Abstract
Dyslexia is a severe and persistent reading and spelling disorder caused by impairment to manipulate speech sounds. Here, we combine functional magnetic resonance brain imaging with multi-voxel pattern analysis and functional and structural connectivity analysis to disentangle whether dyslexics’ phonological deficits are caused by poor quality of the phonetic representations or by difficulties in accessing intact phonetic representations. We show that phonetic representations are hosted bilaterally in primary and secondary auditory cortices, and that their neural quality is intact in adults with dyslexia. However, the functional and structural connectivity between bilateral auditory cortices and left inferior frontal gyrus (a region involved in higher-level phonological processing) is significantly hampered in dyslexics, suggesting deficient access to otherwise intact phonetic representations.
Speech perception involves the mapping of spectrally complex and rapidly changing acoustic signals onto discrete and abstract phonetic sound categories or phonemes (1). Developmental dyslexia is a hereditary neurological disorder characterized by severe and persistent reading and/or spelling impairments (2). Individuals with dyslexia perform poorly on tasks that require phonological awareness, verbal short-term memory and lexical access. Performance on these phonological tasks predicts reading acquisition in both normal and dyslexic readers (3). One view is that success on these tasks reflects the quality of underlying phonological (phonetic) representations (4) and that these representations of speech sounds are distorted or less well specified in individuals with dyslexia (5). An alternative view holds that representations are intact, but access to the representations is problematic in people with dyslexia (6, 7).
Here, we combine functional magnetic resonance imaging (fMRI) with multi-voxel pattern analysis (MVPA) (8-10) and functional and structural connectivity analysis to disentangle whether dyslexia is caused by poor quality of the phonetic representation or by difficulties in accessing an intact phonetic representation.
We collected whole-brain functional images in 23 adults with a diagnosis of dyslexia and 22 matched normal readers (Table S1, 11-13), while they listened to different versions of four sublexical speech sounds (Fig. S1) and performed an easy phoneme discrimination task. The selection of stimuli allowed us to investigate both vowel and stop consonant discrimination, which relies on spectral versus spectrotemporal acoustic feature processing, respectively. If dyslexia is related to a deficit in the quality of phonetic representations, then we expect that the neural representations would be less robust and distinct in individuals with dyslexia than in normal readers. Given dyslexics’ particular problems processing temporal cues, such as those involved in consonant discrimination (11), we expected the most prominent group differences for neural patterns distinguishing between consonants.
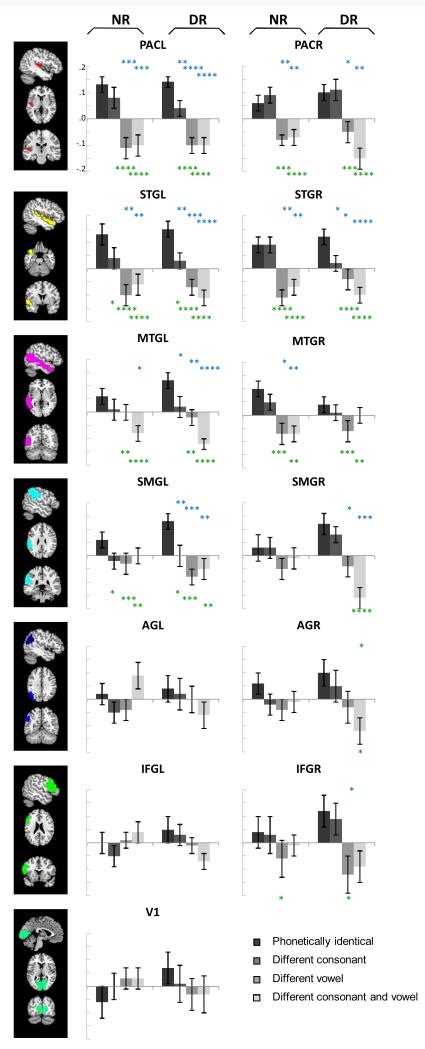
We analyzed the pattern of multi-voxel activity (MVPA analysis) within six left and six right hemisphere regions involved in speech processing and within one non-speech control region (primary visual cortex V1) (Table S2) (8, 14). For each of these regions, we correlated the activity pattern in response to each stimulus in one half of the data with the activity pattern in response to each stimulus in the remaining data (Fig. S2). Fig. 1 displays averaged correlations as a function of the phonetic similarity of the pairs: phonetically identical, differing in consonant, differing in vowel, and differing in both consonant and vowel. Comparison of these correlations indicates to what extent various acoustic realizations of the same phoneme elicit a similar activation pattern while different phonemes elicit a distinct activation pattern. We found significant differences between the four phonetic comparisons in bilateral primary auditory cortex (PAC), superior temporal gyrus (STG), middle temporal gyrus (MTG), and supramarginal gyrus (SMG) (ps < .0003), and unilaterally in right angular gyrus (AG) and right inferior frontal gyrus (IFG) (ps < .03) (repeated-measures ANOVAs with group as between-subject and phonetic comparison as within-subject factor). We found no differences between phonetic comparisons for left AG and left IFG and for area V1 (F < 1). Activity patterns were equally reliable in both groups, except for right SMG where the neural representations of the dyslexic readers were significantly more distinct than those of the normal readers (group×comparison interaction: p = .024). Focusing merely on the most crucial comparison entailing temporal cues, we observed that speech sounds differing in consonant could be differentiated in left PAC, STG, MTG and SMG (ps < .02; for all other regions ps > .16). Activity patterns differentiating between consonants were equally reliable in both groups (all group×comparison interactions: p > .28), except for right STG where differentiation between consonants was feasible for dyslexic (p = .037) but not for normal readers (p = .977). Across all regions and for both reading groups, left hemisphere regions were significantly more sensitive than right hemisphere regions to differences in consonants (p = .017) (consistent with the left-hemisphere bias for temporal cues as described in the literature, 10, 15). We found no lateralization for vowel decoding in either normal or dyslexic readers (F < 1).
Fig. 1.
Quality of phonetic representations as derived from multivoxel pattern analysis. Average correlations between the (normalized) activity patterns elicited by phonetically identical syllables, syllables differing in consonant, syllables differing in vowel, and syllables differing in both consonant and vowel for dyslexic (DR) and normal readers (NR) in each of the anatomical regions (error bars represent 1 SE). The larger the overall quality of the phonetic representations, the larger the differences between the baseline correlation (between phonetically identical syllables) and the other correlations. Correlations differing from this baseline correlation are indicated with blue asterisks (paired t-tests) and green asterisks (repeated-measures ANOVA, Tukey-corrected post-hoc t-tests) (* p < .05, ** p < .01, *** p < .001, **** p < .0001). On the left: a representation of the left hemisphere anatomical regions.
To ensure that we did not overlook any brain region hosting superior representations in normal as compared to dyslexic readers, a whole-brain searchlight MVPA was performed (16). A spherical ‘searchlight’ was moved across the entire brain and for every local region we calculated how well the response patterns differentiated between speech sounds. This analysis confirmed that phonetic representations are primarily hosted bilaterally in primary and secondary auditory cortices and that both normal and dyslexic readers shared similar quality of these representations (Fig. S4).
Thus, we have no indication of poorer quality of phonetic representations in dyslexic readers. The MVPA results show that phonetic representations of dyslexic readers were at least as robust and distinct, or even slightly more, as those of normal readers. It may be that dyslexic readers achieve normal neural representations through greater than normal effort. Indeed, on the phoneme discrimination task administered during scanning, dyslexic readers achieved normal accuracy (as such we avoid accuracy confounds when comparing the two groups), but at slower speed (Table S1). Attention may modulate brain activity and alter brain activity profiles (17). To investigate phonetic representations under conditions that elicit less compensational processing, we recalculated the previous analyses using only the activity pattern in response to speech sounds that were less relevant for the task at hand (the third speech sound in a stimulus block of four). As these stimuli were processed less intentionally, they yielded less brain activity (Table S3). Nevertheless, even under this more stringent condition, speech sounds could be differentiated in left and right PAC, STG, MTG and SMG (ps < .05). And again, although dyslexic readers overall presented less activation (Table S3), the quality of the phonetic representations was equal in both groups (all group×comparison interactions: p > .25) (Fig. S5).
Thus, we found no difference in the neural quality of phonetic representations between dyslexic and normal readers. Although we cannot rule out that dyslexics’ neural representations may have been less well specified at a younger age or would follow a different temporal trajectory detectable through techniques such as EEG (18), our results indicate that the phonetic representations can be intact in adult dyslexics despite persisting reading difficulties (6, 7). Therefore, we sought to investigate the alternative hypothesis of impaired access to phonetic representations.
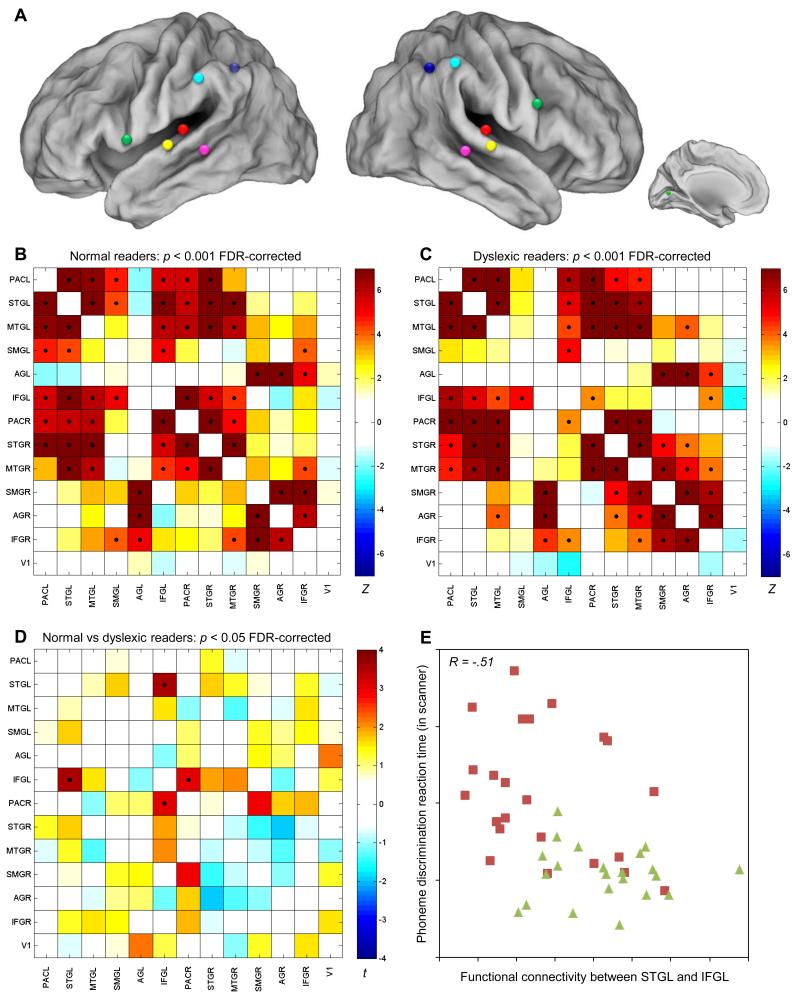
Several studies have shown that Broca’s area, particularly left IFG pars opercularis, is involved in sensory-motor integration and effortful phonological processing (19, 20). Hence, this area, which itself does not host phonetic representations, has to access the representations in primary and secondary auditory cortices to compute the required phonological manipulations. We investigated the efficiency of access or the quality of interregional brain communication by assessing intrinsic functional connectivity between each of the 13 anatomical regions (21). In each region we selected the most active cluster during task performance, and we calculated the correlations between the residualized signal-intensity time series of each pair of these 13 predefined seed regions (Fig. 2) (22). Both groups showed equally strong connectivity among bilateral temporal areas (primary and secondary auditory cortices) across both hemispheres. Bilateral temporal areas were functionally connected with left IFG, but this connection was smaller in the dyslexic group, in particular for left STG and right PAC (ps < .005, corrected for multiple comparisons). Without multiple testing correction, the group difference in functional connectivity between left IFG and right STG (p = .067) and between left IFG and right MTG (near to superior temporal sulcus) (p = .051) also approached significance. Individual differences in the strength of functional connectivity between left STG and left IFG correlated with behavioral indices of word reading (r = .40), nonword reading (r = .48), spelling (r = .53), phonological awareness (r = .46), verbal short-term memory (r = .44) and lexical access (r = .46), as well as with reaction time on the phoneme discrimination task performed in the scanner (r = −.51) (all p < .01).
Fig. 2.
Functional connectivity analysis. (A) Schematic representation of the predefined seed regions. The color-coding corresponds with the one used in Fig. 1. (B-C) Color-coded matrices represent functional connectivity (expressed in Z-scores) among the 13 seed regions in normal and dyslexic readers. Significant correlations (p < .001, FDR-corrected) are indicated by a black dot. (D) Statistical comparison of the functional connectivity between the groups. Significant group differences in functional connectivity are indicated by a black dot (independent t-test, p < .05, FDR-corrected). (E) Scatterplot of the association between reaction time on the phoneme discrimination task in the scanner (y-axis) and intrinsic functional connectivity between left STG and left IFG (x-axis). Dyslexic readers are depicted by red squares, normal readers by green triangles.
At a neuroanatomical level, adequate communication between left IFG and left STG is effected by the left arcuate fasciculus, the major language tract which ensures an efficient signal transmission between Wernicke’s and Broca’s area. We recently collected diffusion tensor imaging data in a subsample (N = 32) of our participants and delineated the left arcuate fasciculus and its three constituent segments (direct, anterior and posterior) on the basis of whole-brain tractography (12). For the present report, we complemented these data and analyses to comprise the full sample (N = 45). Group comparisons (Table S1) revealed significantly reduced white matter integrity of the left arcuate fasciculus in dyslexics (p = .019), in particular in the segment that directly connects posterior temporal and frontal areas (p = .038), hence providing neuroanatomical evidence that corroborates the deficiency in functional connectivity between left IFG and left STG. The functional and structural connectivity measures were not mutually related (r = .06, p = .70). This is in line with recent evidence highlighting the differences between both types of connectivity measures (21, 23), and suggests that both measures are complementary, each capturing a different aspect of the communication between left frontal and temporal language areas. Together, the functional and structural connectivity measures accounted for 35% of the variance in reading and spelling ability, and they predicted reading status (dyslexic versus normal reader) with an accuracy of 73%. This finding adds to the growing recognition of dyslexia as a disconnection syndrome (24, 25).
Our results indicate that deficient phonetic representations are not the core problem in dyslexia. Does this imply that it is time to abandon the influential phonological deficit hypothesis? No, certainly not. The behavioral data of our dyslexic participants reveal that they do show severe deficits in the traditional phonological domains including phonological awareness, verbal short-term memory and lexical access (Table S1) (11, 13). Yet, our neuroimaging findings suggest that it is not a deficit in underlying representations what characterizes dyslexia. Instead, our results suggest that a dysfunctional connection between frontal and temporal language areas impedes efficient access to otherwise intact representations of speech sounds, thus hampering a person’s ability to manipulate them fluently.
Supplementary Material
Acknowledgments
Work supported by KU Leuven (OT/07/034, IDO/10/003), Research Foundation Flanders (G0331.08, postdoctoral fellowship of BB and MV), European Research Council (grant ERC-2011-Stg-284101), Federal Research Action (IUAP/PAI P7/11), Queen Fabiola Foundation, Welcome Trust (Sir Henry Welcome fellowship of CRG (098771/Z/12/Z) and Sir Henry Dale fellowship of DM (101253/Z/13/Z)), and the Royal Society (DM: 101253/Z/13/Z). Matlab scripts are available in the Supplementary Materials. We thank R. Peeters and H. Poelmans for assistance.
BB, HOdB, MV, SKS, SS, JW and PG designed the study. BB and MV collected the data. BB analyzed the data and wrote the manuscript. HOdB and JB contributed to MVPA analyses, MV and SS contributed to DTI analyses, CRG and DM contributed to functional connectivity analyses. All authors discussed the results and commented on the manuscript.
Footnotes
Materials and Methods
Supplementary Text
Figures S1-S5
Tables S1-S4
References (26-30)
External Database S1: Matlab scripts
References and Notes
- 1.Chang EF, et al. Nat. Neurosci. 2010;13:1428. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. J. Child Psychol. Psyc. 2004;45:2. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- 3.Boets B, et al. Br. J. Dev. Psychol. 2010;28:5. doi: 10.1348/026151010x485223. [DOI] [PubMed] [Google Scholar]
- 4.Wagner RK, Torgesen JK. Psychological Bulletin. 1987;101:192. [Google Scholar]
- 5.Goswami U. Dyslexia. 2000;6:133. doi: 10.1002/(SICI)1099-0909(200004/06)6:2<133::AID-DYS160>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Ramus F, Szenkovits G. Q. J. Exp. Psychol. 2008;61:129. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- 7.Ramus F, Ahissar M. Cogn. Neuropsychol. 2012;29:104. doi: 10.1080/02643294.2012.677420. [DOI] [PubMed] [Google Scholar]
- 8.Norman KA, Polyn SM, Detre GJ, Haxby JV. Trends Cogn. Sci. 2006;10:424. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Formisano E, de MF, Bonte M, Goebel R. Science. 2008;322:970. doi: 10.1126/science.1164318. [DOI] [PubMed] [Google Scholar]
- 10.Obleser J, Leaver AM, Vanmeter J, Rauschecker JP. Front. Psychol. 2010;1:232. doi: 10.3389/fpsyg.2010.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandermosten M, et al. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10389. doi: 10.1073/pnas.0912858107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandermosten M, et al. Brain. 2012;135:935. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- 13.De Smedt B, Boets B. Neuropsychologia. 2010;48:3973. doi: 10.1016/j.neuropsychologia.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Op de Beeck HP, Torfs K, Wagemans J. J. Neurosci. 2008;28:10111. doi: 10.1523/JNEUROSCI.2511-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zatorre RJ, Gandour JT. Philos. Trans. R. Soc. Lond B Biol. Sci. 2008;363:1087. doi: 10.1098/rstb.2007.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriegeskorte N, Goebel R, Bandettini P. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3863. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jehee JF, Brady DK, Tong F. J. Neurosci. 2011;31:8210. doi: 10.1523/JNEUROSCI.6153-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornickel J, Kraus N. J. Neurosci. 2013;33:3500. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bookheimer S. Annu. Rev. Neurosci. 2002;25:151. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 20.Scott SK, Johnsrude IS. Trends Neurosci. 2003;2:100. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 21.Gillebert CR, Mantini D. The Neuroscientist. 2013;19:509. doi: 10.1177/1073858412463168. [DOI] [PubMed] [Google Scholar]
- 22.Fair DA, et al. Neuroimage. 2007;35:396. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyszka JM, Kennedy DP, Adolphs R, Paul LK. J. Neurosci. 2011;31:15154. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandermosten M, Boets B, Wouters J, Ghesquiere P. Neurosci. Biobehav. Rev. 2012;36:1532. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 25.van der Mark S, et al. Neuroimage. 2011;54:2426. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Hickok G, Poeppel D. Nat. Rev. Neurosci. 2007;8:393. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 27.Eickhoff SB, et al. Neuroimage. 2005;25:1325. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Morosan P, et al. Neuroimage. 2001;13:684. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- 29.Tzourio-Mazoyer N, et al. Neuroimage. 2002;15:273. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 30.Op de Beeck HP. Neuroimage. 2010;49:1943. doi: 10.1016/j.neuroimage.2009.02.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.