Abstract
CD1 molecules bind and present lipid-based antigens to T cells. Humans express both Group 1 (CD1a, CD1b and CD1c) and Group 2 (CD1d) CD1 molecules with nonredundant functions in the human immune response. Studies of Group 1 CD1 molecules and the T cells that respond to them have lagged behind Group 2 due to the lack of a suitable model system. However, recent work has thrust the Group 1 CD1s into the limelight, revealing their importance in tissue surveillance and microbial defense. Here I review recent advances in Group 1 CD1 lipid presentation, the T cell populations that respond to them and the role of CD1 molecules in engagement of human γδ T cells.
Introduction
Antigen processing and presentation by MHC and MHC-like proteins is a cornerstone of the immune system of jawed vertebrates. CD1 molecules represent one class of MHC molecule that have evolved the capability to present lipid or lipid-based molecules. Some species have a limited CD1 repertoire; the mouse for example expresses only one isotype of CD1 molecule, CD1d. However, many species express a diverse repertoire of these molecules, some in multiple copies [1]. In humans, four CD1 isoforms function to present lipids, CD1a, CD1b, CD1c and CD1d. A fifth, CD1e, functions as a lipid chaperone and has not been shown to present lipids to T cells [2,3**]. CD1a, b and c are considered “Group 1” CD1 molecules while CD1d itself comprises the Group 2 CD1. Each of the lipid-presenting isoforms has evolved a particular molecular architecture that specifies what types of lipids are presented (Figure 1). In addition, there are notable differences in cellular expression, intracellular trafficking and exposure to lipid-transfer chaperones [4], all which dictate the lipid repertoire to which these molecules have access.
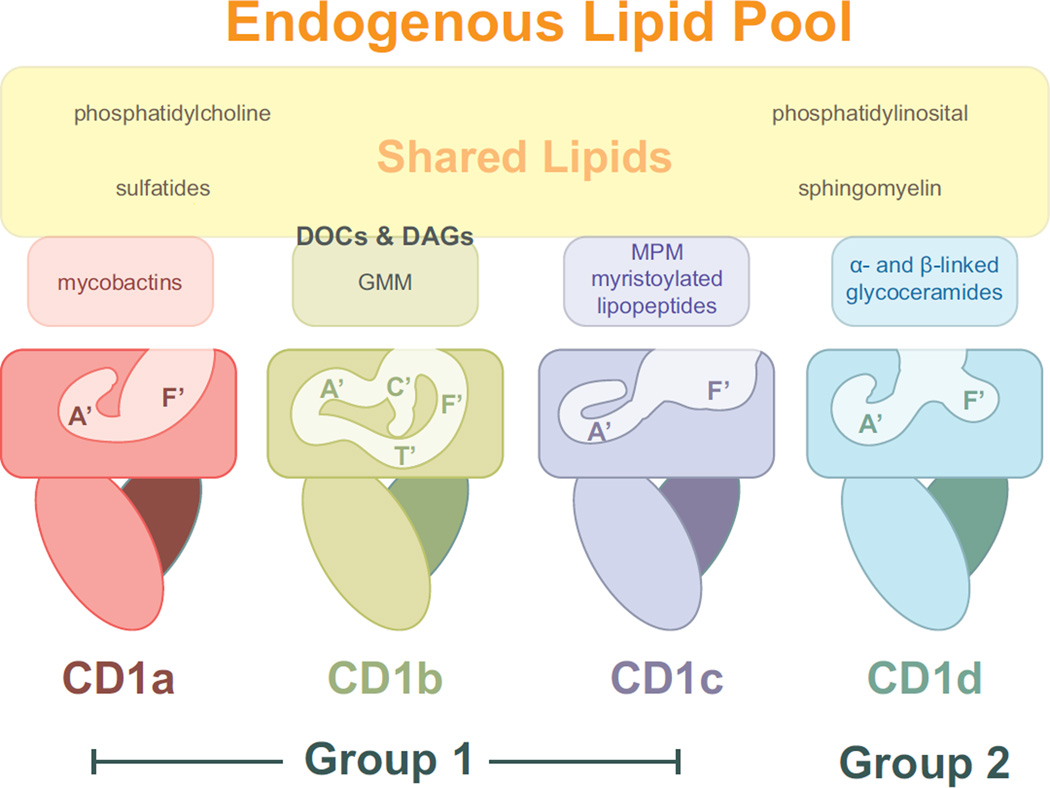
Figure 1.
The four human CD1 isoforms adopt unique three-dimensional structures that dictate the repertoire of lipid ligands they present. Shown are cartoon representations of CD1a, CD1b, CD1c and CD1d with approximated tunnel structures. As discussed in the text, each isoform presents unique lipid types (small boxes colored as the CD1 molecule), however the overall presented endogenous lipidome is highly overlapping (large box, colored yellow). CD1b, in particular, is reliant upon highly hydrophobic scaffolding lipids such as diacylglycerols (DAGs) and deoxyceramides (DOCs) in order to present much of its endogenous lipids, these are shown at the interface between shared and unique CD1b lipid antigens.
The information ingrained in CD1 lipid presentation is of course translated through T cell recognition. Much of our knowledge of T cell recognition of CD1 molecules comes from studies in mice and therefore is focused on the CD1d isoform and the invariant Natural Killer T (iNKT) cell population that is restricted to it [5,6]. However, more recent advances in tetramer-staining and T cell isolation technologies has enabled a new focus on CD1 molecules in humans, revealing that human CD1 molecules provide antigen to many different αβ T cell populations including iNKT cells, NKT cells that express diverse TCRs, and the more recently characterized Group 1 reactive T cells. Less well studied, yet of potentially great importance, is the involvement of CD1 presentation on the human γδ T cell population. Here we review the recent advances in human CD1 lipid presentation with an emphasis on the Group 1 CD1 molecules, and the newly described human T cell populations that respond to CD1 molecules.
Presentation of Endogenous Lipids
The three-dimensional structures of all the human CD1 molecules revealed different sizes and architectures to the two main internal hydrophobic cavities, which are generally described as the A’ and F’ pockets (Figure 1) [7]. The volume of these cavities varies extensively between isoforms with the general trend being CD1a < CD1d < CD1c <CD1b (~1,350 Å3, ~1400 Å3, ~1780 Å3, and ~2,200Å3 respectively). The size and shape of these cavities, which are mostly lined with hydrophobic amino acids, correlates well with the size of the hydrophobic chains of the lipids they have been found to present (Figure 1). CD1a, for example, is known to present lipids with short or single hydrocarbon chains, such as dideoxymycobactin lipopeptides, which contain a single acyl chain [8]. CD1b, in contrast, can present lipids containing incredibly long hydrocarbon chains, up to 80 carbons in length due to a network of tunnels that exist within its internal space [9]. CD1c has a unique structural feature which may related to its ability to present acylated lipopeptides, its F’ pocket is open and exposed to solvent, well suited to presentation of the long peptide portion of N-terminally acylated peptides [10].
However the Group 1 molecules also present overlapping repertoires of lipids, as is noted by their ability to all present the lipid sulfatide [11]. Thus, there has been interest in characterizing the endogenous lipid repertoire pool for these molecules in order to define the chemical and structural parameters of the lipids presented. Much of the work on lipid characterization for the Group 1 molecules has focused on specific exogenous or mycobacterial lipids and has been recently reviewed [12]; work on characterizing the endogenous lipid pool has been more challenging due to difficulties in protein isolation and purification. Isolation of native CD1 proteins with intact intracellular trafficking signals requires detergents, which can alter the bound lipid repertoire during protein purification, however CD1 constructs that are engineered to be secreted for purification from supernatant are not recycled through their respective endocytic compartments and therefore do not contain representatives of the lipid repertoire to which they are ultimately exposed. Yet dissecting the lipid repertoire available to these proteins during their initial synthesis and trafficking through the ER and Golgi provides important insight into the primary lipid repertoire that is presented on the cell surface. This is the first step in understanding how these lipids are competed out, modified or presented in conjunction with exogenous and endogenous lipids available upon recycling in the endocytic pathway.
Mass Spectrometry (MS) approaches have be recently used to characterize the lipid pool bound by CD1c and has further characterized the diversity of lipids bound by the other Group 1 isoforms. Three classes of lipids were found bound by CD1c: sphingomyelin (SM), phoshatidylcholine (PC) and phosphatidylinositol (PI), some of which contained polyunsaturated acyl chains [13*]. So despite having an exposed F’ pocket, CD1c efficiently presents lipids with multiple hydrocarbon chains, consistent with the present of spacer fatty acids observed in the original crystal structure [10]. This study also examined CD1d and found gangliosides, globo/isoglobosides, sulfatide, sphingomyelins, and phosphatidylcholines, the diversity of which contrasted with some past studies that found a much more restricted lipid repertoire. Using a broad comparative lipodomics approach, Huang and colleagues examined all four CD1 isoforms and found diverse lipid antigen pools for each CD1 isoform [14**], with considerable repertoire overlap (Figure 1). For CD1b the lipids isolated were similarly sized to those isolated from other isoforms, however unusually hydrophobic lipids were also identified that filled the excess cavity space. This has been proposed previously from crystallographic data [9] and demonstrated via MS [15], but Huang and colleagues identified two particular classes of lipids, deoxyceramides and diacylglycerols (DAGs) which were unlike simple fatty acids. They further show that addition of one of these isolated “scaffold” lipids, DAG, could modulate the T cell response to differently sized mycobacterial lipids (C32 GMM or C80 GMM): enhancing T cell reactivity by serving as a scaffold for C32 GMM or inhibiting through space-competition with C80 GMM. Therefore, the scaffold lipids present in CD1b can have a significant effect on what endogenous and exogenous lipids are presented during endosomal recycling.
T cell reactivity to Group 1 CD1s
Autoreactive T cell populations are at high frequency in blood
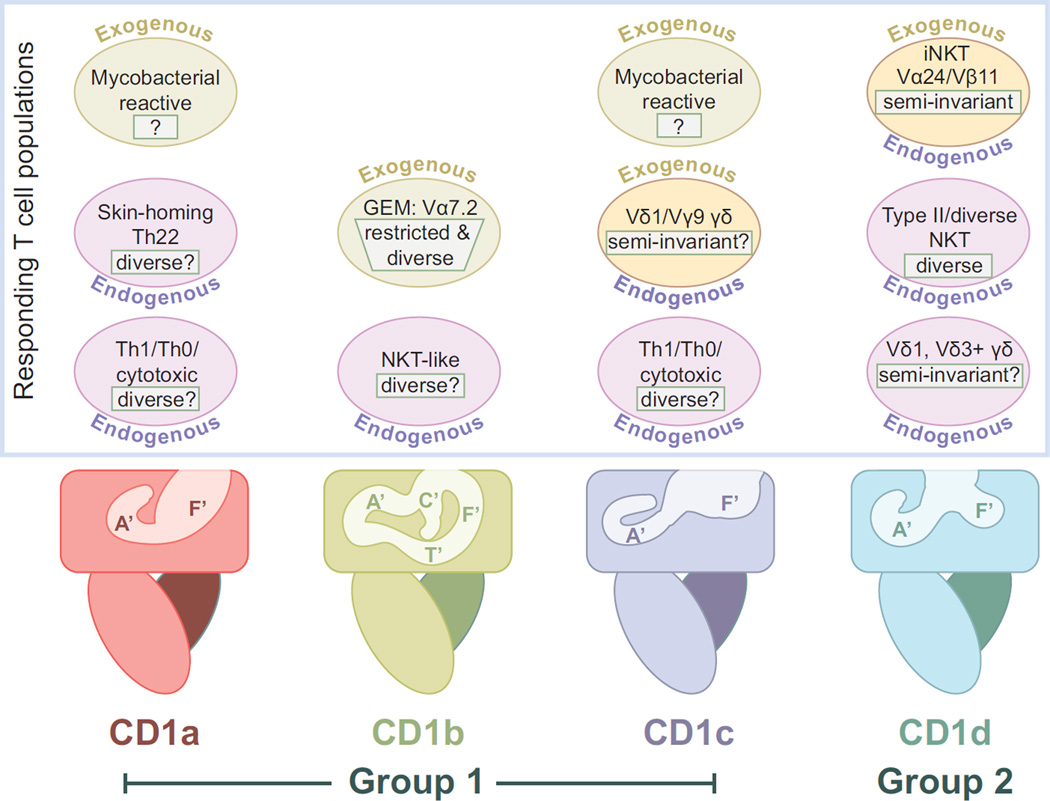
T cell autoreactivity, or reactivity to CD1 molecules presenting endogenous lipid ligands, has been known for some time [16], including the characterization of specific T cell clones [17] (Figure 2). Yet our understanding of the frequency and relevance of these T cells has been limited. One of the first studies to shed light on the significance of CD1 autoreactivity focused on an autoreactive population of αβ T cells expressing diverse TCRs that are responsive to endogenous lipids presented by CD1a molecules [18**] (Figure 2). These T cells were found in all donors examined and were at a surprisingly high frequency of total T cells exhibiting autoreactivity to autologous DCs (~1 in every 50 autoreactive T cells, estimated to be ~0.02% to 0.4% of memory T cells in the blood). These T cells express CCR4, CCR6 and CCR10, homing receptors to the skin, and secrete IL-22 in a CD1a dependent fashion. This is important because IL-22 activates skin keratinocytes to release anti-microbial peptides and promotes proliferation and survival responses [19]. Tied to this, of course, is that CD1a is expressed in epithelial Langerhans cells, suggesting that CD1a reactive T cells function in skin homeostasis, reminiscent of the DETC γδ T cells in the mouse [20].
Figure 2.
The T cell populations that recognize human Group 1 and Group 2 CD1 molecules. Cartoon representations of each of the CD1 isoforms are shown as in Figure 1. Above each isoform are circles representing the known responding T cell groups or populations. Auto-reactive T cell populations, or those responding to endogenously presented lipid antigens, are labeled with “Endogenous” and are colored in light green. T cells responding to exogenous antigens, such as those from mycobacteria, are colored pink and labeled with “Exogenous”. T cell populations known to respond to both endogenous and exogenous lipid antigens are colored yellow and labeled with both terms. V usage is indicated, where known and the TCR diversity of the population is indicated in a white box. In many cases is unclear the degree of overlap between endogenous and exogenous reactive populations, they are therefore represented separately for lack of better information.
Using a different antigen presenting cell (C1R) but a similar approach, De Lalla and colleagues investigated the frequency and effector phenotype of CD1 autoreactive T cells in adult donors and cord blood [21**] (Figure 2). Overall, the estimated frequency of CD4+ or double negative (DN) T cells responsive to CD1 expressing C1R cells was 1/10 to 1/300, much higher than the estimated frequency of human CD1d-restricted iNKT cells and similar to that of MHC alloreactive αβ T cells. Within the CD1s, CD1a and CD1c reactive T cells were of the highest percentage, with an estimated frequency of CD1c reactive T cells at ~0.5 to 1% of total blood T cells. Similar overall frequencies of these cells were also found in cord blood, although there the cells exhibited a naïve phenotype (CD45RA+) whereas adult samples had an increased memory population (CD45RO+). The effector phenotype of these cells was diverse, with clones exhibiting either Th1 or Th2 cytokine profiles and some with demonstrable cytotoxic activity. What remains unclear in both these studies is the nature of the endogenous lipid antigen(s) that trigger these T cell responses and how these ligands are regulated to prevent autoimmunity and promote proper host surveillance.
While autoreactive T cells have been identified for CD1b [22], they were found to be in the minority in the above study and thus were not characterized more fully. Use of a Group 1 humanized mouse model [23] has shed some light into CD1b autoreactive T cells. A transgenic CD1b autoreactive T cell (HJ1) was used to make an HJ1 transgenic in Group 1 CD1 humanized mice [24*]. Characterization of the HJ1 population revealed features similar to iNKT cells, such as expression of the PLZF transcription factor, an activated effector phenotype and a subset of cells that express NK1.1 and is enriched in the liver (Figure 2). Furthermore, expression of the TCR and CD3 signaling components were found to be more down-regulated in HJ1tg/hCD1 mice in comparison to HJ1tg control mice, suggesting a plausible mechanism to explain avoidance of negative selection in the thymus and auto-immunity in the periphery.
Group 1 CD1 T cell response to Mycobacterium tuberculosis
The role of Group 1 CD1 molecules in mycobacterial antigen presentation has been well established however our understanding of the phenotype of the responding cell population was initially restricted to in vitro-derived T cell clones or limited in the scope of effector-function characterization. Recently, studying T cell reactivity to a specific CD1/lipid complex has become more feasible with the expansion of CD1-tetramer technology. This enables the direct isolation of antigen-specific T cells from blood and tissue without the need for antigen-presenting cells and circumventing the possibility of intracellular lipid processing. This technique has been used to more fully characterize the lipid-specific T cell response against mycobacteria mediated by CD1b and CD1c.
CD1b tetramers loaded with the mycobacterial lipid GMM brightly stained T cells from Mtb infected individuals but not T cells from healthy patients [25*], suggesting an expansion of a specific population of mycobacterial responsive T cells had occurred in response to Mtb infection. These cells were found to be predominantly CD4+ and appeared to express a restricted TCR repertoire. Further characterization of these T cells, now called “GEM” T cells for ‘germline-encoded, mycoyl-reactive’, revealed a population expressing highly restricted TCRs composed of a TRAV1-2 (Vα7.2) V gene segment (also found in MAIT cells) rearranged with TRAJ9, with limited CDR3α diversity [26**]. The Vβ domains were also found to be of limited diversity, with only TRBV6-2 and TRBV30 domains represented in the clones characterized. While the semi-invariant nature of this population’s TCR repertoire is reminiscent of iNKT or MAIT TCR repertoires, this population is at very low frequency in Mtb naïve individuals (~0.0024% of total PBMCs) suggesting a significant antigen-specific expansion of this population in response to Mtb infection, more akin to “public” TCRs observed for classical MHC restricted responses. A second, tetramer-intermediate staining population was also noted, expressing a more diverse TCR repertoire that likely recognized CD1d-GMM with lower apparent affinity; these cells may have a baseline reactivity to CD1b that can be enhanced with other mycobacterial lipid antigens.
A similar strategy has been employed to examine mycobacterial-reactive CD1c restricted T cells. The role of CD1c in mycobacterial surveillance, primarily Mtb, has been well established [12], including the identity of some of the natural ligands recognized. These lipids were identified as branched chain phospholipids including mannosyl phosphodolichols (MPDs) and mycobacterial mannosyl-β1-phosphomycoketide (MPM) [27]. T cells clones have been characterized that respond well to these antigens, such as the DN6 and CD8-1 cell lines which respond well to MPD [27]. Yet further examination of the specific reactivity of DN6 to Mtb lysates revealed an interesting broad reactivity; DN6 responded well to unglycosylated and β-mannosylated phosphomycoketides [28**]. Dissection of this response revealed the true specificity of DN6 to be to naked phosphomycoketide (PM), and the previously observed broad reactivity was discovered to be due to removal of the mannose during processing of the MPM within the antigen-presenting cells. CD1c-tetramers loaded with PM were then used to estimate the frequency of reactive T cells in latently infected Mtb. donors at ~0.0017% of total peripheral blood T cells. Clones derived from this analysis exhibited similar specificity to DN6, recognizing both glycosylated and unglycosylated mycoketides when presented by antigen presenting cells, indicating that processing of this antigen was likely occurring for stimulation of these additional clones.
γδ T cell recognition of CD1 molecules (insert Box 1 in this section)
Box 1.
Human γδ T cell populations are distinguished by their Vδ chain usage and tissue residence. Despite the similarity in γδ TCR expression, γδ T cells are quite different between mouse and human in the ligands they recognize and the composition of their TCRs. Indeed, there has yet to be a single conserved γδ T cell population identified in both species, thus it is likely that these cells have evolved to recognize species-specific ligands. In humans, most blood γδ T cells express a Vγ9Vδ2 TCR and respond to small molecular weight phosphoantigens. Vδ1 expressing γδ T cells are found at low frequency in the blood, but predominate in the periphery, residing in barrier tissues such as the gut, lung and reproductive tract. The identity of the specific antigens that directly engage human γδ TCRs are mostly unknown or controversial, however the discovery that CD1 molecules engage Vδ1+ γδ T cells has opened up a new area of investigation into how Vδ1+ γδ T cells are regulated in a CD1-specific manner and how CD1 presentation of lipids to these cells may play a role in diseases of the tissue in which they reside.
CD1 molecules are unique in that they have been found to be ligands for significant populations of both αβ and γδ T cells. CD1c was the first CD1 isoform to have γδ T cell responses characterized [29] and T cells isolated from the duodenum were later found to be broadly reactive to all four CD1 isoforms presenting endogenous antigens [30]. All of these γδ T cells expressed the Vδ1 domain, found at low frequency in blood γδ T cells but represents the majority of γδ T cells in the gut. Recently, a tetramer approach similar to that described above revealed consistent and reproducible γδ reactivity to CD1d presenting the endogenous antigen sulfatide [31**]. These γδ T cells, derived from peripheral blood of healthy human donors, were found in all individuals examined and in some donors 100% of the T cells stained by this tetramer were Vδ1+ γδ. All the CD1d-sulfatide staining γδ T cells expressed the Vδ1 chain consistent with the results from the duodenum. While our understanding of Vδ1 restriction to CD1 molecules remains limited, CD1 molecules have the potential to be one of the few bon fide ligands for human γδ T cells as other ligands remain undefined or are controversial. CD1d, in particular, is expressed in intestinal epithelial cell (IEC) in the gut [32] and in MS lesions [33], both sites where Vδ1+ γδ T cells have been found [34]. CD1d was also found to be recognized by blood Vδ3+ γδ T cells [35*], suggesting this CD1 isoform may provide antigenic signals to two of the three major γδ populations in humans.
Conclusions and future directions
Characterizing the endogenous lipid pool for CD1 molecules has provided insight into: 1) the chemical and structural requirements of lipids for presentation in a particular CD1 isoform; 2) the potential identity of lipids that provide selection signals for CD1-reactive T cells in the thymus and 3) the nature of the lipid repertoire to which auto-reactive, CD1 specific T cells, are responding to in the periphery. All of the studies that have contributed to the identity of these lipids have provided candidates to test for each of these important topics. Progress is being made on understanding the T cell repertoire that recognizes human CD1 molecules; from what is currently known it is clear that CD1 molecules provide signals to an unexpectedly large proportion of blood T cells and may engage even more in the periphery. Understanding the lipid signals that mediate the observed auto-reactivity and how it is modulated in the periphery to circumvent auto-immunity is a key aim for current and future CD1-related investigations. In terms of microbial surveillance, expanding on current studies that have clearly demonstrated the role of CD1b and CD1c molecules in presenting microbial derived antigens to T cells will be of great importance. Do CD1 molecules present other lipid antigens from Mtb or from other microbial species? What is the nature of the T cells that respond to these signals; do T cells that respond to a particular CD1 isoform have commonalities that we have yet to see in current studies? Finally, the engagement of CD1 molecules by human γδ T cells may represent a major breakthrough in understanding the role of γδ T cells in the periphery and how their functions are regulated.
Highlights.
Human CD1 molecules present a diverse, overlapping repertoire of endogenous lipids.
CD1 specific T cells comprise a significant percentage of blood αβ T cells.
Many CD1 specific T cells exhibit auto-reactivity to endogenous lipid antigens.
Acknowledgements
I sincerely apologize to colleagues whose work I have not cited due to space constraints or oversight. I thank members of my laboratory and departmental and external colleagues for helpful discussions. This work was supported by the National Institutes of Health R01 Grant AI073922.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dascher CC. Evolutionary biology of CD1. Curr Top Microbiol Immunol. 2007;314:3–26. doi: 10.1007/978-3-540-69511-0_1. [DOI] [PubMed] [Google Scholar]
- 2.de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Maitre B, Mourey L, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 3. Facciotti F, Cavallari M, Angenieux C, Garcia-Alles LF, Signorino-Gelo F, Angman L, Gilleron M, Prandi J, Puzo G, Panza L, et al. Fine tuning by human CD1e of lipid-specific immune responses. Proc Natl Acad Sci U S A. 2011;108:14228–14233. doi: 10.1073/pnas.1108809108. ** This study futher defines the role of CD1e as a chaperone in loading lipids into CD1b, CD1c and CD1d molecules by facilitating both rapid formation CD1/lipid complexes and their subsequent turn-over. The authors show how CD1e can hone an antigen-specific response through biasing lipid presentation.
- 4.Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7:929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 6.Gapin L, Godfrey DI, Rossjohn J. Natural Killer T cell obsession with self-antigens. Curr Opin Immunol. 2013;25:168–173. doi: 10.1016/j.coi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams EJ, Luoma AM. The adaptable major histocompatibility complex (MHC) fold: structure and function of nonclassical and MHC class I-like molecules. Annu Rev Immunol. 2013;31:529–561. doi: 10.1146/annurev-immunol-032712-095912. [DOI] [PubMed] [Google Scholar]
- 8.Zajonc DM, Crispin MD, Bowden TA, Young DC, Cheng TY, Hu J, Costello CE, Rudd PM, Dwek RA, Miller MJ, et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity. 2005;22:209–219. doi: 10.1016/j.immuni.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, Schmidt RR, Jones EY, Cerundolo V. Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat Immunol. 2002;3:721–726. doi: 10.1038/ni821. [DOI] [PubMed] [Google Scholar]
- 10.Scharf L, Li NS, Hawk AJ, Garzon D, Zhang T, Fox LM, Kazen AR, Shah S, Haddadian EJ, Gumperz JE, et al. The 2.5 a structure of CD1c in complex with a mycobacterial lipid reveals an open groove ideally suited for diverse antigen presentation. Immunity. 2010;33:853–862. doi: 10.1016/j.immuni.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamshiev A, Gober HJ, Donda A, Mazorra Z, Mori L, De Libero G. Presentation of the same glycolipid by different CD1 molecules. J Exp Med. 2002;195:1013–1021. doi: 10.1084/jem.20011963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Rhijn I, Ly D, Moody DB. CD1a, CD1b, and CD1c in immunity against mycobacteria. Adv Exp Med Biol. 2013;783:181–197. doi: 10.1007/978-1-4614-6111-1_10. [DOI] [PubMed] [Google Scholar]
- 13. Haig NA, Guan Z, Li D, McMichael A, Raetz CR, Xu XN. Identification of self-lipids presented by CD1c and CD1d proteins. J Biol Chem. 2011;286:37692–37701. doi: 10.1074/jbc.M111.267948. * This manuscript describes lipids extracted from secreted, highly purified CD1c and CD1d molecules using a rigorous Mass spectrometry approach.
- 14. Huang S, Cheng TY, Young DC, Layre E, Madigan CA, Shires J, Cerundolo V, Altman JD, Moody DB. Discovery of deoxyceramides and diacylglycerols as CD1b scaffold lipids among diverse groove-blocking lipids of the human CD1 system. Proc Natl Acad Sci U S A. 2011;108:19335–19340. doi: 10.1073/pnas.1112969108. ** This study uses comparative lipidomics to compare and contrast the endogenous lipid repertoire of all four human CD1 molecules and identifies specific scaffolding lipids that function during lipid presentation of CD1b.
- 15.Garcia-Alles LFVK, Maveyraud L, Vallina AT, Sansano S, Bello NF, Gober HJ, Guillet V, de la Salle H, Puzo G, Mori L, Heck AJ, De Libero G, Mourey L. Endogenous phosphatidylcholine and a long spacer ligand stabilize the lipid-binding groove of CD1b. EMBO J. 2006;25:3684–3692. doi: 10.1038/sj.emboj.7601244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8- cytolytic T lymphocytes. Nature. 1989;341:447–450. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 17.Vincent MS, Xiong X, Grant EP, Peng W, Brenner MB. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J Immunol. 2005;175:6344–6351. doi: 10.4049/jimmunol.175.10.6344. [DOI] [PubMed] [Google Scholar]
- 18. de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB. CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nature immunology. 2010;11:1102–1109. doi: 10.1038/ni.1956. ** This study identifies an αβ T cell population specific to human CD1a bound with endogenous lipid antigens. These T cells were initially identified from the blood but were also found resident in the skin. Upon CD1a-dependent stimulation, these cells secrete IL-22, a cytokine involved in homeostasis and surveillance of the skin epithelium.
- 19.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 20.Macleod AS, Havran WL. Functions of skin-resident gammadelta T cells. Cell Mol Life Sci. 2011;68:2399–2408. doi: 10.1007/s00018-011-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, Mori L, De Libero G, Dellabona P, Casorati G. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. European journal of immunology. 2011;41:602–610. doi: 10.1002/eji.201041211. ** This study explores the frequency and effector-functions of human T cells responsive to the four CD1 isoforms. In adult donors, they show that CD1a and CD1c have the highest frequency of responsive cells, similar to that found in cord blood, yet adult T cells have a higher proportion of effector-memory phenotype.
- 22.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29:1667–1675. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Felio K, Nguyen H, Dascher CC, Choi HJ, Li S, Zimmer MI, Colmone A, Moody DB, Brenner MB, Wang CR. CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. 2009;206:2497–2509. doi: 10.1084/jem.20090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li S, Choi HJ, Felio K, Wang CR. Autoreactive CD1b-restricted T cells: a new innate-like T-cell population that contributes to immunity against infection. Blood. 2011;118:3870–3878. doi: 10.1182/blood-2011-03-341941. * This study uses a humanized mouse model for the Group 1 CD1s to understand the development and effector-phenotype of a CD1b autoreactive T cell (HJ1).
- 25. Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, et al. CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J Exp Med. 2011;208:1741–1747. doi: 10.1084/jem.20110665. * This study uses CD1b/antigen tetramers to identify a unique population of CD1b reactive, mycobacterial antigen-specific T cells.
- 26. Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14:706–713. doi: 10.1038/ni.2630. **This study characterizes the CD1b tetramer-positive population initially identified in Ref 25, showing its expansion in humans latently infected with Mtb. Tetramer-high staining T cells express a restricted TCR repertoire whereas tetramer-intermediate staining T cells express diverse TCRs, suggesting the potential for responses against other CD1b-presented antigens.
- 27.Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–888. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 28. Ly D, Kasmar AG, Cheng TY, de Jong A, Huang S, Roy S, Bhatt A, van Summeren RP, Altman JD, Jacobs WR, Jr, et al. CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J Exp Med. 2013;210:729–741. doi: 10.1084/jem.20120624. ** This study is the first to demonstrate that intracellular processing of lipid antigens presented by CD1c molecules plays a role in the CD1c specific T cell response against Mtb.
- 29.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, Porcelli SA, Spinozzi F. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal gammadelta+ T lymphocytes. J Immunol. 2007;178:3620–3626. doi: 10.4049/jimmunol.178.6.3620. [DOI] [PubMed] [Google Scholar]
- 31. Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, Adams EJ, Savage PB, Bendelac A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol. 2012;42:2505–2510. doi: 10.1002/eji.201242531. **This study characterizes a CD1d-sulfatide specific γδ T cell population from healthy human donors. These γδ T cells all expressed the Vδ1+ domain, similar to findings from other groups showing Vδ1+ restriction to CD1 molecules. This suggests that CD1 molecules may be a key ligand used in γδ T cell surveillance.
- 32.Perera L, Shao L, Patel A, Evans K, Meresse B, Blumberg R, Geraghty D, Groh V, Spies T, Jabri B, et al. Expression of nonclassical class I molecules by intestinal epithelial cells. Inflamm Bowel Dis. 2007;13:298–307. doi: 10.1002/ibd.20026. [DOI] [PubMed] [Google Scholar]
- 33.Hoftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14:43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci U S A. 1992;89:4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mangan BA, Dunne MR, O'Reilly VP, Dunne PJ, Exley MA, O'Shea D, Scotet E, Hogan AE, Doherty DG. Cutting edge: CD1d restriction and Th1/Th2/Th17 cytokine secretion by human Vdelta3 T cells. J Immunol. 2013;191:30–34. doi: 10.4049/jimmunol.1300121. *This study demonstrates that the majority of Vδ3+ γδ T cells in human blood respond to CD1d and secrete a broad cytokine spectrum.