Nature. 1987;330:444. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 55.Ragsdale CW, Jr, Petkovich M, Gates PB, Chambon P, Brockes JP. Nature. 1989;341:654. doi: 10.1038/341654a0. [DOI] [PubMed] [Google Scholar]
- 56.Hamada K, Gleason SL, Levi BZ, Hirschfeld S, Appella E, Ozato K. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8289. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leid M, Kastner P, Lyons R, Nakshatri H, Saunders M, Zacharewski T, Chen JY, Staub A, Garnier JM, Mader S, Chambon P. Cell. 1992;68:377. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- 58.Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nature. 1990;345:224. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- 59.Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM. Genes Dev. 1992;6:329. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 60.Liu Q, Linney E. Mol. Endocrinol. 1993;7:651. doi: 10.1210/mend.7.5.8391126. [DOI] [PubMed] [Google Scholar]
- 61.Lefebvre P, Benomar Y, Staels B. Trends Endocrinol. Metab. 2010;21:676. doi: 10.1016/j.tem.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Brocard J, Kastner P, Chambon P. Biochem. Biophys. Res. Commun. 1996;229:211. doi: 10.1006/bbrc.1996.1782. [DOI] [PubMed] [Google Scholar]
- 63.Fleischhauer K, Park JH, DiSanto JP, Marks M, Ozato K, Yang SY. Nucleic Acids Res. 1992;20:1801. doi: 10.1093/nar/20.7.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. Nature. 2008;456:350. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berrabah W, Aumercier P, Lefebvre P, Staels B. FEBS Lett. 2011;585:1640. doi: 10.1016/j.febslet.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 66.Khorasanizadeh S, Rastinejad F. Trends Biochem. Sci. 2001;26:384. doi: 10.1016/s0968-0004(01)01800-x. [DOI] [PubMed] [Google Scholar]
- 67.Rastinejad F, Perlmann T, Evans RM, Sigler PB. Nature. 1995;375:203. doi: 10.1038/375203a0. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Q, Chasse SA, Devarakonda S, Sierk ML, Ahvazi B, Rastinejad F. J. Mol. Biol. 2000;296:509. doi: 10.1006/jmbi.1999.3457. [DOI] [PubMed] [Google Scholar]
- 69.Rastinejad F, Wagner T, Zhao Q, Khorasanizadeh S. EMBO J. 2000;19:1045. doi: 10.1093/emboj/19.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Devarakonda S, Harp JM, Kim Y, Ozyhar A, Rastinejad F. EMBO J. 2003;22:5827. doi: 10.1093/emboj/cdg569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wurtz JM, Bourguet W, Renaud JP, Vivat V, Chambon P, Moras D, Gronemeyer H. Nat. Struct. Biol. 1996;3:87. doi: 10.1038/nsb0196-87. [DOI] [PubMed] [Google Scholar]
- 72.Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Mol. Cell. 2000;5:289. doi: 10.1016/s1097-2765(00)80424-4. [DOI] [PubMed] [Google Scholar]
- 73.Egea PF, Mitschler A, Moras D. Mol. Endocrinol. 2002;16:987. doi: 10.1210/mend.16.5.0823. [DOI] [PubMed] [Google Scholar]
- 74.Egea PF, Mitschler A, Rochel N, Ruff M, Chambon P, Moras D. EMBO J. 2000;19:2592. doi: 10.1093/emboj/19.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klaholz BP, Mitschler A, Belema M, Zusi C, Moras D. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6322. doi: 10.1073/pnas.97.12.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klaholz BP, Mitschler A, Moras D. J. Mol. Biol. 2000;302:155. doi: 10.1006/jmbi.2000.4032. [DOI] [PubMed] [Google Scholar]
- 77.Klaholz BP, Renaud JP, Mitschler A, Zusi C, Chambon P, Gronemeyer H, Moras D. Nat. Struct. Biol. 1998;5:199. doi: 10.1038/nsb0398-199. [DOI] [PubMed] [Google Scholar]
- 78.Renaud JP, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Nature. 1995;378:681. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 79.Avantaggiato V, Acampora D, Tuorto F, Simeone A. Dev. Biol. 1996;175:347. doi: 10.1006/dbio.1996.0120. [DOI] [PubMed] [Google Scholar]
- 80.Durston AJ, Timmermans JP, Hage WJ, Hendriks HF, de Vries NJ, Heideveld M, Nieuwkoop PD. Nature. 1989;340:140. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- 81.Rhinn M, Dolle P. Development. 2012;139:843. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 82.Mark M, Ghyselinck NB, Chambon P. Nucl. Recept. Signal. 2009;7:e002. doi: 10.1621/nrs.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kastner P, Messaddeq N, Mark M, Wendling O, Grondona JM, Ward S, Ghyselinck N, Chambon P. Development. 1997;124:4749. doi: 10.1242/dev.124.23.4749. [DOI] [PubMed] [Google Scholar]
- 84.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Decimo D, Krezel W, Dierich A, Chambon P. Genes Dev. 1996;10:80. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 85.Krezel W, Dupe V, Mark M, Dierich A, Kastner P, Chambon P. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9010. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dejean A, Bougueleret L, Grzeschik KH, Tiollais P. Nature. 1986;322:70. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- 87.Peng X, Maruo T, Cao Y, Punj V, Mehta R, Das Gupta TK, Christov K. Cancer Res. 2004;64:8911. doi: 10.1158/0008-5472.CAN-04-1810. [DOI] [PubMed] [Google Scholar]
- 88.Liao WL, Tsai HC, Wang HF, Chang J, Lu KM, Wu HL, Lee YC, Tsai TF, Takahashi H, Wagner M, Ghyselinck NB, Chambon P, Liu FC. Proc. Natl. Acad Sci. U. S. A. 2008;105:6765. doi: 10.1073/pnas.0802109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krezel W, Ghyselinck N, Samad TA, Dupe V, Kastner P, Borrelli E, Chambon P. Science. 1998;279:863. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- 90.Krust A, Kastner P, Petkovich M, Zelent A, Chambon P. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5310. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, Grippo JF. Nature. 1992;355:359. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 92.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. Cell. 1992;68:397. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 93.Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, Rosenberger M, Lovey A, Kastner P, Grippo JF, Chambon P, Levin AA. Proc. Natl. Acad. Sci. U. S. A. 1993;90:30. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clagett-Dame M, DeLuca HF. Annu. Rev. Nutr. 2002;22:347. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 95.Mic FA, Molotkov A, Benbrook DM, Duester G. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7135. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolf G. Nutr. Rev. 2006;64:532. doi: 10.1111/j.1753-4887.2006.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 97.Kane MA. Biochim. Biophys. Acta. 2012;1821:10. doi: 10.1016/j.bbalip.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS. J. Biol. Chem. 1995;270:17850. doi: 10.1074/jbc.270.30.17850. [DOI] [PubMed] [Google Scholar]
- 99.Horton C, Maden M. Dev. Dyn. 1995;202:312. doi: 10.1002/aja.1002020310. [DOI] [PubMed] [Google Scholar]
- 100.Ulven SM, Gundersen TE, Weedon MS, Landaas VO, Sakhi AK, Fromm SH, Geronimo BA, Moskaug JO, Blomhoff R. Dev. Biol. 2000;220:379. doi: 10.1006/dbio.2000.9634. [DOI] [PubMed] [Google Scholar]
- 101.Werner EA, DeLuca HF. Arch. Biochem. Biophys. 2001;393:262. doi: 10.1006/abbi.2001.2495. [DOI] [PubMed] [Google Scholar]
- 102.Urbach J, Rando RR. FEBS Lett. 1994;351:429. doi: 10.1016/0014-5793(94)00090-5. [DOI] [PubMed] [Google Scholar]
- 103.Urbach J, Rando RR. Biochem. J. 1994;299(Pt 2):459. doi: 10.1042/bj2990459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Science. 2000;290:2140. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 105.Neuringer M, Anderson GJ, Connor WE. Annu. Rev. Nutr. 1988;8:517. doi: 10.1146/annurev.nu.08.070188.002505. [DOI] [PubMed] [Google Scholar]
- 106.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Mol. Endocrinol. 2007;21:77. doi: 10.1210/me.2006-0225. [DOI] [PubMed] [Google Scholar]
- 107.Kitareewan S, Burka LT, Tomer KB, Parker CE, Deterding LJ, Stevens RD, Forman BM, Mais DE, Heyman RA, McMorris T, Weinberger C. Mol. Biol. Cell. 1996;7:1153. doi: 10.1091/mbc.7.8.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oppenheimer JH. Science. 1979;203:971. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- 109.Yen PM. Physiol. Rev. 2001;81:1097. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 110.Baxter JD, Webb P. Nat. Rev. Drug Discov. 2009;8:308. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- 111.Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. Nature. 1986;324:635. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 112.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. Nature. 1986;324:641. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 113.Weinberger C, Giguere V, Hollenberg S, Rosenfeld MG, Evans RM. Cold Spring Harb. Symp. Quant. Biol. 1986;51(Pt 2):759. doi: 10.1101/sqb.1986.051.01.089. [DOI] [PubMed] [Google Scholar]
- 114.Burke LJ, Baniahmad A. FASEB J. 2000;14:1876. doi: 10.1096/fj.99-0943rev. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J, Lazar MA. Annu. Rev. Physiol. 2000;62:439. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 116.Hsu JH, Brent GA. Trends Endocrinol. Metab. 1998;9:103. doi: 10.1016/s1043-2760(98)00026-5. [DOI] [PubMed] [Google Scholar]
- 117.Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH. Endocrinology. 2000;141:3057. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- 118.Amorim BS, Ueta CB, Freitas BC, Nassif RJ, Gouveia CH, Christoffolete MA, Moriscot AS, Lancelloti CL, Llimona F, Barbeiro HV, de Souza HP, Catanozi S, Passarelli M, Aoki MS, Bianco AC, Ribeiro MO. J. Endocrinol. 2009;203:291. doi: 10.1677/JOE-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD, Fletterick RJ. Nature. 1995;378:690. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 120.Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ. Mol. Endocrinol. 2001;15:398. doi: 10.1210/mend.15.3.0608. [DOI] [PubMed] [Google Scholar]
- 121.Webb P, Nguyen NH, Chiellini G, Yoshihara HA, Cunha Lima ST, Apriletti JW, Ribeiro RC, Marimuthu A, West BL, Goede P, Mellstrom K, Nilsson S, Kushner PJ, Fletterick RJ, Scanlan TS, Baxter JD. J. Steroid Biochem. Mol. Biol. 2002;83:59. doi: 10.1016/s0960-0760(02)00270-4. [DOI] [PubMed] [Google Scholar]
- 122.Putcha BD, Wright E, Brunzelle JS, Fernandez EJ. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6084. doi: 10.1073/pnas.1119852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ito M, Roeder RG. Trends Endocrinol. Metab. 2001;12:127. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- 124.Rachez C, Freedman LP. Curr. Opin. Cell. Biol. 2001;13:274. doi: 10.1016/s0955-0674(00)00209-x. [DOI] [PubMed] [Google Scholar]
- 125.DeLuca HF. Am. J. Clin. Nutr. 2004;80:1689S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 126.Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, Selznick SH, Whitfield GK. J. Endocrinol. 1997;154(Suppl):S57. [PubMed] [Google Scholar]
- 127.Jones G, Strugnell SA, DeLuca HF. Physiol. Rev. 1998;78:1193. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y, Zhu J, DeLuca HF. Arch. Biochem. Biophys. 2012;523:123. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 129.Carlberg C, Molnar F, Mourino A. Expert Opin. Ther. Pat. 2012;22:417. doi: 10.1517/13543776.2012.673590. [DOI] [PubMed] [Google Scholar]
- 130.Orlov I, Rochel N, Moras D, Klaholz BP. EMBO J. 2012;31:291. doi: 10.1038/emboj.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rochel N, Ciesielski F, Godet J, Moman E, Roessle M, Peluso-Iltis C, Moulin M, Haertlein M, Callow P, Mely Y, Svergun DI, Moras D. Nat. Struct. Mol. Biol. 2011;18:564. doi: 10.1038/nsmb.2054. [DOI] [PubMed] [Google Scholar]
- 132.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. Mol. Cell. Biol. 1994;14:7025. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. Genes Dev. 1995;9:1033. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 134.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. Nature. 1996;383:728. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 135.Kalaany NY, Mangelsdorf DJ. Annu. Rev. Physiol. 2006;68:159. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 136.Reschly EJ, Ai N, Welsh WJ, Ekins S, Hagey LR, Krasowski MD. J. Steroid Biochem. Mol. Biol. 2008;110:83. doi: 10.1016/j.jsbmb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cell. 1998;93:693. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 138.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O'Connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. Cell. 2004;119:299. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 139.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. Cell. 2008;134:97. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Mol. Cell. 2010;38:576. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boergesen M, Pedersen TA, Gross B, van Heeringen SJ, Hagenbeek D, Bindesboll C, Caron S, Lalloyer F, Steffensen KR, Nebb HI, Gustafsson JA, Stunnenberg HG, Staels B, Mandrup S. Mol. Cell. Biol. 2012;32:852. doi: 10.1128/MCB.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pehkonen P, Welter-Stahl L, Diwo J, Ryynanen J, Wienecke-Baldacchino A, Heikkinen S, Treuter E, Steffensen KR, Carlberg C. BMC Genomics. 2012;13:50. doi: 10.1186/1471-2164-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. Pharmacol. Rev. 2006;58:726. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 144.Willson TM, Brown PJ, Sternbach DD, Henke BR. J. Med. Chem. 2000;43:527. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 145.Smith SA. Biochem Soc. Trans. 1997;25:1242. doi: 10.1042/bst0251242. [DOI] [PubMed] [Google Scholar]
- 146.Bocher V, Pineda-Torra I, Fruchart JC, Staels B. Ann. N. Y. Acad. Sci. 2002;967:7. doi: 10.1111/j.1749-6632.2002.tb04258.x. [DOI] [PubMed] [Google Scholar]
- 147.Lehrke M, Lazar MA. Cell. 2005;123:993. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 148.Duffy D, Rader DJ. Curr. Opin. Cardiol. 2005;20:301. doi: 10.1097/01.hco.0000168532.69342.26. [DOI] [PubMed] [Google Scholar]
- 149.Lazar MA. Biochimie. 2005;87:9. doi: 10.1016/j.biochi.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 150.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. J. Clin. Invest. 2007;117:3930. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3444. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Cell. 2003;113:159. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 153.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barish GD, Narkar VA, Evans RM. J. Clin. Invest. 2006;116:590. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Science. 1999;284:1365. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 156.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Science. 1999;284:1362. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 157.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Cell. 1995;81:687. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 158.Mi LZ, Devarakonda S, Harp JM, Han Q, Pellicciari R, Willson TM, Khorasanizadeh S, Rastinejad F. Mol. Cell. 2003;11:1093. doi: 10.1016/s1097-2765(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 159.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Cell. 2000;102:731. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 160.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. Genes Dev. 1998;12:3195. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. J. Clin. Invest. 1998;102:1016. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. Cell. 1998;92:73. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 163.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3369. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3375. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jonker JW, Liddle C, Downes M. J. Steroid Biochem. Mol. Biol. 2012;130:147. doi: 10.1016/j.jsbmb.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. Science. 2001;292:2329. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- 167.Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. J. Biol. Chem. 2000;275:15122. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 168.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Mol. Pharmacol. 2002;62:638. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 169.Suino K, Peng L, Reynolds R, Li Y, Cha JY, Repa JJ, Kliewer SA, Xu HE. Mol. Cell. 2004;16:893. doi: 10.1016/j.molcel.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 170.Vincent J, Shan L, Fan M, Brunzelle JS, Forman BM, Fernandez EJ. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2005;61:156. doi: 10.1107/S1744309104032762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shan L, Vincent J, Brunzelle JS, Dussault I, Lin M, Ianculescu I, Sherman MA, Forman BM, Fernandez EJ. Mol. Cell. 2004;16:907. doi: 10.1016/j.molcel.2004.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu RX, Lambert MH, Wisely BB, Warren EN, Weinert EE, Waitt GM, Williams JD, Collins JL, Moore LB, Willson TM, Moore JT. Mol. Cell. 2004;16:919. doi: 10.1016/j.molcel.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 173.Germain P, Iyer J, Zechel C, Gronemeyer H. Nature. 2002;415:187. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- 174.Chambon P. Mol. Endocrinol. 2005;19:1418. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- 175.Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr, Heyman RA. Nature. 1997;386:407. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 176.Glass CK, Rosenfeld MG. Genes Dev. 2000;14:121. [PubMed] [Google Scholar]
- 177.Lefebvre P, Martin PJ, Flajollet S, Dedieu S, Billaut X, Lefebvre B. Vitam. Horm. 2005;70:199. doi: 10.1016/S0083-6729(05)70007-8. [DOI] [PubMed] [Google Scholar]
- 178.Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Nat. Rev. Genet. 2010;11:109. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- 179.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. Cell. 2004;116:511. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 180.Chen JD, Evans RM. Nature. 1995;377:454. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 181.Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, Rosenfeld MG. Nature. 1995;377:397. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 182.Watson PJ, Fairall L, Schwabe JW. Mol. Cell. Endocrinol. 2012;348:440. doi: 10.1016/j.mce.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Germain P, Gaudon C, Pogenberg V, Sanglier S, Van Dorsselaer A, Royer CA, Lazar MA, Bourguet W, Gronemeyer H. Chem. Biol. 2009;16:479. doi: 10.1016/j.chembiol.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 184.Chambon P. FASEB J. 1996;10:940. [PubMed] [Google Scholar]
- 185.Rosenfeld MG, Lunyak VV, Glass CK. Genes Dev. 2006;20:1405. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 186.Xu J, Li Q. Mol. Endocrinol. 2003;17:1681. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- 187.York B, O'Malley BW. J. Biol. Chem. 2010;285:38743. doi: 10.1074/jbc.R110.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.L'Horset F, Dauvois S, Heery DM, Cavailles V, Parker MG. Mol. Cell Biol. 1996;16:6029. doi: 10.1128/mcb.16.11.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cavailles V, Dauvois S, L'Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG. EMBO J. 1995;14:3741. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. Cell. 2005;122:835. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 191.Nagy L, Schwabe JW. Trends Biochem. Sci. 2004;29:317. doi: 10.1016/j.tibs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 192.de Lera AR, Bourguet W, Altucci L, Gronemeyer H. Nat. Rev. Drug Discov. 2007;6:811. doi: 10.1038/nrd2398. [DOI] [PubMed] [Google Scholar]
- 193.Nahoum V, Perez E, Germain P, Rodriguez-Barrios F, Manzo F, Kammerer S, Lemaire G, Hirsch O, Royer CA, Gronemeyer H, de Lera AR, Bourguet W. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17323. doi: 10.1073/pnas.0705356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Nature. 1997;389:753. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 195.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. Cell. 1998;95:927. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 196.Williams S, Bledsoe RK, Collins JL, Boggs S, Lambert MH, Miller AB, Moore J, McKee DD, Moore L, Nichols J, Parks D, Watson M, Wisely B, Willson TM. J. Biol. Chem. 2003;278:27138. doi: 10.1074/jbc.M302260200. [DOI] [PubMed] [Google Scholar]
- 197.Xu HE, Stanley TB, Montana VG, Lambert MH, Shearer BG, Cobb JE, McKee DD, Galardi CM, Plunket KD, Nolte RT, Parks DJ, Moore JT, Kliewer SA, Willson TM, Stimmel JB. Nature. 2002;415:813. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 198.Perlmann T, Rangarajan PN, Umesono K, Evans RM. Genes Dev. 1993;7:1411. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- 199.Mader S, Leroy P, Chen JY, Chambon P. J. Biol. Chem. 1993;268:591. [PubMed] [Google Scholar]
- 200.Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, Sigler PB. Nature. 1991;352:497. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- 201.Schwabe JW, Chapman L, Finch JT, Rhodes D. Cell. 1993;75:567. doi: 10.1016/0092-8674(93)90390-c. [DOI] [PubMed] [Google Scholar]
- 202.Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4758. doi: 10.1073/pnas.0401123101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Roemer SC, Donham DC, Sherman L, Pon VH, Edwards DP, Churchill ME. Mol. Endocrinol. 2006;20:3042. doi: 10.1210/me.2005-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wilson TE, Fahrner TJ, Milbrandt J. Mol. Cell. Biol. 1993;13:5794. doi: 10.1128/mcb.13.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Harding HP, Lazar MA. Mol. Cell. Biol. 1993;13:3113. doi: 10.1128/mcb.13.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Harding HP, Lazar MA. Mol. Cell. Biol. 1995;15:4791. doi: 10.1128/mcb.15.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Giguere V, McBroom LD, Flock G. Mol. Cell Biol. 1995;15:2517. doi: 10.1128/mcb.15.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Charles JP, Shinoda T, Chinzei Y. Eu.r J. Biochem. 1999;266:181. doi: 10.1046/j.1432-1327.1999.00842.x. [DOI] [PubMed] [Google Scholar]
- 209.Zhao Q, Khorasanizadeh S, Miyoshi Y, Lazar MA, Rastinejad F. Mol. Cell. 1998;1:849. doi: 10.1016/s1097-2765(00)80084-2. [DOI] [PubMed] [Google Scholar]
- 210.Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld MG, Glass CK. Nature. 1995;377:451. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 211.Balmer JE, Blomhoff R. J. Lipid Res. 2002;43:1773. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 212.Hamza MS, Pott S, Vega VB, Thomsen JS, Kandhadayar GS, Ng PW, Chiu KP, Pettersson S, Wei CL, Ruan Y, Liu ET. PLoS One. 2009;4:e4907. doi: 10.1371/journal.pone.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nielsen R, Pedersen TA, Hagenbeek D, Moulos P, Siersbaek R, Megens E, Denissov S, Borgesen M, Francoijs KJ, Mandrup S, Stunnenberg HG. Genes Dev. 2008;22:2953. doi: 10.1101/gad.501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Meyer MB, Goetsch PD, Pike JW. J. Steroid Biochem. Mol. Biol. 2010;121:136. doi: 10.1016/j.jsbmb.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ, Jr, Liu XS, Lazar MA. Genes Dev. 2008;22:2941. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moutier E, Ye T, Choukrallah MA, Urban S, Osz J, Chatagnon A, Delacroix L, Langer D, Rochel N, Moras D, Benoit G, Davidson I. J. Biol. Chem. 2012;287:26328. doi: 10.1074/jbc.M112.361790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ratman D, Vanden Berghe W, Dejager L, Libert C, Tavernier J, Beck IM, De Bosscher K. Mol. Cell. Endocrinol. 2013;380:41. doi: 10.1016/j.mce.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 218.Zechel C, Shen XQ, Chen JY, Chen ZP, Chambon P, Gronemeyer H. EMBO J. 1994;13:1425. doi: 10.1002/j.1460-2075.1994.tb06396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zechel C, Shen XQ, Chambon P, Gronemeyer H. EMBO J. 1994;13:1414. doi: 10.1002/j.1460-2075.1994.tb06395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Khorasanizadeh S, Rastinejad F. Curr. Biol. 1999;9:R456. doi: 10.1016/s0960-9822(99)80281-4. [DOI] [PubMed] [Google Scholar]
- 221.Sierk ML, Zhao Q, Rastinejad F. Biochemistry. 2001;40:12833. doi: 10.1021/bi011086r. [DOI] [PubMed] [Google Scholar]
- 222.Gampe RT, Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. Mol. Cell. 2000;5:545. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 223.Svensson S, Ostberg T, Jacobsson M, Norstrom C, Stefansson K, Hallen D, Johansson IC, Zachrisson K, Ogg D, Jendeberg L. EMBO J. 2003;22:4625. doi: 10.1093/emboj/cdg456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chandra V, Huang P, Potluri N, Wu D, Kim Y, Rastinejad F. Nature. 2013;495:394. doi: 10.1038/nature11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kersten S, Kelleher D, Chambon P, Gronemeyer H, Noy N. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8645. doi: 10.1073/pnas.92.19.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kersten S, Pan L, Noy N. Biochemistry. 1995;34:14263. doi: 10.1021/bi00043a034. [DOI] [PubMed] [Google Scholar]
- 227.Kersten S, Pan L, Chambon P, Gronemeyer H, Noy N. Biochemistry. 1995;34:13717. doi: 10.1021/bi00042a001. [DOI] [PubMed] [Google Scholar]
- 228.Kersten S, Reczek PR, Noy N. J. Biol. Chem. 1997;272:29759. doi: 10.1074/jbc.272.47.29759. [DOI] [PubMed] [Google Scholar]
- 229.Kersten S, Dong D, Lee W, Reczek PR, Noy N. J. Mol. Biol. 1998;284:21. doi: 10.1006/jmbi.1998.2168. [DOI] [PubMed] [Google Scholar]
- 230.Dong D, Noy N. Biochemistry. 1998;37:10691. doi: 10.1021/bi980561r. [DOI] [PubMed] [Google Scholar]
- 231.Mangelsdorf DJ, Umesono K, Kliewer SA, Borgmeyer U, Ong ES, Evans RM. Cell. 1991;66:555. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- 232.Chen H, Privalsky ML. Proc. Natl. Acad. Sci. U. S. A. 1995;92:422. doi: 10.1073/pnas.92.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gampe RT, Jr, Montana VG, Lambert MH, Wisely GB, Milburn MV, Xu HE. Genes Dev. 2000;14:2229. doi: 10.1101/gad.802300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang H, Chen L, Chen J, Jiang H, Shen X. J. Biol. Chem. 2011;286:24593. doi: 10.1074/jbc.M111.245498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Peet DJ, Doyle DF, Corey DR, Mangelsdorf DJ. Chem. Biol. 1998;5:13. doi: 10.1016/s1074-5521(98)90083-7. [DOI] [PubMed] [Google Scholar]
- 236.Perez E, Bourguet W, Gronemeyer H, de Lera AR. Biochim. Biophys. Acta. 2012;1821:57. doi: 10.1016/j.bbalip.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 237.Germain P, Kammerer S, Perez E, Peluso-Iltis C, Tortolani D, Zusi FC, Starrett J, Lapointe P, Daris JP, Marinier A, de Lera AR, Rochel N, Gronemeyer H. EMBO Rep. 2004;5:877. doi: 10.1038/sj.embor.7400235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.le Maire A, Teyssier C, Erb C, Grimaldi M, Alvarez S, de Lera AR, Balaguer P, Gronemeyer H, Royer CA, Germain P, Bourguet W. Nat. Struct. Mol. Biol. 2010;17:801. doi: 10.1038/nsmb.1855. [DOI] [PubMed] [Google Scholar]
- 239.Newcomer ME. Structure. 1993;1:7. doi: 10.1016/0969-2126(93)90004-z. [DOI] [PubMed] [Google Scholar]
- 240.Newcomer ME, Pappas RS, Ong DE. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9223. doi: 10.1073/pnas.90.19.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kleywegt GJ, Bergfors T, Senn H, Le Motte P, Gsell B, Shudo K, Jones TA. Structure. 1994;2:1241. doi: 10.1016/s0969-2126(94)00125-1. [DOI] [PubMed] [Google Scholar]
- 242.Yamakawa T, Kagechika H, Kawachi E, Hashimoto Y, Shudo K. J. Med. Chem. 1990;33:1430. doi: 10.1021/jm00167a024. [DOI] [PubMed] [Google Scholar]
- 243.Teng M, Duong TT, Klein ES, Pino ME, Chandraratna RA. J. Med. Chem. 1996;39:3035. doi: 10.1021/jm9603532. [DOI] [PubMed] [Google Scholar]
- 244.Dragnev KH, Rigas JR, Dmitrovsky E. Oncologist. 2000;5:361. doi: 10.1634/theoncologist.5-5-361. [DOI] [PubMed] [Google Scholar]
- 245.Reynolds CP, Lemons RS. Hematol. Oncol. Clin. North Am. 2001;15:867. doi: 10.1016/s0889-8588(05)70256-2. [DOI] [PubMed] [Google Scholar]
- 246.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wang ZY. Blood. 1988;72:567. [PubMed] [Google Scholar]
- 247.Warrell RP, Jr, Frankel SR, Miller WH, Jr, Scheinberg DA, Itri LM, Hittelman WN, Vyas R, Andreeff M, Tafuri A, Jakubowski A, Gabrilove J, Gordon MS, Dmitrovsky E. N. Engl. J. Med. 1991;324:1385. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- 248.Tallman MS, Altman JK. Hematology Am. Soc. Hematol. Educ. Program. 2008;1:391. doi: 10.1182/asheducation-2008.1.391. [DOI] [PubMed] [Google Scholar]
- 249.Niles RM. Nutrition. 2000;16:1084. doi: 10.1016/s0899-9007(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 250.Moon RC, Steele VE, Kelloff GJ, Thomas CF, Detrisac CJ, Mehta RG, Lubet RA. Anticancer Res. 1994;14:889. [PubMed] [Google Scholar]
- 251.Lippman SM, Parkinson DR, Itri LM, Weber RS, Schantz SP, Ota DM, Schusterman MA, Krakoff IH, Gutterman JU, Hong WK. J Natl. Cancer Inst. 1992;84:235. doi: 10.1093/jnci/84.4.235. [DOI] [PubMed] [Google Scholar]
- 252.Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, Schantz SP, Kramer AM, Lotan R, Peters LJ, Dimery IW, Brown WB, Goepfert H. N Engl. J. Med. 1990;323:795. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 253.Delacroix L, Moutier E, Altobelli G, Legras S, Poch O, Choukrallah MA, Bertin I, Jost B, Davidson I. Mol. Cell Biol. 2010;30:231. doi: 10.1128/MCB.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, Fofonoff S, Byers R, Atkinson EN, Vaughan C, Toth BB, Kramer A, Dimery IW, Skipper P, Strong S. N. Engl. J. Med. 1986;315:1501. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 255.Pastorino U, Infante M, Maioli M, Chiesa G, Buyse M, Firket P, Rosmentz N, Clerici M, Soresi E, Valente M, Belloni PA, Gianni R. J. Clin. Oncol. 1993;11:1216. doi: 10.1200/JCO.1993.11.7.1216. [DOI] [PubMed] [Google Scholar]
- 256.Takai K, Okuno M, Yasuda I, Matsushima-Nishiwaki R, Uematsu T, Tsurumi H, Shiratori Y, Muto Y, Moriwaki H. Intervirology. 2005;48:39. doi: 10.1159/000082093. [DOI] [PubMed] [Google Scholar]
- 257.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Cancer Lett. 2003;197:185. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 258.Reynolds CP. Curr. Oncol. Rep. 2000;2:511. doi: 10.1007/s11912-000-0104-y. [DOI] [PubMed] [Google Scholar]
- 259.Meyskens FL, Jr, Surwit E, Moon TE, Childers JM, Davis JR, Dorr RT, Johnson CS, Alberts DS. J. Natl. Cancer Inst. 1994;86:539. doi: 10.1093/jnci/86.7.539. [DOI] [PubMed] [Google Scholar]
- 260.Ng KW, Livesey SA, Collier F, Gummer PR, Martin TJ. Cancer Res. 1985;45:5106. [PubMed] [Google Scholar]
- 261.Thein R, Lotan R. Cancer Res. 1982;42:4771. [PubMed] [Google Scholar]
- 262.Malik SM, Collins B, Pishvaian M, Ramzi P, Marshall J, Hwang J. Clin. Lung Cancer. 2011;12:231. doi: 10.1016/j.cllc.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 263.Okusaka T, Ueno H, Ikeda M, Takezako Y, Morizane C. Cancer Sci. 2012;103:1524. doi: 10.1111/j.1349-7006.2012.02334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dominguez J, Hojyo MT, Celayo JL, Dominguez-Soto L, Teixeira F. Int. J. Dermatol. 1998;37:54. doi: 10.1046/j.1365-4362.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 265.Krautheim A, Gollnick H. Clin. Pharmacokinet. 2003;42:1287. doi: 10.2165/00003088-200342140-00005. [DOI] [PubMed] [Google Scholar]
- 266.Thielitz A, Abdel-Naser MB, Fluhr JW, Zouboulis CC, Gollnick H. J. Dtsch. Dermatol. Ges. 2008;6:1023. doi: 10.1111/j.1610-0387.2008.06741.x. [DOI] [PubMed] [Google Scholar]
- 267.Thielitz A, Gollnick H. Am. J. Clin. Dermatol. 2008;9:369. doi: 10.2165/0128071-200809060-00003. [DOI] [PubMed] [Google Scholar]
- 268.Gustafson CJ, Watkins C, Hix E, Feldman SR. Am. J. Clin. Dermatol. 2013;14:9. doi: 10.1007/s40257-012-0003-7. [DOI] [PubMed] [Google Scholar]
- 269.Lebwohl M. Cutis. 1998;61:27. [PubMed] [Google Scholar]
- 270.Dawson MI, Xia Z. Biochim. Biophys. Acta. 2012;1821:21. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tanaka T, De Luca LM. Cancer Res. 2009;69:4945. doi: 10.1158/0008-5472.CAN-08-4407. [DOI] [PubMed] [Google Scholar]
- 272.Kagechika H, Shudo K. J. Med. Chem. 2005;48:5875. doi: 10.1021/jm0581821. [DOI] [PubMed] [Google Scholar]
- 273.Vu-Dac N, Gervois P, Torra IP, Fruchart JC, Kosykh V, Kooistra T, Princen HM, Dallongeville J, Staels B. J. Clin. Invest . 1998;102:625. doi: 10.1172/JCI1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gervois P, Fruchart JC, Staels B. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:145. doi: 10.1038/ncpendmet0397. [DOI] [PubMed] [Google Scholar]
- 275.Cummins CL, Volle DH, Zhang Y, McDonald JG, Sion B, Lefrancois-Martinez AM, Caira F, Veyssiere G, Mangelsdorf DJ, Lobaccaro JM. J. Clin. Invest. 2006;116:1902. doi: 10.1172/JCI28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Haraguchi G, Suzuki J, Kosuge H, Ogawa M, Koga N, Muto S, Itai A, Kagechika H, Shudo K, Isobe M. J. Mol. Cell. Cardiol. 2006;41:885. doi: 10.1016/j.yjmcc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 277.Nishimaki-Mogami T, Tamehiro N, Sato Y, Okuhira K, Sai K, Kagechika H, Shudo K, Abe-Dohmae S, Yokoyama S, Ohno Y, Inoue K, Sawada J. Biochem. Pharmacol. 2008;76:1006. doi: 10.1016/j.bcp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 278.Ishida S, Shigemoto-Mogami Y, Kagechika H, Shudo K, Ozawa S, Sawada J, Ohno Y, Inoue K. Mol. Cancer Ther. 2003;2:49. [PubMed] [Google Scholar]
- 279.Leibowitz MD, Ardecky RJ, Boehm MF, Broderick CL, Carfagna MA, Crombie DL, D'Arrigo J, Etgen GJ, Faul MM, Grese TA, Havel H, Hein NI, Heyman RA, Jolley D, Klausing K, Liu S, Mais DE, Mapes CM, Marschke KB, Michellys PY, Montrose-Rafizadeh C, Ogilvie KM, Pascual B, Rungta D, Tyhonas JS, Urcan MS, Wardlow M, Yumibe N, Reifel-Miller A. Endocrinology. 2006;147:1044. doi: 10.1210/en.2005-0690. [DOI] [PubMed] [Google Scholar]
- 280.Michellys PY, Ardecky RJ, Chen JH, Crombie DL, Etgen GJ, Faul MM, Faulkner AL, Grese TA, Heyman RA, Karanewsky DS, Klausing K, Leibowitz MD, Liu S, Mais DA, Mapes CM, Marschke KB, Reifel-Miller A, Ogilvie KM, Rungta D, Thompson AW, Tyhonas JS, Boehm MF. J. Med. Chem. 2003;46:2683. doi: 10.1021/jm020340q. [DOI] [PubMed] [Google Scholar]
- 281.Yamauchi T, Waki H, Kamon J, Murakami K, Motojima K, Komeda K, Miki H, Kubota N, Terauchi Y, Tsuchida A, Tsuboyama-Kasaoka N, Yamauchi N, Ide T, Hori W, Kato S, Fukayama M, Akanuma Y, Ezaki O, Itai A, Nagai R, Kimura S, Tobe K, Kagechika H, Shudo K, Kadowaki T. J. Clin. Invest. 2001;108:1001. doi: 10.1172/JCI12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jetten AM, Joo JH. Adv. Dev. Biol. 2006;16:313. doi: 10.1016/S1574-3349(06)16010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bois-Joyeux B, Chauvet C, Nacer-Cherif H, Bergeret W, Mazure N, Giguere V, Laudet V, Danan JL. DNA Cell. Biol. 2000;19:589. doi: 10.1089/104454900750019344. [DOI] [PubMed] [Google Scholar]
- 284.Raspe E, Mautino G, Duval C, Fontaine C, Duez H, Barbier O, Monte D, Fruchart J, Fruchart JC, Staels B. J. Biol. Chem. 2002;277:49275. doi: 10.1074/jbc.M206215200. [DOI] [PubMed] [Google Scholar]
- 285.Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C. J. Biol. Chem. 1994;269:28531. [PubMed] [Google Scholar]
- 286.Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, Evans RM. Mol. Endocrinol. 1994;8:1253. doi: 10.1210/mend.8.9.7838158. [DOI] [PubMed] [Google Scholar]
- 287.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. Immunity. 2008;28:29. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Huh JR, Littman DR. Eur. J. Immunol. 2012;42:2232. doi: 10.1002/eji.201242740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR. Nature. 2011;472:486. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, Schurer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. Nature. 2011;472:491. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Cell. 2006;126:801. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 292.Kang HS, Angers M, Beak JY, Wu X, Gimble JM, Wada T, Xie W, Collins JB, Grissom SF, Jetten AM. Physiol. Genomics. 2007;31:281. doi: 10.1152/physiolgenomics.00098.2007. [DOI] [PubMed] [Google Scholar]
- 293.Dussault I, Fawcett D, Matthyssen A, Bader JA, Giguere V. Mech. Dev. 1998;70:147. doi: 10.1016/s0925-4773(97)00187-1. [DOI] [PubMed] [Google Scholar]
- 294.Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crepel F, Mariani J, Sotelo C, Becker-Andre M. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3960. doi: 10.1073/pnas.95.7.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, Kruglyak L, Lander ES. Nature. 1996;379:736. doi: 10.1038/379736a0. [DOI] [PubMed] [Google Scholar]
- 296.Raspe E, Duez H, Gervois P, Fievet C, Fruchart JC, Besnard S, Mariani J, Tedgui A, Staels B. J. Biol. Chem. 2001;276:2865. doi: 10.1074/jbc.M004982200. [DOI] [PubMed] [Google Scholar]
- 297.Schaeren-Wiemers N, Andre E, Kapfhammer JP, Becker-Andre M. Eur. J. Neurosci. 1997;9:2687. doi: 10.1111/j.1460-9568.1997.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 298.Andre E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-Andre M. EMBO J. 1998;17:3867. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. Structure. 2002;10:1697. doi: 10.1016/s0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- 300.Stehlin C, Wurtz JM, Steinmetz A, Greiner E, Schule R, Moras D, Renaud JP. EMBO J. 2001;20:5822. doi: 10.1093/emboj/20.21.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. J. Biol. Chem. 2004;279:14033. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- 302.Boukhtouche F, Mariani J, Tedgui A. Arterioscler Thromb. Vasc. Biol. 2004;24:637. doi: 10.1161/01.ATV.0000119355.56036.de. [DOI] [PubMed] [Google Scholar]
- 303.Wada T, Kang HS, Jetten AM, Xie W. Exp. Biol. Med. (Maywood) 2008;233:1191. doi: 10.3181/0802-MR-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y. Mol. Endocrinol. 2010;24:923. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hirose T, Smith RJ, Jetten AM. Biochem. Biophy.s Res. Commun. 1994;205:1976. doi: 10.1006/bbrc.1994.2902. [DOI] [PubMed] [Google Scholar]
- 306.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Science. 2000;288:2369. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 307.Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, Moras D, Schule R. Nat. Struct. Biol. 2003;10:820. doi: 10.1038/nsb979. [DOI] [PubMed] [Google Scholar]
- 308.Jetten AM. Nucl. Recept. Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Berry DC, Noy N. PPAR Res. 2007;2007:73256. doi: 10.1155/2007/73256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Berry DC, Noy N. Mol. Cell. Biol. 2009;29:3286. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, Xu Y, Wang L, Tsai SY, Tsai MJ, Xu HE. PLoS Biol. 2008;6:e227. doi: 10.1371/journal.pbio.0060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhou XE, Suino-Powell KM, Xu Y, Chan CW, Tanabe O, Kruse SW, Reynolds R, Engel JD, Xu HE. J. Biol. Chem. 2011;286:2877. doi: 10.1074/jbc.M110.168740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Calleja C, Messaddeq N, Chapellier B, Yang H, Krezel W, Li M, Metzger D, Mascrez B, Ohta K, Kagechika H, Endo Y, Mark M, Ghyselinck NB, Chambon P. Genes Dev. 2006;20:1525. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Weston AD, Blumberg B, Underhill TM. J Cell Biol. 2003;161:223. doi: 10.1083/jcb.200211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Weston AD, Chandraratna RA, Torchia J, Underhill TM. J. Cell. Biol. 2002;158:39. doi: 10.1083/jcb.200112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Underhill TM, Sampaio AV, Weston AD. Novartis Found Symp. 2001;232:171. doi: 10.1002/0470846658.ch12. [DOI] [PubMed] [Google Scholar]
- 317.Haffner CD, Lenhard JM, Miller AB, McDougald DL, Dwornik K, Ittoop OR, Gampe RT, Jr, Xu HE, Blanchard S, Montana VG, Consler TG, Bledsoe RK, Ayscue A, Croom D. J. Med. Chem. 2004;47:2010. doi: 10.1021/jm030565g. [DOI] [PubMed] [Google Scholar]