Abstract
Dicer is a multifunctional protein that is essential across species for the generation of microRNAs, a function that is highly conserved across the plant and animal kingdoms. Intriguingly, Dicer exhibits antiviral functions in lower organisms including Drosophila melanogaster and Caenorhabditis elegans. Antiviral activity occurs via small interfering RNA production following cytoplasmic sensing of viral dsRNA. Notably, such antiviral activity has not yet been clearly demonstrated in higher organisms such as mammals. Here, we review the evidence for Dicer as an innate antiviral across species.
Introduction
The discovery of Toll in Drosophila melanogaster and the subsequent characterization of the mammalian Toll-like receptors illustrated how ancient and highly conserved the pathways of innate immunity truly are. Orthologous pattern recognition receptors have since been found in species as evolutionarily divergent as plants and humans, highlighting the importance and evolutionary age of innate immune proteins. Likewise, Dicer and RNA interference (RNAi) are crucial components of a conserved antiviral response in a diverse array of eukaryotic organisms including D. melanogaster (reviewed in [1]). Dicer is a large, multi-domain enzyme whose RNase III activity is responsible for producing small RNA species from larger precursors. Specifically, Dicer is the enzyme responsible for cleaving pre-miRNAs into mature miRNAs and dsRNAs into siRNAs, and is thus a crucial component of the RNAi gene regulatory network.
Dicer is an indispensible antiviral protein in many organisms including plants and invertebrate animals. In these lower eukaryotes, Dicer is directly involved in binding and cleaving foreign dsRNA, which includes virus-derived RNA, and loading it into the host organism’s RNAi pathway and thereby mediating viral suppression. In this way, Dicer acts as a sensor of viral RNA, capable of inducing a specific and directed antiviral response.
The structure of Dicer is highly conserved across animals [2] (Figure 1). Yet relatively little is known about Dicer’s antiviral capacity in mammals. In this review, we discuss how Dicer functions as a cytoplasmic sensor of viral RNA in the model organisms Caenorhabditis elegans and D. melanogaster, and discuss recent evidence that suggests that mammalian Dicer may act similarly.
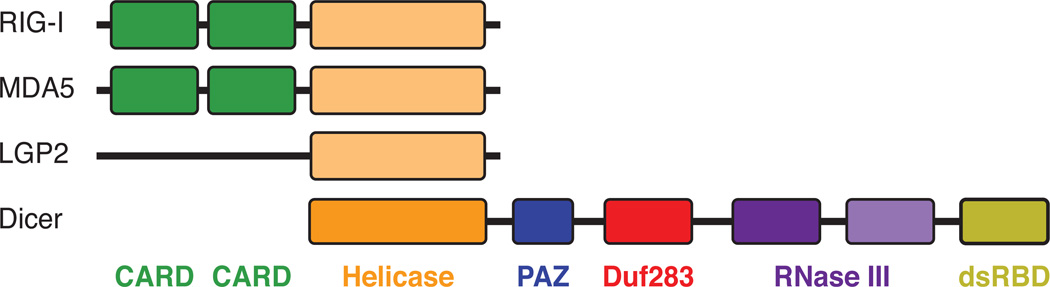
Figure 1. Conservation of Dicer domains across species.
The domain organization of Dicer from Caenorhabditis elegans, Drosophila melanogaster, and Homo sapiens. The domain structure of Dicer proteins from each of the species is highly conserved. D. melanogaster has two Dicer molecules: Dicer-1 lacks a functional helicase domain and is responsible for processing pre-miRNAs into mature miRNAs, and Dicer-2 has a functional helicase domain and is responsible for processing dsRNAs into siRNAs. For this reason, Dicer-2 selectively handles antiviral RNAi processing. DExD/H Helicase, a highly conserved helicase domain; PAZ, Piwi Argonaute Zwille domain; DUF283, Domain of Unknown Function 283; RNase III; dsRBD, double-stranded RNA-Binding Domain.
Small RNA antiviral immunity in invertebrates
Worms and insects can detect, process, and target foreign RNA using their RNAi pathways. While some important differences exist between these species, the general RNAi pathway is well conserved. Foreign RNA is detected in the cytoplasm and cleaved by the RNA-processing enzyme Dicer into small RNAs. These Dicer-derived small RNAs are then incorporated into RNA-induced silencing complexes (RISCs) and used to produce a direct and specific RNAi response against other RNA from the same virus.
In C. elegans, virus-derived dsRNA is cleaved by Dicer to create primary viral small interfering RNAs (vsiRNAs). Dicer’s DExD/H helicase domain appears to be important for the processing of siRNA from a dsRNA precursor, while being dispensable in the processing of pre-miRNAs into miRNAs [3,4]. These primary vsiRNAs are then thought to recruit RNA-dependent RNA polymerase (RdRP) complexes to viral RNA [5–7]. RdRP complexes produce large amounts of single-stranded anti-sense secondary vsiRNA, which are then incorporated in RISCs, where they can target viral RNA for silencing [8]. Worms lacking an active Dicer-dependent RNAi pathway are susceptible to infection by several natural and non-natural viral pathogens, including single-stranded RNA viruses such as insect Flock house virus, mammalian vesicular stomatitis virus (VSV), and Orsay virus, which naturally infects C. elegans [9–13].
In contrast to C. elegans, D. melanogaster possesses two homologous yet functionally distinct Dicer molecules, Dicer-1 and Dicer-2. Dicer-1, which lacks a functional DExD/H helicase domain, is responsible for processing hairpin-structured pre-miRNAs, while Dicer-2, which has a functional DExD/H helicase domain, processes dsRNA substrates and therefore selectively handles antiviral RNAi processing [14,15]. In addition, production of vsiRNAs in D. melanogaster occurs without RdRP and secondary vsiRNAs. The overall antiviral pathway of Dicer-2 in D. melanogaster closely resembles that of C. elegans. Viral dsRNA is cleaved by Dicer-2 to create vsiRNAs, which are incorporated into RISCs, where they then can target viral RNA for inhibition [16,17]. Dicer-2 has been shown to induce the expression of certain antiviral genes following infection in flies [18].
Without a functional Dicer-2, flies succumb rapidly to infection by a number of viral pathogens, demonstrating the importance of this Dicer-2-mediated antiviral response [15–17,19,20].
Dicer proteins clearly function as important mediators of the cytoplasmic antiviral response in both C. elegans and D. melanogaster. In both organisms, an active helicase domain allows Dicer to process non-pre-miRNA substrates, i.e., RNA molecules missing the 2-nt 3’ overhang characteristic of pre-miRNAs, while also allowing it to function processively, cleaving off small RNA molecules as it moves along the length of dsRNA [3,4]. Collectively, these studies indicate that Dicer’s helicase domain is important for Dicer to function in a dsRNA-specific reaction for antiviral responses in both worms and flies.
Dicer's role in mammalian innate immunity against viruses
Evidence that mammalian Dicer may function as a viral RNA sensor has been steadily increasing. Mammalian Dicer is closely related to the Dicer enzymes in C. elegans and D. melanogaster (Figure 1). As in these other species, the mammalian Dicer enzyme processes both pre-miRNAs and dsRNAs into mature miRNAs and siRNAs, respectively [21]. Mammalian Dicer has a DExH/D helicase domain, a Piwi Argonaute Zwille (PAZ) domain, a Domain of Unknown Function 283 (DUF283), a double-stranded RNA binding domain (dsRBD), and two RNase III domains. The RNase III domains are each responsible for cleaving a strand of substrate RNA, while the PAZ domain binds to the 5’ phosphate and 3’ end of these substrate RNAs and positions them properly for cleavage within the enzyme [22]. The DUF283 and dsRBD domains are likely important in binding to RNA substrates [23,24]. Even though the RNA helicase domain appears to be dispensable for pre-miRNA processing, it is essential for binding and processing dsRNA substrates and for binding the Dicer-partner proteins TAR RNA-binding protein (TRBP) and protein kinase RNA activator (PACT) [4,25].
How can mammalian Dicer act as an antiviral?
Many herpesviruses produce miRNAs that can regulate host or viral gene expression. In addition, viral infection induces significant changes in host miRNA expression (reviewed by [26,27]). These miRNAs may have direct antiviral functions or may target the antiviral immune response. Following infection, specific miRNA expression patterns have been observed, suggesting virus-specific and tissue-specific miRNA responses to both DNA and RNA viruses [28,29]. Furthermore, insertion of endogenous miRNA target sites into a viral genome can attenuate virus replication, demonstrating that host miRNAs have the capacity to interfere with viral RNA [30]. RNA viruses can also be engineered to express virus-derived cytosolic pri-miRNA which are processed into functional miRNA via a non-canonical, Dicer- and Drosha-dependent mechanism [31,32]. Both virus-derived and host-derived miRNAs have the potential to regulate viral RNA levels. Thus, by carrying out its canonical and non-canonical functions as the mature miRNA-processing enzyme, mammalian Dicer does affect the expression of viral and antiviral proteins (Figure 2A).
Figure 2. Three potential mechanisms by which Dicer could be involved in the innate antiviral response.
A. Dicer is the key miRNA-producing enzyme and affects innate signaling pathways via its role in miRNA production.
B. Dicer can process virus-derived RNA and feed it into the RNAi pathway, resulting in direct interference of viral transcripts or viral genomes. Dicer is known to cleave dsRNA and Dicer-cleavage products have been found in cells infected with various viruses.
C. Dicer functions as part of larger viral-RNA bound detection complex along with the RLRs. These proteins all have helicase domains that bind to dsRNA. By forming a complex with RLRs, Dicer may enhance their antiviral effects.
RISC, RNA-induced silencing complex; LGP2, laboratory of genetics and physiology-2; IFN, interferon.
Mammalian Dicer may also exhibit a miRNA-independent antiviral function. Dicer has the potential to generate small virus-derived RNAs (Figure 2B). In fact, RNA molecules resembling Dicer-cleavage products have been found in mammalian cells infected with a wide array of human viruses [28,33–36]. However, levels of viral small RNAs (vsRNAs) are often very low and sRNA silencing of viruses has not been observed in mammalian cells suggesting that sRNA-mediated gene silencing as an anti-viral strategy is limited to non-chordates (reviewed in [26]). Two exciting recent reports demonstrate that Dicer-cleavage products of viral RNA are produced at significant levels in certain virus-infected mammalian cells. Li et al. found significant amounts of vsRNAs in baby hamster kidney cells and suckling mice infected with a mutant strain of Nodamura virus that lacks B2, a suppressor of RNAi [37]. Many of these small RNAs mapped to the ends of the viral RNA genome and had the length and 2-nt overhang hallmarks of Dicer products. Maillard et al. showed a similar accumulation of vsRNAs following the infection of mouse embryonic stem cells (mESC) with encephalomyocarditis virus or the same mutant Nodamura virus [38]. These RNAs were generated in a Dicer-dependent manner, and were found to associate with RISC proteins. Upon differentiation, mESC lost the capacity to accumulate these virus-derived small RNAs. Further studies will reveal the function and effect of these small RNAs and elucidate whether they represent a cell-type specific mammalian antiviral RNAi response.
Mammalian Dicer could also directly interact with the RIG-I-like receptor (RLR) viral-RNA sensing pathway (Figure 2C). Mammalian Dicer possesses a conserved and functional DExH/D helicase domain that is evolutionarily related to the RNA helicase domains found on RLRs [39] (Figure 3). These RLRs are a family of cytoplasmic antiviral sensors – retinoic acid-inducible gene 1 (RIG-I), melanoma differentiation-associated gene 5 (MDA5), and laboratory of genetics and physiology-2 (LGP2) – each of which is critical in detecting and responding to foreign RNA within the cytoplasm (reviewed by [40]). Recent evidence suggests that Dicer can differentially bind dsRNA and pre-miRNA, with dsRNA being an important byproduct of infection by RNA and DNA viruses [41]. One potential way in which Dicer acts as an antiviral is as part of a dsRNA-detection complex interacting with one or all of the RLRs through their binding to viral RNAs. For example, by forming a complex with LGP2, Dicer could promote MDA5 or RIG-I signaling, thus enhancing interferon (IFN) induction following viral infection. LGP2 has been shown to directly interact with mammalian Dicer. Proteomic analysis of human type I IFN inducing pathways in a HEK293 cell over-expression system has revealed that human Dicer does associate with LGP2 following stimulation with the dsRNA analogue polyI:C [42].
Figure 3. The domain organization of the human retinoic-acid-inducible-gene-1-like receptors (RIG-I-like receptors, or RLRs) and human Dicer.
Mammalian Dicer shares a DExD/H helicase domain with the RLR family of cytosolic RNA sensors. The RLR family consists of RIG-I (retinoic acid-inducible gene 1), MDA5 (melanoma differentiation-associated gene 5), and LGP2 (laboratory of genetics and physiology-2). CARD, caspase activation and recruitment domain; Helicase, DExD/H helicase domain; PAZ, Piwi Argonaute Zwille domain; DUF283, Domain of Unknown Function 283; RNase III; dsRBD, double-stranded RNA-binding domain.
Interestingly, Dicer is known to interact with orthologous DExD/H helicases in C. elegans, which are critical components of the antiviral RNAi response [43]. In C. elegans, the RLR orthologue and Dicer-associated protein, Dicer-related helicase 1 (DRH-1), is essential for the Dicer-dependent cleavage of viral dsRNA into primary vsiRNAs. Guo et al. recently demonstrated that worms lacking DRH-1 are unable to mount an antiviral RNAi response against Orsay virus [44]. However, the antiviral RNAi response in these worms can be rescued with a chimeric DRH-1 protein consisting of the helicase and C-terminal domains of human RIG-I and a worm-specific N-terminal domain [44]. These data suggest that DRH-1 may act as a viral sensor.
Further investigation is needed to determine if human Dicer interacts with, recruits, or requires different partner proteins during its processing of dsRNA when compared to pre-miRNAs, and/or whether its interaction with known viral RNA sensors like LGP2 is dependent upon a dsRNA ligand or other proteins as part of a larger complex. Numerous viral proteins have been found to directly inhibit Dicer and other members of the RNAi pathway (reviewed in [45]). Together these findings suggest that Dicer does more than simply process miRNAs during the course of a viral infection.
What models are available for studying mammalian Dicer?
Investigating the role of Dicer in mammalian innate immunity has been complicated by the fact that Dicer is an essential gene required for embryogenesis and cellular homeostasis. Knocking out Dicer in mice results in embryonic lethality due to a defect in angiogenesis [46]. Likewise, knocking out Dicer in cells leads to senescence [47]. This can be overcome by SV40 transformation of Dicer knock-out cell lines [48]. Such knockout cell lines have been used in seminal studies to understand Dicer function [28,30,49]. However SV40 encodes its own miRNAs that could complicate results [50]. For these reasons, Dicer’s specific contribution to the antiviral response in mammals has been difficult to investigate.
Otsuka et al. developed and characterized a unique Dicer hypomorphic mouse that is able to escape embryonic lethality. Created by inserting a gene-trap element into the Dicer gene, this Dicer hypomorphic mouse expresses Dicer during embryogenesis. However, once full-grown, the gene-trap mice exhibit significantly reduced levels of Dicer expression in all tissues examined – apart from the testes – making it a useful tool for probing the function of Dicer in the context of viral infection [51]. Dicer hypomorphic mice come with several limitations. First, they are not a true knockout since cells retain the ability to express Dicer, albeit at reduced levels. Second, the gene-trapped allele has the potential to express a truncated N-terminal form of the Dicer protein – despite lacking an active RNase domain, this could still retain other functionalities of the N-terminal helicase and/or DUF domains.
Dicer hypomorphic mice have been found to be more susceptible to infection by several viruses. These mice exhibit decreased survival to infection with VSV and produce higher titers of virus in comparison to wild-type mice [51]. Macrophages collected from the Dicer hypomorphic and wild-type mice were shown to produce equivalent levels of type I IFN following infection with VSV. Type I IFN levels in the animals following VSV infection were not shown [51]. Like VSV, murine cytomegalovirus (mCMV) results in increased mortality in Dicer hypomorphic mice along with elevated levels of viral replication. However, infection of Dicer hypomorphic mice with mCMV induced significantly lower levels of IFN-β protein in the serum and interferon-stimulated genes (ISG) in the spleen compared to wild-type mice [52]. Further analysis of splenocytes from Dicer hypomorphic mice showed a substantial dysregulation of many ISGs, even before infection [52]. The different type I IFN findings in these two infection models might be due to different levels of expression of wild-type Dicer from the gene-trapped allele in the cell types examined. Alternatively, Dicer could have a tissue- or cell-specific effect on the IFN response.
Knockdown studies in cell lines have also demonstrated a role for mammalian Dicer in controlling virus infections. A study by Matskevich and Moelling investigated the effect of Dicer knockdown on influenza A viral replication in interferon-unresponsive, monkey-derived Vero cells and in A549 human lung adenocarcinoma cells. Knockdown of Dicer increased viral replication in both Vero cells and also in A549 cells following antibody neutralization of their type I IFN response [53]. Similarly, knockdown of Dicer in Huh7 cells resulted in increased replication of dengue virus [1,54]. Whether the antiviral effect in Dicer knockdown cells is mediated by miRNA processing or a more direct antiviral functionality needs additional clarification.
Conclusion
Collectively, a number of important studies demonstrating altered viral susceptibility in Dicer-deficient systems suggest that mammalian Dicer plays a crucial role in the antiviral response by directly curbing viral replication and/or inducing a type I IFN response. Dicer’s role in miRNA processing is well known and vitally important for regulating essentially every pathway within a given cell. Yet Dicer still remains an enigmatic protein, and any roles of mammalian Dicer beyond miRNA production are difficult to discern. Recent studies have begun to elucidate a role for Dicer in antiviral pathways that is distinct from its miRNA-processing functionality. Mammalian Dicer was found to interact with dsRNA in a different way than it interacts with miRNA precursors, further suggesting that Dicer may interact uniquely with virus-derived RNA. Evidence also suggests that Dicer and RLR pathways intersect at the protein level, and that Dicer may participate in the assembly and/or function of the RLR antiviral sensor complex.
Future studies will reveal if mammalian Dicer indeed functions as a direct protein-mediator of antiviral innate immunity.
Highlights.
Dicer has important antiviral functions in D. melanogaster and C. elegans
Dicer binds to dsRNA
Models of mammalian Dicer are in development
Mammalian Dicer interacts with homologous antiviral RLRs
Dicer is highly conserved among organisms
Acknowledgments
We would like to thank Dr. Melanie Trombly for her assistance in preparing this manuscript. This work was supported by NIH grants T32AI095213 to CRM, R01AI092105 to JPW, and P01AI083215 to EKJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Ding S-W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010;10:632–644. doi: 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee K, Campos H, Kolaczkowski B. Evolution of animal and plant dicers: early parallel duplications and recurrent adaptation of antiviral RNA binding in plants. Mol. Biol. Evol. 2013;30:627–641. doi: 10.1093/molbev/mss263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welker NC, Pavelec DM, Nix DA, Duchaine TF, Kennedy S, Bass BL. Dicer's helicase domain is required for accumulation of some, but not all, C. elegans endogenous siRNAs. RNA. 2010;16:893–903. doi: 10.1261/rna.2122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Welker NC, Maity TS, Ye X, Aruscavage PJ, Krauchuk AA, Liu Q, Bass BL. Dicer's helicase domain discriminates dsRNA termini to promote an altered reaction mode. Mol. Cell. 2011;41:589–599. doi: 10.1016/j.molcel.2011.02.005. The authors present evidence that the Dicer enzymes of D. melanogaster and C. elegans interact with dsRNAs in a different manner than pre-miRNAs.
- 5.Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 6.Sijen T, Steiner FA, Thijssen KL, Plasterk RHA. Secondary siRNAs result from unprimed RNA synthesis and form a distinct class. Science. 2007;315:244–247. doi: 10.1126/science.1136699. [DOI] [PubMed] [Google Scholar]
- 7.Pak J, Fire A. Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science. 2007;315:241–244. doi: 10.1126/science.1132839. [DOI] [PubMed] [Google Scholar]
- 8. Lu R, Yigit E, Li W-X, Ding S-W. An RIG-I-Like RNA helicase mediates antiviral RNAi downstream of viral siRNA biogenesis in Caenorhabditis elegans. PLoS Pathog. 2009;5:e1000286. doi: 10.1371/journal.ppat.1000286. Using cryo-electron microscopy and domain-specific labeling, the authors were able to map the domains of human Dicer and establish a structural image of Dicer that fits experimental findings.
- 9.Lu R, Maduro M, Li F, Li HW, Broitman-Maduro G, Li WX, Ding SW. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature. 2005;436:1040–1043. doi: 10.1038/nature03870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkins C, Dishongh R, Moore SC, Whitt MA, Chow M, Machaca K. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature. 2005;436:1044–1047. doi: 10.1038/nature03957. [DOI] [PubMed] [Google Scholar]
- 11.Schott DH, Cureton DK, Whelan SP, Hunter CP. An antiviral role for the RNA interference machinery in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18420–18424. doi: 10.1073/pnas.0507123102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Zhang R, Wang J, Lu R. Antiviral RNA silencing initiated in the absence of RDE-4, a double-stranded RNA binding protein, in Caenorhabditis elegans. J. Virol. 2013;87:10721–10729. doi: 10.1128/JVI.01305-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Félix M-A, Ashe A, Piffaretti J, Wu G, Nuez I, Bélicard T, Jiang Y, Zhao G, Franz CJ, Goldstein LD, et al. Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS Biol. 2011;9:e1000586. doi: 10.1371/journal.pbio.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct Roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA Silencing Pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 15.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler J-L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat. Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- 16.van Rij RP, Saleh M-C, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaste. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X-H, Aliyari R, Li W-X, Li H-W, Kim K, Carthew R, Atkinson P, Ding S-W. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, Imler J-L. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat. Immunol. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- 19. Kemp C, Mueller S, Goto A, Barbier V, Paro S, Bonnay F, Dostert C, Troxler L, Hetru C, Meignin C, et al. Broad RNA interference-mediated antiviral immunity and virusspecific inducible responses in Drosophila. J. Immunol. 2013;190:650–658. doi: 10.4049/jimmunol.1102486. Demonstrates a virus-specific requirement of Dicer-2 and the Jak/STAT pathway in the protection of Drosophila from infection.
- 20.Sabin LR, Zheng Q, Thekkat P, Yang J, Hannon GJ, Gregory BD, Tudor M, Cherry S. Dicer-2 processes diverse viral RNA species. PLoS ONE. 2013;8:e55458. doi: 10.1371/journal.pone.0055458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Kolb FA, Brondani V, Billy E, Filipowicz W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002;21:5875–5885. doi: 10.1093/emboj/cdf582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau P-W, Guiley KZ, De N, Potter CS, Carragher B, MacRae IJ. The molecular architecture of human Dicer. Nat. Struct. Mol. Biol. 2012;19:436–440. doi: 10.1038/nsmb.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dlakić M. DUF283 domain of Dicer proteins has a double-stranded RNA-binding fold. Bioinformatics. 2006;22:2711–2714. doi: 10.1093/bioinformatics/btl468. [DOI] [PubMed] [Google Scholar]
- 24.Wostenberg C, Lary JW, Sahu D, Acevedo R, Quarles KA, Cole JL, Showalter SA. The role of human Dicer-dsRBD in processing small regulatory RNAs. PLoS ONE. 2012;7:e51829. doi: 10.1371/journal.pone.0051829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y, Hur I, Park S-Y, Kim Y-K, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.tenOever BR. RNA viruses and the host microRNA machinery. Nat. Rev. Microbiol. 2013;11:169–180. doi: 10.1038/nrmicro2971. [DOI] [PubMed] [Google Scholar]
- 27.Cullen BR. MicroRNAs as mediators of viral evasion of the immune system. Nat. Immunol. 2013;14:205–210. doi: 10.1038/ni.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parameswaran P, Sklan E, Wilkins C, Burgon T, Samuel MA, Lu R, Ansel KM, Heissmeyer V, Einav S, Jackson W, et al. Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog. 2010;6:e1000764. doi: 10.1371/journal.ppat.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng X, Gralinski L, Ferris MT, Frieman MB, Thomas MJ, Proll S, Korth MJ, Tisoncik JR, Heise M, Luo S, et al. Integrative deep sequencing of the mouse lung transcriptome reveals differential expression of diverse classes of small RNAs in response to respiratory virus infection. MBio. 2011;2:e00198-11. doi: 10.1128/mBio.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez JT, Pham AM, Lorini MH, Chua MA, Steel J, tenOever BR. MicroRNA-mediated species-specific attenuation of influenza A virus. Nat. Biotechnol. 2009;27:572–576. doi: 10.1038/nbt.1542. [DOI] [PubMed] [Google Scholar]
- 31.Langlois RA, Shapiro JS, Pham AM, tenOever BR. In vivo delivery of cytoplasmic RNA virus-derived miRNAs. Mol. Ther. 2012;20:367–375. doi: 10.1038/mt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro JS, Langlois RA, Pham AM, tenOever BR. Evidence for a cytoplasmic microprocessor of pri-miRNAs. RNA. 2012;18:1338–1346. doi: 10.1261/rna.032268.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schopman NCT, Willemsen M, Liu YP, Bradley T, van Kampen A, Baas F, Berkhout B, Haasnoot J. Deep sequencing of virus-infected cells reveals HIV-encoded small RNAs. Nucleic Acids Res. 2012;40:414–427. doi: 10.1093/nar/gkr719. Discovery and mapping of HIV-derived small RNAs, and a demonstration that antagonism of some of these small RNAs results in increased viral replication.
- 34.Xu N, Segerman B, Zhou X, Akusjärvi G. Adenovirus virus-associated RNAII-derived small RNAs are efficiently incorporated into the RNA-induced silencing complex and associate with polyribosomes. J. Virol. 2007;81:10540–10549. doi: 10.1128/JVI.00885-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aparicio O, Razquin N, Zaratiegui M, Narvaiza I, Fortes P. Adenovirus virus-associated RNA is processed to functional interfering RNAs involved in virus production. J. Virol. 2006;80:1376–1384. doi: 10.1128/JVI.80.3.1376-1384.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson MG, Haasnoot PCJ, Xu N, Berenjian S, Berkhout B, Akusjärvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J. Virol. 2005;79:9556–9565. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, Lu J, Han Y, Fan X, Ding S-W. RNA Interference Functions as an Antiviral Immunity Mechanism in Mammals. Science. 2013;342:231–234. doi: 10.1126/science.1241911. A demonstration that virus-derived small RNAs accumulate in a cell line and young mice following infection with a mutant Nodamura virus.
- 38. Maillard PV, Ciaudo C, Marchais A, Li Y, Jay F, Ding SW, Voinnet O. Antiviral RNA Interference in Mammalian Cells. Science. 2013;342:235–238. doi: 10.1126/science.1241930. A demonstration that dicer-dependent and virus-derived small RNAs accumulate following the infection of murine embryonic stem cells with two viruses.
- 39.Zou J, Chang M, Nie P, Secombes CJ. Origin and evolution of the RIG-I like RNA helicase gene family. BMC Evol. Biol. 2009;9:85. doi: 10.1186/1471-2148-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fullam A, Schröder M. DExD/H-box RNA helicases as mediators of anti-viral innate immunity and essential host factors for viral replication. Biochim. Biophys. Acta. 2013;1829:854–865. doi: 10.1016/j.bbagrm.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor DW, Ma E, Shigematsu H, Cianfrocco MA, Noland CL, Nagayama K, Nogales E, Doudna JA, Wang H-W. Substrate-specific structural rearrangements of human Dicer. Nat. Struct. Mol. Biol. 2013;20:662–670. doi: 10.1038/nsmb.2564. Building off the work of Lau et al., the authors demonstrate that human Dicer adopts different conformations depending on the nature of its substrate.
- 42. Li S, Wang L, Berman M, Kong Y-Y, Dorf ME. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity. 2011;35:426–440. doi: 10.1016/j.immuni.2011.06.014. A remarkable proteomic analysis of the protein:protein interactions present in the human type I interferon induction pathways. Of note, Dicer is presented in the figures and the supplemental material as an interacting protein.
- 43.Duchaine TF, Wohlschlegel JA, Kennedy S, Bei Y, Conte D, Pang K, Brownell DR, Harding S, Mitani S, Ruvkun G, et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 44. Guo X, Zhang R, Wang J, Ding S-W, Lu R. Homologous RIG-I-like helicase proteins direct RNAi-mediated antiviral immunity in C. elegans by distinct mechanisms. Proc. Natl. Acad. Sci. U.S.A. 2013;110:16085–16090. doi: 10.1073/pnas.1307453110. The authors show that the RLR-orthologue DRH-1 is critical for antiviral RNAi in C. elegans, and that certain domain of human RIG-I are able to function in this pathway when expressed in C. elegans.
- 45.Bivalkar-Mehla S, Vakharia J, Mehla R, Abreha M, Kanwar JR, Tikoo A, Chauhan A. Viral RNA silencing suppressors (RSS): novel strategy of viruses to ablate the host RNA interference (RNAi) defense system. Virus Res. 2011;155:1–9. doi: 10.1016/j.virusres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 47.Mudhasani R, Zhu Z, Hutvagner G, Eischen CM, Lyle S, Hall LL, Lawrence JB, Imbalzano AN, Jones SN. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J. Cell Biol. 2008;181:1055–1063. doi: 10.1083/jcb.200802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan GS, Garchow BG, Liu X, Yeung J, Morris JP, Cuellar TL, McManus MT, Kiriakidou M. Expanded RNA-binding activities of mammalian Argonaute 2. Nucleic Acids Res. 2009;37:7533–7545. doi: 10.1093/nar/gkp812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Jin D-Y, McManus MT, Mourelatos Z. Precursor microRNA-programmed silencing complex assembly pathways in mammals. Mol. Cell. 2012;46:507–517. doi: 10.1016/j.molcel.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–686. doi: 10.1038/nature03576. [DOI] [PubMed] [Google Scholar]
- 51.Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, Kang YJ, Jiang Z, Du X, Cook R, et al. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity. 2007;27:123–134. doi: 10.1016/j.immuni.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 52. Ostermann E, Tuddenham L, Macquin C, Alsaleh G, Schreiber-Becker J, Tanguy M, Bahram S, Pfeffer S, Georgel P. Deregulation of type I IFN-dependent genes correlates with increased susceptibility to cytomegalovirus acute infection of dicer mutant mice. PLoS ONE. 2012;7:e43744. doi: 10.1371/journal.pone.0043744. The authors demonstrate a clear susceptibility of Dicer hypomorphic mice and implicate Dicer in the induction of type I interferon.
- 53.Matskevich AA, Moelling K. Dicer is involved in protection against influenza A virus infection. J. Gen. Virol. 2007;88:2627–2635. doi: 10.1099/vir.0.83103-0. [DOI] [PubMed] [Google Scholar]
- 54. Kakumani PK, Ponia SS, S RK, Sood V, Chinnappan M, Banerjea AC, Medigeshi GR, Malhotra P, Mukherjee SK, Bhatnagar RK. Role of RNA Interference (RNAi) in Dengue Virus Replication and Identification of NS4B as an RNAi Suppressor. J. Virol. 2013;87:8870–8883. doi: 10.1128/JVI.02774-12. Implicates Dicer and RNAi in curbing dengue virus replication in human cells, while also identifying a viral inhibitor of RNAi. These findings are of particular interest since dengue virus is an insect-borne human pathogen.