Abstract
Recognition of peptides bound to class I major histocompatibility complex (MHC) molecules by specific receptors on T cells regulates the development and activity of the cellular immune system. We have designed and synthesized de novo cyclic peptides that incorporate PEG in the ring structure for binding to class I MHC molecules. The large PEG loops are positioned to extend out of the peptide binding site, thus creating steric effects aimed at preventing the recognition of class I MHC complexes by T-cell receptors. Peptides were synthesized and cyclized on polymer support using high molecular weight symmetrical PEG dicarboxylic acids to link the side chains of lysine residues substituted at positions 4 and 8 in the sequence of the HLA-A2-restricted human T-lymphotrophic virus type I Tax peptide. Cyclic peptides promoted the in vitro folding and assembly of HLA-A2 complexes. Thermal denaturation studies using circular dichroism spectroscopy showed that these complexes are as stable as complexes formed with antigenic peptides.
Full text
PDF
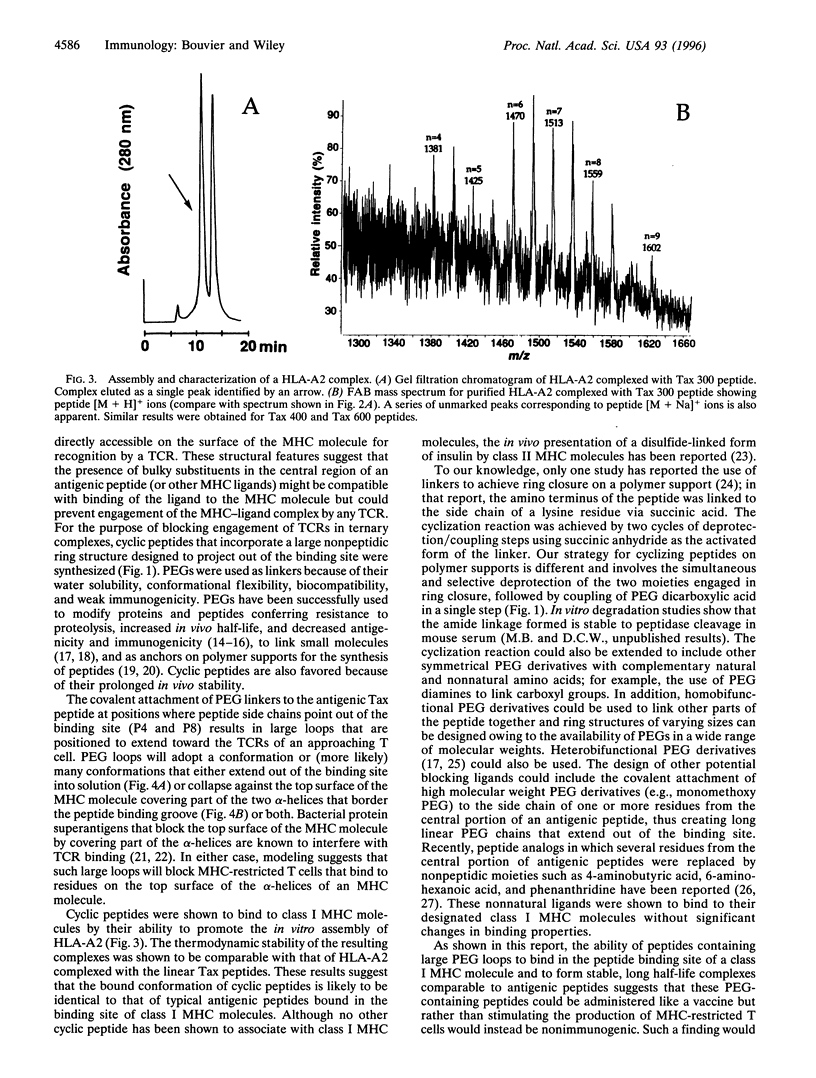

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., McCoy J. R., Palczuk N. C., van Es T., Davis F. F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977 Jun 10;252(11):3582–3586. [PubMed] [Google Scholar]
- Beauchamp C. O., Gonias S. L., Menapace D. P., Pizzo S. V. A new procedure for the synthesis of polyethylene glycol-protein adducts; effects on function, receptor recognition, and clearance of superoxide dismutase, lactoferrin, and alpha 2-macroglobulin. Anal Biochem. 1983 May;131(1):25–33. doi: 10.1016/0003-2697(83)90131-8. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Bouvier M., Wiley D. C. Importance of peptide amino and carboxyl termini to the stability of MHC class I molecules. Science. 1994 Jul 15;265(5170):398–402. doi: 10.1126/science.8023162. [DOI] [PubMed] [Google Scholar]
- Fahnestock M. L., Tamir I., Narhi L., Bjorkman P. J. Thermal stability comparison of purified empty and peptide-filled forms of a class I MHC molecule. Science. 1992 Dec 4;258(5088):1658–1662. doi: 10.1126/science.1360705. [DOI] [PubMed] [Google Scholar]
- Forquet F., Hadzija M., Semple J. W., Speck E., Delovitch T. L. Naturally processed heterodimeric disulfide-linked insulin peptides bind to major histocompatibility class II molecules on thymic epithelial cells. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3936–3940. doi: 10.1073/pnas.91.9.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garboczi D. N., Hung D. T., Wiley D. C. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisin B. F. The monitoring of reactions in solid-phase peptide synthesis with picric acid. Anal Chim Acta. 1972 Jan;58(1):248–249. doi: 10.1016/S0003-2670(00)86882-8. [DOI] [PubMed] [Google Scholar]
- Glick G. D., Toogood P. L., Wiley D. C., Skehel J. J., Knowles J. R. Ligand recognition by influenza virus. The binding of bivalent sialosides. J Biol Chem. 1991 Dec 15;266(35):23660–23669. [PubMed] [Google Scholar]
- Jardetzky T. S., Brown J. H., Gorga J. C., Stern L. J., Urban R. G., Chi Y. I., Stauffacher C., Strominger J. L., Wiley D. C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994 Apr 21;368(6473):711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Kim J., Urban R. G., Strominger J. L., Wiley D. C. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science. 1994 Dec 16;266(5192):1870–1874. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- Lu Y. A., Felix A. M. Pegylated peptides. II. Solid-phase synthesis of amino-, carboxy- and side-chain pegylated peptides. Int J Pept Protein Res. 1994 Feb;43(2):127–138. [PubMed] [Google Scholar]
- Madden D. R., Garboczi D. N., Wiley D. C. The antigenic identity of peptide-MHC complexes: a comparison of the conformations of five viral peptides presented by HLA-A2. Cell. 1993 Nov 19;75(4):693–708. doi: 10.1016/0092-8674(93)90490-h. [DOI] [PubMed] [Google Scholar]
- Madden D. R. The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- Plaué S. Synthesis of cyclic peptides on solid support. Application to analogs of hemagglutinin of influenza virus. Int J Pept Protein Res. 1990 Jun;35(6):510–517. doi: 10.1111/j.1399-3011.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Rognan D., Scapozza L., Folkers G., Daser A. Rational design of nonnatural peptides as high-affinity ligands for the HLA-B*2705 human leukocyte antigen. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):753–757. doi: 10.1073/pnas.92.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. Antigenic peptide binding by class I and class II histocompatibility proteins. Structure. 1994 Apr 15;2(4):245–251. doi: 10.1016/s0969-2126(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Utz U., Koenig S., Coligan J. E., Biddison W. E. Presentation of three different viral peptides, HTLV-1 Tax, HCMV gB, and influenza virus M1, is determined by common structural features of the HLA-A2.1 molecule. J Immunol. 1992 Jul 1;149(1):214–221. [PubMed] [Google Scholar]
- Weiss G. A., Collins E. J., Garboczi D. N., Wiley D. C., Schreiber S. L. A tricyclic ring system replaces the variable regions of peptides presented by three alleles of human MHC class I molecules. Chem Biol. 1995 Jun;2(6):401–407. doi: 10.1016/1074-5521(95)90221-x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Walter R., Tsuru D. Proline-specific endopeptidase from Flavobacterium. Purification and properties. J Biol Chem. 1980 May 25;255(10):4786–4792. [PubMed] [Google Scholar]
- Zalipsky S. Functionalized poly(ethylene glycol) for preparation of biologically relevant conjugates. Bioconjug Chem. 1995 Mar-Apr;6(2):150–165. doi: 10.1021/bc00032a002. [DOI] [PubMed] [Google Scholar]