Abstract
A decade of work shows that the core function of phagocytosis in engulfment and destruction of microorganisms is only a small facet of the full spectrum of roles for phagocytosis in the immune system. The regulation of phagocytosis and its outcomes by inflammatory pattern recognition receptors (PRRs) is now followed by new studies strengthening this concept and adding further complexity to the relationship between phagocytosis and innate immune signaling. Phagocytosis forms the platform for activation of distinct members of the Toll-like receptor family, and even dictates their signaling outcomes. In many cases, phagocytosis is a necessary precedent to the activation of cytosolic PRRs and assembly of canonical and non-canonical inflammasomes, leading to strong pro-inflammatory responses and inflammatory cell death.
Introduction
Phagocytosis constitutes the first line of defense deployed by the organism when it encounters microbes. Discovered late in the nineteenth century by Elie Metchnikoff, phagocytosis is conducted by a specialized group of innate immune cells called phagocytes, and involves actin cytoskeleton-dependent internalization of cargo larger than 0.5μm in diameter. Phagocytes comprise macrophages, dendritic cells (DC), monocytes and neutrophils. After internalization, microbes are confined to intracellular vesicles called phagosomes, which undergo a series of interactions with endosomes and lysosomes in a process called phagosome maturation. Phagocytosis also serves to clear cells dying as a result of infection [1]. Besides clearance and neutralization, two critical outcomes of phagocytosis are: First, processing and presentation of microbial peptides within major histocompatibility complex (MHC) molecules, which are recognized by T cell receptors in a T cell co-stimulatory context, leading to activation of CD4+ and CD8+ T cells of the adaptive immune system [2]. Second, phagocytosis of microorganisms and infected dying cells is accompanied by the production of inflammatory cytokines that recruit innate immune cells to the site of infection, and determine the nature of the adaptive immune response. These inflammatory cytokines are made in direct response to the engagement of pattern recognition receptors (PRRs) by microbial components during the course of phagocytosis [3]. While PRRs critically impact phagocytosis and its consequences [4,5], emerging evidence shows that phagocytosis in turn determines the outcome of signal transduction from these PRRs. Here, we examine the intimate links between phagocytosis and the cell autonomous signaling pathways of host defense.
Signal dependent induction of phagocytosis and phagosome maturation
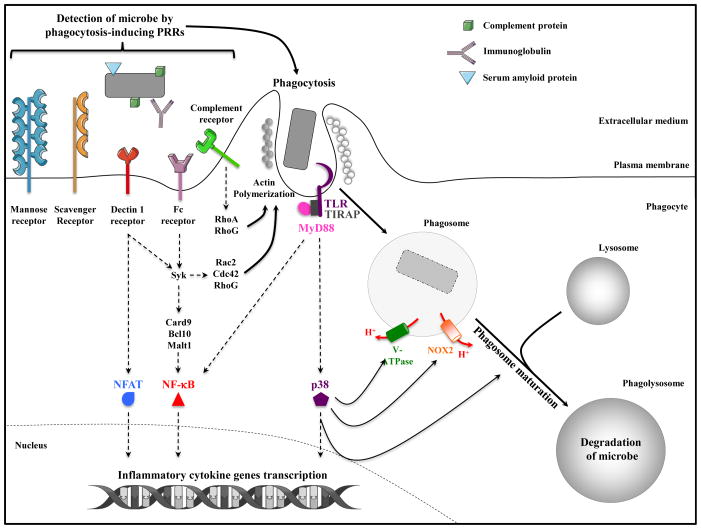
Three categories of PRRs can be engaged during phagocytosis: 1) PRRs that mediate phagocytosis, 2) PRRs that initiate inflammatory signal transduction, and 3) PRRs that do both. In the first category, the mannose-receptor exemplifies a phagocytic receptor: it recognizes mannose residues on the surface of microorganisms and induces their phagocytosis without inflammatory signaling capabilities of its own [6]. Instead, this receptor appears to modulate signaling by PRRs of the second category, such as Toll-like receptor (TLR) 2. Other receptors such as scavenger receptors, including SR-A, MARCO and CD36, also function primarily as phagocytic receptors binding to a variety of microbial components, and can modulate inflammatory signaling by TLRs [7]. As such, scavenger receptors and mannose receptor mediate non-opsonic phagocytosis. This contrasts with opsonic phagocytosis where opsonization of microorganisms with IgG, complement, or pentraxins such as C-reactive protein and serum amyloid P component, enhances phagocytosis by binding to Fcγ receptor (FcγR) or complement receptors expressed at the surface of phagocytes [8] (Figure 1).
Figure 1. Signal-dependent induction of phagocytosis and phagosome maturation.
Phagocytosis is an actin-driven, receptor mediated process initiated upon recognition of microorganisms by Pattern Recognition Receptors (PRRs) expressed at the plasma membrane of phagocytes. Microorganisms can also be opsonized by immunoglobulins, serum amyloid P component or complement proteins, which engage specific PRRs and trigger opsonic phagocytosis. Some receptors like scavenger receptors and mannose receptor serve as phagocytic PRRs, while others like Dectin-1 and FcγR serve dual roles transmitting inflammatory signals receptor that activate NF-κB and/or NFAT transcription factors, and triggering actin polymerization via Rac2, Cdc42, and RhoG [75]. Toll-like receptors (TLRs) are signaling PRRs engaged by microbial components during phagocytosis leading to the activation of NF-κB, MAPK and other transcription factors. TLR signals also trigger an inducible rate of phagocytosis and phagosome maturation via MyD88-dependent activation of the MAPK p38. Signals from TLRs also induce assembly of the NADPH oxidase NOX2 and the vacuolar v-ATPase.
PRRs of the third category directly trigger both phagocytosis and inflammatory signal transduction. For example, engagement of the FcγR by IgG-opsonized microorganisms triggers Src family kinase-mediated phosphorylation of tyrosine residues within the immunoreceptor tyrosine based activation motif (ITAM). Subsequent recruitment of the typrosine kinase Syk, activation of the phophatidylinositol-3-kinase (PI3-kinase) and the small GTPases, Rac2 and Cdc42, direct actin cytoskeletal rearrangement and engulfment [9]. Syk also links into the CARD9-BCL10-MALT1 (CBM) complex culminating in the activation of mitogen-activated protein kinases (MAPK) and NF-κB [10,11]. Similar events take place downstream of the C-type lectin receptor Dectin1, which recognizes β-glucan in fungal cell walls and initiates phagocytosis and inflammatory signaling using the Syk-CBM-NF-κB pathway [11], but also the transcription factor NFAT [10] (Figure 1).
Receptors of the second category specialize in signal transduction, and here TLRs are the best example. TLRs are strategically placed on the plasma membrane and along the phagocytic pathway where they patrol for the presence of microorganisms and their components. TLRs alert the immune system by signaling via two adaptors – TRIF and MyD88 – and downstream MAPK, interferon regulatory factor (IRF) and NF-κB pathways [3]. While TLR signals do not initiate phagocytosis, they play a dominant role in triggering an inducible rate of phagocytosis and phagosome maturation, commensurate with the urgent need to alert the cell to the microbial nature of the cargo it has encountered [12]. Phosphorylation of p38 MAPK downstream of MyD88 is important for this process [12] (Figure 1). Similar studies with the soluble TLR4 ligand lipopolysaccharide (LPS) demonstrated enhanced macropinocytosis, the process of internalizing large amounts of extracellular fluid, upon engagement of TLR4 [13]. This enhancement was dependent on signals later shown to involve the ribosomal s6 kinase (RsK) [14]. A soluble form of MD-2, which binds LPS in the TLR4 membrane complex, has been shown to opsonize Gram-negative bacteria and enhance their phagocytosis by neutrophils, likely also because of the engagement of TLR4 signaling [15].
TLR signaling critically modulates phagosome function. Besides increasing antigen processing and presentation [2], there is some evidence that TLRs enhance phagosomal assembly and microbicidal function of the NADPH oxidase complex NOX2 in both macrophages and DC [12,16,17]. One mechanism is through MyD88-dependent phosphorylation of the cytosolic p47phox component of NOX2 [16]. Because ROS production consumes protons countering the activity of the v-ATPase and causing phagosome alkalinization, enhancing NOX2 activity by TLRs appears contradictory to TLR-regulated phagosome acidification [12,18,19]. The dynamic nature of phagosome maturation is conducive to kinetic recruitment of distinct components to mediate unique functions (NOX2 and v-ATPase, for example), and this is likely also tailored according to cell type – macrophage, DC or different subsets thereof. By regulating phagosome maturation, TLR signals could well orchestrate this sequence of events. In neutrophils, NADPH oxidase activity has a finite duration with rapid onset and decline as the phagosome matures and the pH drops [20]. Early NOX2 recruitment in DC serves to raise phagosomal pH preserving peptides derived from phagocytic cargo for cross-presentation to CD8+ T cells [21]. Therefore, TLR regulation of NOX2 and v-ATPase activity could be temporal beginning with alkalinization and followed by acidification, which would then serve other functions such as cargo degradation and MHC class II antigen presentation.
Phagocytosis modulates innate immune signaling
The phagosome is a signaling compartment for Toll-like receptors
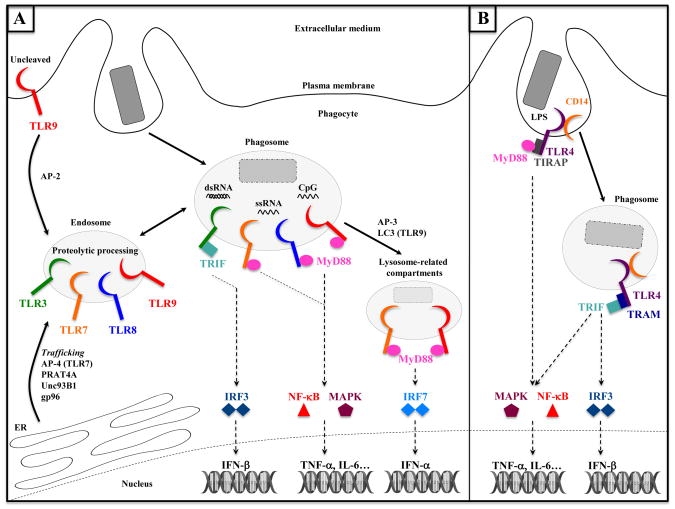
Among the TLR family, it is well appreciated that some members are localized specifically to endosomes and phagosomes. These include TLR3 (sensing double-stranded RNA), TLR7 and TLR8 (sensing single-stranded RNA) and TLR9 (sensing unmethylated CpG DNA) [3,22] (Figure 2). It has been proposed that the intracellular location of nucleic acid sensing TLRs serves to ensure their engagement only by nucleic acids derived from internalized viruses or bacteria, and not by self nucleic acids that may be present in interstitial fluids [22].
Figure 2. The phagosome is a signaling compartment for TLRs: cases of endosomal TLRs and TLR4.
(A) TLR3, TLR7, TLR8 and TLR9 are endosomal receptors. Trafficking of these receptors from the endoplasmic reticulum (ER) to endosomes requires chaperone proteins such as UNC93B1, gp96, AP-4 (for trafficking TLR7 directly to endosomes) or AP-2 (for trafficking TLR9 from the intermediate plasma membrane step between ER and endosomes). Microbial nucleic acids released upon degradation of microorgamisms activate endosomal TLRs delivered to phagosomes. TLR3 is activated by dsRNA and signals through TRIF to activate IRF3 and IFN-β transcription. TLR7, TLR8 (both activated by ssRNA) and TLR9 (sensing CpG DNA) signal through MyD88 and activate NF-κB and MAPK signaling pathways, leading to the transcription of genes such as TNF-α or IL-6. AP-3 and LC3 enable trafficking and signaling of TLR7 and TLR9 to lysosome-related compartments, where these receptors specifically activate the transcription of IFN-α genes in a MyD88- and IRF7-dependent manner.
(B) At the plasma membrane, TLR4 recognizes LPS and signals through MyD88 to activate NF-κB and MAPK. CD14 mediates the endocytosis of TLR4, which undergoes a signaling switch from plasma membrane MyD88 to endosomal/phagosomal TRIF signaling and activation of the IRF3-dependent transcription of IFN-β genes.
How is the intracellular localization of endosomal TLRs determined? The protein Uncoordinated 93 homolog B1 (Unc93B1) exerts its hallmark role in endosomal TLR signaling [23] by binding to the transmembrane domains of endosomal TLRs when they are still in the endoplasmic reticulum (ER), and packaging them into COPII vesicles en route to endosomes [23,24]. At this point, Unc93B1 differentially controls endosomal delivery of TLR7 and TLR9 by mediating recruitment of the adaptor proteins AP-4 to TLR7, and AP-2 to TLR9 [24]. Chaperoned by Unc93B1, TLR9 traffics to the plasma membrane, where AP-2 is recruited and subsequently delivers TLR9 to endosomes [24]. Delivery to endosomes subjects endosomal TLRs 3, 7 and 9 to proteolytic processing by cathepsins and asparagine-endopeptidases, a step that is critical for the ability of these TLRs to recognize their ligands [23]. It is not clear whether the Unc93B1-TLR complex is targeted specifically to endosomes and phagosomes that contain TLR ligand, or whether constitutive endosomal delivery ensures receptor engagement and signaling whenever ligand is detected. Besides Unc93B1, other proteins such as the ER chaperone gp96 and a protein associated with TLR4 (PRAT4A) have been implicated in trafficking TLRs from the ER [23] (Figure 2).
Yet another adaptor protein AP-3 is necessary for the trafficking of TLR7 and TLR9 from endosomes to lysosome-related compartments in plasmacytoid DC [25], where tumor necrosis associated factor 3 (TRAF3) links MyD88 signaling to an IRF7-dependent type I interferon response downstream of these receptors [25,26] (Figure 2). The production of MyD88-NF-κB-dependent cytokines, on the other hand, proceeds independently of AP-3 function [25]. The studies above collectively demonstrate how the signaling outcome of endosomal TLRs is dictated by sorting to proper compartments where proteolytic enzymes and specialized signaling proteins reside.
A similar scenario is observed for TLR4 such that plasma membrane-localized TLR4 recruits MyD88 – via the sorting adaptor TIRAP – and engages MAPK and NF-κB signaling [27,28] while endosomal TLR4 recruits TRIF – via the sorting adaptor TRAM – and initiates an IRF3 dependent type I IFN response [29–32] (Figure 2). Movement of TLR4 from the plasma membrane to endosomal compartments is controlled by CD14, another LPS-binding molecule in the TLR4 complex [33]. After stimulation, CD14 activates Syk and downstream phospholipase γC2 (PLCγ2), and thus promotes TLR4 endocytosis and subsequent IRF3 activation [33,34]. Furthermore, and independently of TLR4 endocytosis, intracellular pools of TLR4 residing in Rab11a-positive endosomes can be recruited to nascent phagosomes to initiate TLR4-TRIF signaling [35]. In conventional DC, AP-3 has also been implicated in trafficking TLR4 and MyD88 to phagosomes, influencing subsequent pro-inflammatory cytokine production but not IFN-β [36]. This is contrary to the role of AP-3 in facilitating TRAF-3 and IRF7 dependent signaling and IFN-β production by plasmacytoid DC, and may be a reflection of cell type specific specialization.
Such a switch in signaling and inflammatory response from one cellular location to another could constitute an important part of the biology of other plasma membrane TLRs. Characterization of the TLR2 dependent type I IFN response of Ly6Chi inflammatory monocytes to the DNA viruses, vaccinia virus and MCMV, implicated the importance of TLR2 internalization for type I IFN but not TNF-α responses [37]. Indeed, surface TLR2 has been shown to be internalized into endosomes [38] and has been observed around phagosomes [39].
A few years ago, microtubule-associated protein 1 light chain 3α (LC3)-associated phagocytosis (LAP) was described as a process where autophagy-related proteins (notably lipidated LC3, a marker of autophagy) are recruited to phagosomes in a TLR dependent manner, accelerating fusion of phagosomes with lysosomes and subsequent degradation of phagocytosed microbes [18]. LAP differs from canonical autophagy where a characteristic double-membrane structure – called an autophagosome – forms within the cytoplasm, recruits lipidated LC3, sequesters cytosolic components or microbes, and eventually fuses with lysosomes for degradation [40]. A recent study demonstrated that LAP is critical for TLR9-MyD88-IRF-7 dependent IFN-α production in response to phagocytosed large DNA-immune complexes [41]. Notably, TLR9 recruitment was normal in the absence of LAP, but phagosomes failed to acquire LAMP-1 impairing acidification and consequent TLR9 activation. Recruitment of TLR9, UNC93B1 and even LC3 to phagosomes were dependent on engagement of Fcγ receptor, and regardless of whether phagocytosed immune complexes contained DNA or not [41]. These findings suggest that surface engagement of Fc receptor may signal specific recruitment of TLR9 (and LC3) to phagosomes [41,42]. Unlike the critical role of AP-3 in TLR9 dependent type I IFN responses to endocytosed CpG-A [25], the response to phagocytosed DNA-immune complexes was unaffected by the absence of AP-3 [41]. Perhaps, the differential roles of LC3 and AP-3 in promoting type I IFN signaling is dictated by whether cargo is phagocytosed or endocytosed, respectively [42] (Figure 2).
Phagocytosis participates in the activation of inflammasomes
During innate immune response to infection, cytosolic PRRs can also be involved in the recognition of microbial derived molecules. While some of these cytosolic receptors trigger subsequent transcriptional initiation of inflammatory immune response genes, others such as AIM2 (a HIN200 protein family member) and distinct members of the Nod-like receptor (NLR) family initiate the assembly of a cytosolic multiprotein complex termed the inflammasome (reviewed in [43]). Core components of inflammasomes are: sensor receptor, adaptor ASC and pro-caspase-1 proteins. When stimulated, inflammasomes activate caspase-1, which undergoes autocatalytic cleavage and in turn cleaves pro-forms of IL-1β and IL-18 leading to secretion of the mature pro-inflammatory cytokines. Active caspase-1 can also induce an inflammatory form of cell death called pyroptosis [43,44]. Six inflammasomes have been identified so far: the NLRP1, NLRP3, NLRC4, NLRP6, NLRP12 and AIM2 inflammasomes [43]. Besides these canonical inflammasomes, a non-canonical cytosolic pathway leading to pyroptosis, HMGB1 and IL-1α secretion proceeds independently of NLRP3/NLRC4 and ASC, and is mediated by caspase-11 in response to distinct stimuli such as cholera toxin B (CTB), E. coli, C. rodentium and V. cholera [45]. This pathway could also lead to caspase-1 cleavage with subsequent IL-1β and IL-18 secretion [45]. The activating ligands of inflammasomes can be brought to the cytoplasm after phagocytosis of microbes [44].
Phagocytosis and the canonical inflammasomes
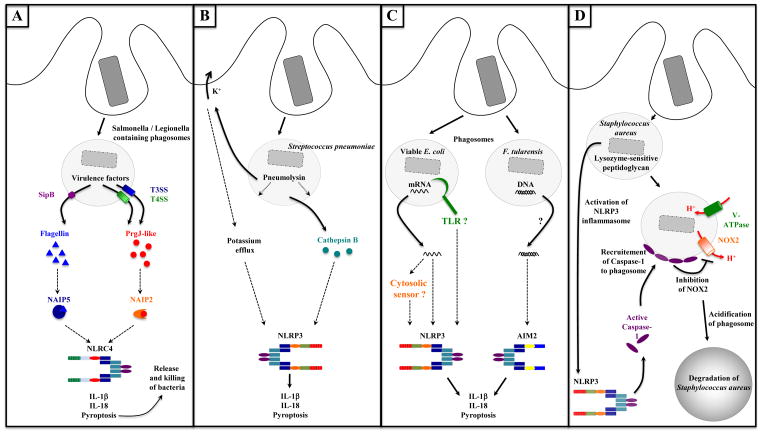
The first indication that phagocytosis enables inflammasome activation came from studies examining the response of macrophages to the activity of virulence factors made by pathogenic bacteria. Phagocytosed Salmonella typhimurium or Legionella pneumophila inject effector proteins into the macrophage cytosol via type III and type IV secretion systems (T3SS and T4SS, respectively) that assemble on host cell membranes in needle-like structures and terminate in a translocation pore [46,47]. Along with effector proteins, T3SS rod proteins and flagellin are also translocated into the cytosol and are responsible for activating the NLRC4 inflammasome [48,49]. Early work had shown that Salmonella deficient in SipB, a T3SS translocon inserted into phagosomal membranes [50,51], were unable to activate caspase-1 cleavage or subsequent pyroptosis [52]. Although the precise mechanism through which bacterial proteins like flagellin access the cytosol has not been pinpointed, in the case of Salmonella, the T3SS translocon SipB may mediate its release, as well as that of other bacterial components, into the cytosol leading to the activation of cytosolic PRRs. Once in the cytosol, flagellin and PrgJ-like proteins do not activate NLRC4 directly, but rather flagellin binds to NAIP5 and PrgJ binds to NAIP2, two other members of the NLR family that are required for activation of the NLRC4 inflammasome [53,54]. How NAIP5 and NAIP2 activate NLRC4 is not known. Activation of these pathways leads to IL-1β and IL-18 production as well as the induction of pyroptosis, which leads to the release of bacteria from macrophages and their subsequent phagocytosis and killing by neutrophils [55] (Figure 3A).
Figure 3. Phagocytosis enables activation of inflammasomes.
A. Activation of the NLRC4 inflammasome through the activity of microbial virulence factors. Phagocytosed Salmonella or Legionella inject proteins into the cytosol of phagocytes via type III and type IV secretion systems (T3SS and T4SS, respectively) or associated translocon proteins such as SipB (from T3SS). Flagellin or PrgJ-like proteins (T3SS rod proteins) are released into the cytosol where they activate the NLRC4 inflammasome. Flagellin binding to NAIP5 and PrgJ binding to NAIP2 are required for NLRC4 activation. Inflammasome-dependent pyroptosis leads to the release of bacteria from macrophages and their subsequent phagocytosis and killing by neutrophils (also the case in panels B, C and D).
B. Activation of the NLRP3 inflammasome by the pore-forming toxin Pneumolysin. Phagocytosed Streptococcus pneumoniae secrete the pore-forming toxin Pneumolysin in phagosomes inducing disruption of phagosomal membranes. Subsequent potassium efflux, or release of the lysosomal protease Cathepsin B into the cytosol can lead to activation of the NLRP3 inflammasome.
C. Activation of NLRP3 and AIM2 inflammasomes by microbial nucleic acids. E. coli mRNA is a signature of microbial viability, which is detected upon or after phagocytosis of bacteria (potentially via a TLR or cytosolic RNA sensor), activates the NLRP3 inflammasome, and triggers IL-1β and IL-18 production and pyroptosis. Phagocytosis of F. tularensis phagocytosis leads to access of bacterial DNA to the cytosol where it activates the AIM2 inflammasome.
D. Interaction between phagosome and NLRP3 inflammasome. The NLRP3 inflammasome can be activated upon phagocytosis of Staphylococcus aureus when its PGN lacks modifications rendering it sensitive to lysozyme degradation. Active caspase-1 has been demonstrated around Staphylococcus-containing phagosomes, where it inhibits the NADPH oxidase NOX2. As a consequence, phagosomes acidify and Staphylococcus degradation in phagosomes is enhanced.
Similar to the translocation of microbial components through T3SS or T4SS, pore-forming toxins (PFT) released by phagocytosed bacterial pathogens can also lead to the formation of pores in phagosomal membranes enabling bacterial components to gain access to the cytosol [56]. So far, it appears that PFT such as Listeriolysin O (LLO) secreted by phagosomal Listeria monocytogenes and pneumolysin secreted by Streptococcus pneumoniae, favor the activation of the NLRP3 inflammasome most likely due to their ability to disrupt phagosomal membranes and perturb levels of intracellular K+ [57,58] (Figure 3B).
In contrast to inflammasome activation in response to the activity of virulence factors like T3SS, T4SS and PFT, activation of the NLRP3 inflammasome – together with induction of a TRIF dependent type I interferon response – is a characteristic immune response to viable, but not dead, avirulent Gram-negative bacteria [59–61]. Bacterial mRNA present in live and lost from dead bacteria is responsible for triggering viability-associated responses leading to its classification as a vita-PAMP [61]. Phagocytosis is the physiological context in which bacterial mRNAs are delivered to the cytosol where they activate the NLRP3 inflammasome leading to pyroptosis and IL-1β secretion [61,62]. Fluorescein-dextran could be demonstrated to gain cytosolic access from phagosomes carrying either live or dead bacteria, and presumably bacterial mRNAs could do so as well [61] (Figure 3C). The mechanism enabling cytosolic access of bacterial mRNAs is not yet clear.
The NLRP3 inflammasome can also be activated in response to meso-diaminopimelic acid (DAP)-type peptidoglycan (PGN) from E. coli or L-Lysine (Lys)-type PGN from Staphylococcus aureus [63,64]. The particulate nature of PGN lends to its phagocytosis by macrophages. Blocking phagocytosis or solubilization of PGN destroyed its ability to activate the NLRP3 inflammasome [64]. Neither the NADPH oxidase nor lysosomal rupture played a role, while inhibiting lysozyme-dependent phagosomal degradation of PGN suppressed IL-1β secretion. Despite the presence of abundant PGN in S. aureus cell walls, these bacteria failed to induce appreciable levels of IL-1β secretion, unless O-acetyltransferase A (OatA), which catalyzes O-acetylation of PGN N-Acetylmuramic acid rendering it lysozyme resistant, was deleted [64]. These data illustrated the importance of phagosomal degradation of PGN upon phagocytosis of S. aureus for triggering subsequent NLRP3 inflammasome activation (possibly again via cytosolic PGN translocation). These observations are in line with the ability of other particulates such as silica, alum, uric acid crystals, and β-amyloid to activate the NLRP3 inflammasome [43,44]. In related observations, active caspase-1 was found to accumulate around Staphylococcus-containing phagosomes upon early NLRP3 inflammasome activation, leading to acidification of phagosomes [65]. Active phagosomal caspase-1 could hydrolyze components of the NOX2, like Rac1 and gp91phox, thus inhibiting the ability of NOX2 to counteract the v-ATPase. As a consequence, phagosomal degradation of Staphylococcus aureus is enhanced leading to NLRP3 inflammasome activation (Figure 3D). Thus, casapse-1 functions here in regulating the pH of phagosomes, and specifically those containing Gram-positive and not Gram-negative bacteria, and independently of its role in processing IL-1β and IL-18. These studies together demonstrate reciprocal regulation of phagocytosis and the NLRP3 inflammasome.
Besides NLRC4 and NLRP3, activation of the AIM2 inflammasome in response to Francisella tularensis was reported to require phagocytosis and phagolysosomal acidification [66,67]. AIM-2 dependent caspase-1 cleavage and pyroptosis were blocked when actin polymerization was blocked with cytochalasin D or phagosomal acidification was inhibited with bafilomycin A and NH4Cl [66]. These effects were not due to decreased bacterial uptake or replication. Notably, Francisella derived DNA was detected in the cytosol of infected cells and co-localized with AIM2 and ASC inducing their oligomerization [66,67]. However, the exact mechanism of DNA delivery into the cytosol was unclear. Because phagosome acidification acts as a cue for escape of F. tularensis into the cytosol, it has been proposed that some cytosolic bacteria lyse during infection releasing DNA into the cytosol [67]. This was based on the observation that AIM2 specks were associated with bright DAPI-staining material, likely leaked DNA, which was present in the vicinity of irregular shaped bacterial remnants [67] (Figure 3C). Notably, a mutant strain of L. pneumophila deficient in a T4SS translocated effector SdhA, which enables establishment of distinct vacuoles that protect Legionella from phagolysosomal fusion, induces elevated caspase-1 cleavage, IL-1β production, and pyroptosis independent of flagellin-NAIP5-NLRC4 and in an AIM2-ASC dependent manner [68]. This correlated with increased cytosolic detection of a Legionella-derived reporter plasmid, implicating a function for SdhA in inhibiting release of Legionella DNA into the cytosol of infected macrophages. Notably, these observations were made in human macrophage cell lines where unlike murine NAIPs, hNAIP does not respond to T3SS rod proteins or flagellin [54]. While hNAIP could turn out to detect other proteins (T3SS needle proteins, for example [54]), DNA-AIM2 rather than flagellin-NLRC4 inflammasome activation may be the dominant response in human cells to flagellated pathogenic bacteria.
Phagocytosis and the non-canonical inflammasome
Unlike the examples discussed above, pyroptosis in response to Burkholderia, a naturally cytosolic Gram-negative bacterial pathogen equipped with both a T3SS and type VI secretion system (T6SS) proceeded independently of all known canonical inflammasomes [69]. While T3SS was necessary for pyroptosis, the virulence-associated T6SS was dispensable suggesting that macrophages detect either phagosomal lysis or bacterial release into the cytosol. Indeed, mutant strains of S. typhimurium and L. pneumophila that expressed neither flagellin (to prevent NLRC4 activation) nor the T3SS and T4SS effectors SifA and SdhA, respectively (to prevent phagosome rupture), induced caspase-11 dependent pyroptosis. Therefore, it appears that caspase-11-mediated pyroptosis is important for inducing a host defence response against cytosolic bacteria such as Burkholderia. Unlike Salmonella and Legionella, Burkholderia do not possess the effectors that inhibit phagosomal rupture, and thus cannot similarly evade caspase-11 activation(Figure 4A).
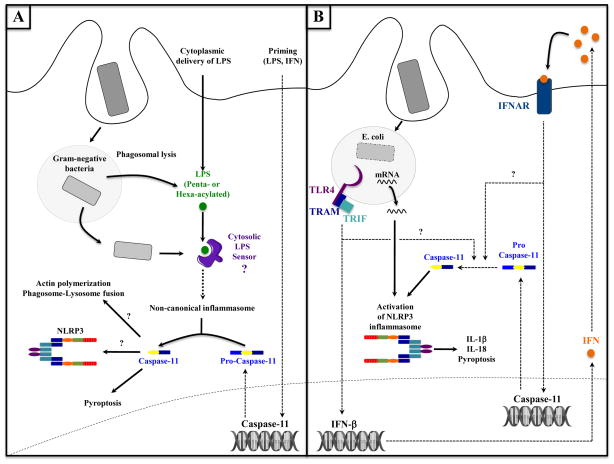
Figure 4. Phagocytosis and activation of non-canonical inflammasome and caspase-11.
A. Activation of the non-canonical inflammasome after phagosomal lysis and leaking of bacterial components. Direct delivery of LPS into the cytosol either experimentally with cholera toxin B or via transfection, or physiologically upon infection with Gram-negative bacteria, enables its detection by an unidentified cytosolic LPS-sensor. This sensor detects specifically hexa- or penta-acylated LPS, and then activates the non-canonical inflammasome pathway and caspase-11 within a few hours following infection. Active caspase-11 then promotes pyroptosis, and could also participate in the activation of the NLRP3 inflammasome or regulate phagosome-lysosome fusion [76]. Note that a priming step is required to induce caspase-11 transcription.
B. Activation of caspase-11 and its role in activation of the NLRP3 inflammasome. Upon phagocytosis of viable E. coli, microbial mRNA induces the assembly and activation of the NLRP3 inflammasome, while TRIF-dependent TLR4 signaling activates the transcription of IFN-β genes. Secreted IFN-β binds to the type I IFN receptor (IFNAR), which induces the transcription of caspase-11 gene. Pro-caspase-11 is then cleaved into active caspase-11 and synergizes with bacterial mRNA in NLRP3 inflammasome. Note that TRIF and IFNAR signaling are involved in late stage (12–16 hours post infection) caspase-11 activation via as yet unidentified steps.
A pathway for caspase-11 mediated pyroptosis and IL-1α secretion that relies on a functional T4SS, but independent of flagellin-NAIP5/NLRC4, was also revealed using ΔFlaA L. pneumophila [70,71]. This pyroptosis proceeded with kinetics that were delayed compared to those induced by flagellin-NAIP5/NLRC4 reflecting the necessity to induce caspase-11 expression. In parallel to the NAIP5/NLRC4 caspase-1 pathway, caspase-11 activation in response to phagocytosed Legionella induced pyroptosis and IL-1α independently of NLRP3, while promoting the canonical NLRP3 dependent pathway of caspase-1 activation. Similar results were obtained with Yersinia pseudotuberculosis that expresses a T3SS but lacks the six known secreted effectors (Δ6 Yp) [71]. These results collectively show the necessity for cytosolic access in activating caspase-11 dependent non-canonical inflammasome pathway independently of the activity of microbial effectors and virulence factors.
A role for caspase-11 has also been shown in orchestrating TRIF-dependent NLRP3 inflammasome activation in response to phagocytosed C. rodentium and enterohemorrhagic E. coli [60]. TRIF dependent IFN-β production downstream of TLR4 engagement by Gram-negative, but not Gram-positive bacteria, led to the subsequent engagement of IFNAR signaling and induction of caspase-11 expression and auto-activation. Active caspase-11 did not regulate assembly of the NLRP3 inflammasome [60], which could be induced in response to bacterial mRNAs released by phagocytosed viable bacteria [61], but rather synergized with the activated NLRP3 inflammasome to mediate IL-1β and IL-18 secretion [60] (Figure 4B). While expression of caspase-11 may be dependent in part on TRIF and IFNAR signaling, it appears the activity of caspase-11 is subject to regulation by an undefined factor, induction of which requires TRIF and IFNAR signaling [72].
Recent studies have shown that caspase-11 dependent pyroptosis could be induced in response to phagocytosed Gram-negative bacteria or LPS that was experimentally delivered into the cytosol via transfection [73,74]. Cholera toxin B naturally translocates a unique serotype of LPS into the cell [74], and presumably LPS from phagosomal Gram-negative bacteria could access the cytosol perhaps by phagosomal rupture, leakage or active translocation. Importantly, these events were preceded by a priming step necessary for inducing caspase-11 expression. The presence of LPS – in particular the hexacylated but not tetraacylated lipid A component – in the cytosol triggered pyroptosis independently of NLRC4 and ASC, and notably in the absence of the classical LPS receptor TLR4. These findings provide compelling evidence for the presence of a new unidentified receptor that senses cytosolic LPS and mobilizes a non-canonical inflammasome pathway of caspase-11 activation [73,74](Figure 4A).
Conclusions
A reciprocal relationship between innate immune signaling and phagocytosis is now evident. It starts from the early steps of phagocytosis where distinct receptors mediating internalization of microbes also engage a cell-autonomous inflammatory response. Phagocytosis enables the activation of certain PRRs, notably TLRs, which signal uniquely from intracellular compartments or induce specific innate immune responses from phagosomes. In turn, signals from TLRs regulate phagocytosis. Moreover, phagocytosis links detection of extracellular microbes to cytosolic PRRs, which trigger inflammasome assembly, while the inflammasome could reciprocally modulate phagosomal function. By many aspects, these interconnections between phagocytosis and innate immune signaling remain intriguing. Deciphering the molecular mechanisms allowing this tight relationship should lead to crucial insights into the regulation of innate immune responses to infection.
Highlights.
Phagocytosis leads to degradation of internalized microbes
Phagocytosis is induced by PRRs, some of which engage immune signaling cascades
Phagocytosis activates endosomal TLRs and subsequent specific signaling pathways
Phagosomal release of microbial nucleic acids or proteins activates inflammasomes
PRR signaling and activated inflammasomes modulate phagosome maturation
Acknowledgments
This work was supported by NIH NIAID grant AI095245 and a Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease award to JMB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torchinsky MB, Garaude J, Blander JM. Infection and apoptosis as a combined inflammatory trigger. Curr Opin Immunol. 2010;22:55–62. doi: 10.1016/j.coi.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair P, Amsen D, Blander JM. Co-ordination of incoming and outgoing traffic in antigen-presenting cells by pattern recognition receptors and T cells. Traffic. 2011;12 :1669–1676. doi: 10.1111/j.1600-0854.2011.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Blander JM. Signalling and phagocytosis in the orchestration of host defence. Cell Microbiol. 2007;9:290–299. doi: 10.1111/j.1462-5822.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 5.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazi U, Martinez-Pomares L. Influence of the mannose receptor in host immune responses. Immunobiology. 2009;214:554–561. doi: 10.1016/j.imbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell Microbiol. 2009;11:1160–1169. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Marjon KD, Mold C, Du Clos TW, Sun PD. Pentraxins and Fc receptors. Immunol Rev. 2012;250:230–238. doi: 10.1111/j.1600-065X.2012.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodridge HS, Underhill DM, Touret N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic. 2012;13:1062–1071. doi: 10.1111/j.1600-0854.2012.01382.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 11.Kingeter LM, Lin X. C-type lectin receptor-induced NF-kappaB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9:105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. This seminal work was the first to demonstrate that phagocytosis could be regulated by TLR signals, which lead to enhanced kinetics of phagolysosomal fusion in a MyD88- and p38 MAPK-dependent manner. [DOI] [PubMed] [Google Scholar]
- 13.West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 14**.Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. Refs. 13 and 14 are notable works demonstrating the regulation of macropinocytosis by TLR4 signals specifically via the MAPK Rsk. [DOI] [PubMed] [Google Scholar]
- 15.Tissieres P, Dunn-Siegrist I, Schappi M, Elson G, Comte R, Nobre V, Pugin J. Soluble MD-2 is an acute-phase protein and an opsonin for Gram-negative bacteria. Blood. 2008;111:2122–2131. doi: 10.1182/blood-2007-06-097782. [DOI] [PubMed] [Google Scholar]
- 16.Laroux FS, Romero X, Wetzler L, Engel P, Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- 17.Vulcano M, Dusi S, Lissandrini D, Badolato R, Mazzi P, Riboldi E, Borroni E, Calleri A, Donini M, Plebani A, et al. Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J Immunol. 2004;173:5749–5756. doi: 10.4049/jimmunol.173.9.5749. [DOI] [PubMed] [Google Scholar]
- 18**.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. This pioneer work identifies LC3-associated phagocytosis. It demonstrates that some components of the autophagy machinery (such as LC3) are recruited to phagosomes, therefore facilitating fusion with lysosomes and enhancing degradation of phagosomal contents. [DOI] [PubMed] [Google Scholar]
- 19.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 20.Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 21.Kotsias F, Hoffmann E, Amigorena S, Savina A. Reactive oxygen species production in the phagosome: impact on antigen presentation in dendritic cells. Antioxid Redox Signal. 2013;18:714–729. doi: 10.1089/ars.2012.4557. [DOI] [PubMed] [Google Scholar]
- 22.Brencicova E, Diebold SS. Nucleic acids and endosomal pattern recognition: how to tell friend from foe? Front Cell Infect Microbiol. 2013;3:37. doi: 10.3389/fcimb.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12:168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. This paper shows that TLR9 trafficking from endosomes to specialized lysosome-related compartments is required for the IRF7-dependent type I interferon production induced by TLR9, and identifies AP-3 as the protein required for this trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 29.Doyle S, Vaidya S, O’Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 30**.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9 :361–368. doi: 10.1038/ni1569. This paper shows that TRIF-dependent TLR4 signaling occurs in endosomes and induces an IFN-β response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. This paper identifies TRIF as the TLR3- and TLR4-associated adaptor responsible for the IRF3-dependent type I interferon response. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 33**.Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147:868–880. doi: 10.1016/j.cell.2011.09.051. This paper shows the critical role for CD14 - one of the LPS-binding protein of the TLR4 complex - in the microbe-induced internalization of TLR4. Along with Ref. 34, this paper further demonstrates the involvement of Syk and downstream phospholipase γC2 in this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang CY, Veckman V, Limmer K, David M. Phospholipase Cgamma-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation. J Biol Chem. 2012;287:3704–3709. doi: 10.1074/jbc.C111.328559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Husebye H, Aune MH, Stenvik J, Samstad E, Skjeldal F, Halaas O, Nilsen NJ, Stenmark H, Latz E, Lien E, et al. The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity. 2010;33 :583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantegazza AR, Guttentag SH, El-Benna J, Sasai M, Iwasaki A, Shen H, Laufer TM, Marks MS. Adaptor protein-3 in dendritic cells facilitates phagosomal toll-like receptor signaling and antigen presentation to CD4(+) T cells. Immunity. 2012;36 :782–794. doi: 10.1016/j.immuni.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsen NJ, Deininger S, Nonstad U, Skjeldal F, Husebye H, Rodionov D, von Aulock S, Hartung T, Lien E, Bakke O, et al. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84:280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 40.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 41**.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. This work is notable because it identifies a role for LAP in facilitating TLR9 dependent IFN-α signaling. It presents evidence for how DNA-immune complexes engage surface FcγR and signal both LC3 and TLR9 phagosomal recruitment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Blander JM. Designing a type I interferon signaling phagosome. Immunity. 2012;37 :947–949. doi: 10.1016/j.immuni.2012.11.009. This commentary about the work in Ref. 41 discusses the possiblity that TLR9 recruitment to phagosomes is regulated taking place in conjunction with a signal from a surface receptor such as FcγR. [DOI] [PubMed] [Google Scholar]
- 43.Bauernfeind F, Hornung V. Of inflammasomes and pathogens--sensing of microbes by the inflammasome. EMBO Mol Med. 2013;5:814–826. doi: 10.1002/emmm.201201771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 48.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 49**.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. Together with Ref. 48, this work shows that detection of cytosolic flagellin leads to NLRC4 (IPAF)-dependent caspase-1 activation after Salmonella infection. [DOI] [PubMed] [Google Scholar]
- 50.Collazo CM, Galan JE. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 51.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 53.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54**.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. This study along with Ref. 53 identifies NAIP5 and NAIP2 proteins as the respective cytosolic receptors for Salmonella flagellin and PrgJ-like proteins, required for NLRC4 inflammasome activation. [DOI] [PubMed] [Google Scholar]
- 55.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 58**.McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010;6:e1001191. doi: 10.1371/journal.ppat.1001191. This work shows that Pneumolysin secreted by Streptococcus pneumoniae synergizes with TLRs to secrete inflammatory cytokines, and is further responsible for activation of NLRP3 inflammasome and subsequent IL-1β secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blander JM, Sander LE. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat Rev Immunol. 2012;12:215–225. doi: 10.1038/nri3167. [DOI] [PubMed] [Google Scholar]
- 60**.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. This study identifies TRIF and IFNAR dependent caspase-11 transcription as a necessary step in the activation of NLRP3 in response to Gram-negative bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Muller M, Blander JM. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–389. doi: 10.1038/nature10072. In this landmark study, the innate immune system is shown to directly sense bacterial viability by detecting bacterial mRNA. Bacterial mRNA is classified as a vita-PAMP signaling via TRIF. First evidence for a link between TRIF and NLRP3 inflammasome activation is presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. Together with Ref. 61, bacterial mRNA is shown to activate the NLRP3 inflammasome. In Ref. 77, this is shown to occur physiologically after phagocytosis of live avirulent Gram-negative bacteria. [DOI] [PubMed] [Google Scholar]
- 63.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14 :1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 64**.Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Gotz F, et al. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe. 2010;7:38–49. doi: 10.1016/j.chom.2009.12.008. This work is notable because it shows that the Gram-positive bacterium Staphylococcus aureus can only trigger activation of the NLRP3 inflammasome when its peptidoglycan is rendered sensitive to degradation within phagosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Sokolovska A, Becker CE, Ip WK, Rathinam VA, Brudner M, Paquette N, Tanne A, Vanaja SK, Moore KJ, Fitzgerald KA, et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat Immunol. 2013;14:543–553. doi: 10.1038/ni.2595. This work shows that active caspase-1 is recruited to Gram-positive but not Gram-negative bacteria-containing phagosomes, where it inhibits the NOX2 NADPH oxidase and thereby enhances phagosome acidification and bacterial degradation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ge J, Gong YN, Xu Y, Shao F. Preventing bacterial DNA release and absent in melanoma 2 inflammasome activation by a Legionella effector functioning in membrane trafficking. Proc Natl Acad Sci U S A. 2012;109:6193–6198. doi: 10.1073/pnas.1117490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, Bradley WP, Fung TC, Flavell RA, Brodsky IE, et al. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog. 2013;9 :e1003400. doi: 10.1371/journal.ppat.1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. Together with Ref. 73, this paper demonstrates that cytosolic LPS can be detected by an unidentified sensor and thereby activates a caspase-11-dependent non-canonical inflammasome leading to pyroptosis. [DOI] [PubMed] [Google Scholar]
- 75.Tzircotis G, Braga VM, Caron E. RhoG is required for both FcgammaR- and CR3-mediated phagocytosis. J Cell Sci. 2011;124:2897–2902. doi: 10.1242/jcs.084269. [DOI] [PubMed] [Google Scholar]
- 76.Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, Abdelaziz DH, Voss OH, Doseff AI, Hassan H, Azad AK, et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity. 2012;37:35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]