Abstract
Background
Type 2 diabetes is one of the most common causes of cardiovascular disease as it causes arterial stiffness changes. The purpose of this study is to characterize, in vivo, carotid arterial structural and functional changes by applying radio frequency and X-strain ultrasound techniques.
Methods
Ninety-one subjects were assigned into two groups; a diabetes group and a control group. Structural and functional changes in the common carotid arterial wall were investigated by quality intima-media thickness (QIMT), quality arterial stiffness (QAS), and X-strain analysis with a Mylab Twice ultrasound instrument. The relationships among variables between the two groups were analyzed in this study.
Results
There was no significant difference in carotid IMT (626.5 ± 169.1 μm vs. 568.5 ± 122.6 μm, P = 0.1506) between two groups. Pulse wave velocity (PWV) and stiffness index (β) were remarkably greater (8.388 ± 3.254 m/s vs. 7.269 ± 1.332 m/s; 12.51 ± 14.16 vs.9.279 ± 2.871), while compliance coefficient (CC) decreased significantly in the diabetes group (0.802 ± 0.3094 mm2/Kpa vs. 0.968 ± 0.3992 mm2/Kpa) (P < 0.05). The displacement difference of radial (RD-D), longitudinal (LD-D) and rotation (ROT-D) directions were significantly different between two groups’ comparison (P = 0.0212, P = 0.0235 and P = 0.0072, respectively). The time of circumferential peak strain difference (CS-DT) and the time of radial peak strain rate (RSR-T) were found to be significantly different between the two groups (341.9 ± 77.56 ms vs. 369.0 ± 78.26 ms, P = 0.0494; 142.7 ± 22.43 ms vs. 136.2 ± 30.70 ms, P = 0.0474). CS-TD and RSR-T were also found to be positively correlated with CC value (r = 0.3908, P < 0.005 and r = 0.3027, P = 0.0326, respectively). Finally, PWV was negatively correlated with CC with (r = –0.6177, P < 0.001).
Conclusions
In type 2 diabetes, the functional changes in CCA can be identified using the methods presented in this article earlier than the structural changes. Arterial stiffness values provided by QAS and X-strain analysis can be used as indicators of CCA functional lesions in patients with type 2 diabetes.
Keywords: Arterial stiffness, Intima-media thickness, Diabetes, Strain
Background
Diabetes is a major contributor to atherosclerosis of the arterial bed [1,2]. Patients with diabetes are at a high risk of artery atherosclerosis leading to cardiovascular disease (CVD), especially coronary heart disease (CHD), which is the most common complication and the principal cause of death in type 2 diabetes patients. The carotid artery can be considered as a model to reflect conditions common to all infected arteries. Detection of structural and functional disorders of the common carotid artery (CCA) by duplex ultrasonography is most favorite method in evaluating systemic artery atherosclerosis.
CCA intima-media thickness (IMT) measurements are considered a strong predictor of future vascular events and a surrogate marker of atherosclerosis [3-5], although there are some recent studies that contest this theory [6,7]. Arterial elasticity assessment is also regarded as an independent predictor of cardiovascular mortality and morbidity in patients with cardiovascular disease as well as in healthy individuals [8,9].
Ultrasound radio frequency (RF) technology is a newly developed modality used for evaluating vascular elasticity of the carotid artery; the measurement of artery elasticity is based on the monitoring of RF signals transmitted by ultrasound. Data acquired by this technology can reflect changes objectively and accurately both from structural and functional aspects. X-strain technology, a vector strain imaging-VSI™, based on angle independent speckle-tracking technology applied to myocardial strain measurements to reduce error compared to Doppler tissue imaging [10,11]. In the present study, we adapt this method for carotid arterial stiffness analysis aiming to assess functional artery changes in patients with type 2 diabetes. In addition, we discuss change tendencies in the carotid artery and how they are identified using this superior technology.
Methods
Ethical approval of the study protocol
All subjects included in the study provided written informed consent. The study protocol was approved by the ethics committee of the Fourth Military Medical University Tangdu Hospital (Xi’an, China).
Patient selection
Between March 2012 and September 2012, 50 patients diagnosed with type 2 diabetes (treatment with diet an oral drugs) by laboratory tests at Tangdu Hospital of the Fourth Military Medical University, were investigated in this study. Patients with hypertension, hyperlipidemia, coronary heart disease, and nephropathy were excluded from the study. In order to avoid errors derived from subject matching, 41 healthy volunteers (22 males and 19 females; age, 40–79 years) were matched with patients based on gender and age. Before ultrasound measurement, we collected data from the physical examination and laboratory tests of each study subject. The following laboratory parameters were obtained: total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides (TG), HbA1c, serum creatinine, uric acid and HAb1c. Other clinical data includes clinical blood pressure determined by performing three systolic blood pressure tests (SBP), and diastolic blood pressure (DBP) measurements, body mass index (BMA) and body surface area (BSA).
Ultrasound examination
Ultrasound examinations were conducted with a Mylab Twice color Doppler ultrasound diagnostic system (Esaote, Firenze, Italy), using a 5-13 MHz vascular probe LA523 with built-in quality intima-media thickness (QIMT), quality arterial stiffness (QAS), and X-strain analysis software. After each subject was placed in the supine position, the right common carotid artery (RCCA), carotid bulb, and portions of the internal carotid arteries on both sides were scanned. The region of interest (ROI) was defined as 30 mm proximal to the beginning of the dilation of the bifurcation bulb.
The CCA examination was performed by two ultrasound physicians with 10 years working experience who had received formal training in vascular screening. The physicians were blinded to any clinical information regarding the subjects.
QIMT analysis
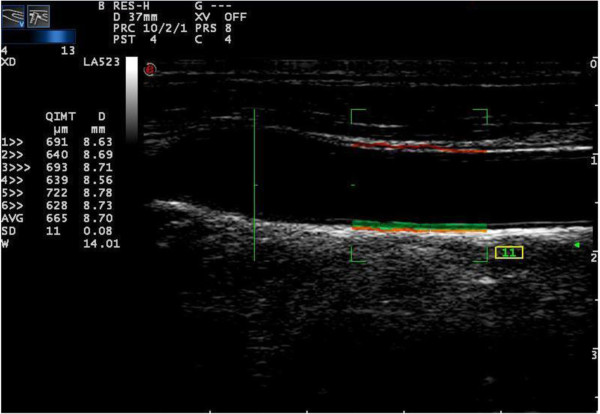
After the subject was placed in the correct position, the CCA was showed in a longitudinal view. The ultrasonographic image was focused on the QIMT measurement site and ensured that the anterior and posterior walls of the CCA were clearly shown. The regions with plaques were avoided in the ROI of CCA. By pressing the “Tools” button and starting the QIMT function, a RF signal tracked the leading edge of lumen intima to the leading edge of media adventitia interface at the posterior wall of the selected vascular segment. The software automatically acquired six cardiac cycle QIMT measurements (Figure 1). When the standard deviation (SD) was less than 15, the image was frozen and stored for further analysis.
Figure 1.
QIMT analysis of the common carotid artery. The red line represents the radiofrequency signal tracking the leading edge of the lumen intima; the green line represents the radiofrequency signal tracking the leading edge of media adventitia interface. The IMT value and vascular diameter was calculated automatically for six cardiac cycles showed on the left side of the picture.
QAS analysis
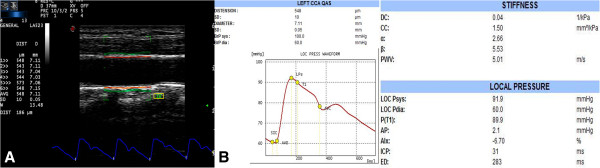
Concurrently with the QIMT measurement, QAS measurements began after pressing the “Tools” button. An RF signal tracked the vascular wall while another signal tracked the motion of vascular wall for at least for six cardiac cycles and calculated the mean and SD values automatically. SD value was controlled under a cutoff value 15 (Figure 2A). QAS data analysis software also calculated the pulse wave velocity (PWV), compliance coefficient (CC), and stiffness index (αand β; Figure 2B).
Figure 2.
QAS analysis of the common carotid artery. (A) The red line represents the radiofrequency signal tracking the leading edge of the lumen intima; the green line represents the radiofrequency signal tracking the leading edge of media adventitia interface. (B) The stiffness value was calculated automatically for six cardiac cycles.
X-strain analysis
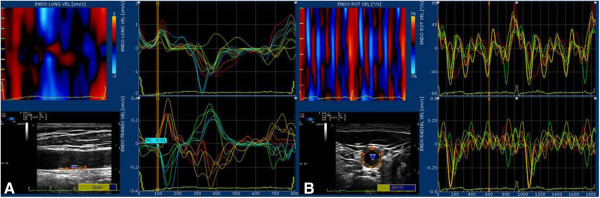
The X-strain analysis site is located 1 cm proximal to the bifurcation. Long and short axis were showed in the same measurement site The sampling sites were placed at the leading edge of the lumen intima and the leading edge of media adventitia interface at the posterior wall. The RF signal tracked both the motion of vascular intima and adventitia for at least three consecutive heart beats. The real-time images were stored and offline analysis was performed using a workstation equipped with Mylab desk analysis software (Figure 3). Depending on the view under analysis the following parameters were calculated as shown in Table 1.
Figure 3.
X-strain analysis of the common carotid artery. Longitudinal (A) and transverse (B) views were shown in the same position of the common carotid artery. The motion of the lumen intima and the adventitia were colorized as shown in the upper side of left column. The curves of different variables by X-strain analysis were traced automatically (the right column). In the pictures, we provide velocity curves analyzed by the X-strain method.
Table 1.
Parameters of different views of X-train analysis
| Parameters of long axis view | Unit |
|---|---|
| Longitudinal displacement |
mm |
| Longitudinal strain |
% |
| Radial strain |
% |
| Longitudinal strain rate |
1/sec |
| Radial strain rate |
1/sec |
|
Parameters of short axis view |
Unit |
| Rotational displacement |
Degree |
| Radial displacement |
mm |
| Circumferential strain |
% |
| Radial strain |
% |
| Circumferential strain rate |
1/sec |
| Radial strain rate | 1/sec |
Except that the radial strain and strain rate was output as the difference of the intima and adventitia values by the software, other parameters were output as the intima and adventitia values respectively. So variables are expressed as D (the intima and adventitia peak value difference), DT (the time of the intima and adventitia peak value difference) and TD (the time difference of the intima and adventitia peak value). Significant difference between the two group and correlations among these variables were evaluated.
Statistical analyses
All data are expressed mean ± SD. Differences between the two groups were tested using unpaired Student t-tests. The reproducibility of the arterial stiffness measurements was tested in the younger subjects and the intra- and inter-observer variability was assessed by linear correlation analysis and Bland-Altman plots. Linear regression analysis was used to assess the correlations among all parameters. Results were considered significant at P < 0.05. The statistical software package SPSS 12.0 (IBM Corporation, Armonk, NY, USA) was used for all data analyses.
Results
Patient characteristics
The main clinical and pathological data for patients at the beginning of the study are presented in Table 2. BSA, DBP, SBP, total cholesterol, LDL cholesterol, TC and HAb1c were higher in patients with diabetes. There were no significant differences between groups with respect to the other characteristics concerned.
Table 2.
Clinical characteristics of the study subjects
| Characteristics | Controls | Patients | P value |
|---|---|---|---|
| Gender (M/F) |
19/22 |
22/28 |
|
| Age (mean ± SD) |
53.05 ± 11.99 |
53.60 ± 9.29 |
0.814 |
| Height (cm) |
164.80 ± 7.49 |
16 6.06 ± 8.26 |
0.471 |
| Weight (kg) |
67.15 ± 8.10 |
70.45 ± 17.98 |
0.290 |
| BMI (kg/m2) |
24.68 ± 2.01 |
25.49 ± 5.83 |
0.407 |
| BSA (m2) |
1.667 ± 0.1746 |
1.754 ± 0.1778 |
0.0257* |
| SBP (mmHg) |
116.11 ± 12.18 |
134.9 ± 18.81 |
0.0007* |
| DBP (mmHg) |
79.84 ± 9.241 |
86.50 ± 12.09 |
0.0036* |
| Duration of diabetes (years) |
/ |
5.47 ± 2.37 |
/ |
| Smoker/duration (%/years) |
32%/8.23 ± 6.74 |
36%/9.72 ± 4.43 |
0.379 |
| TC (mmol/l) |
3.82 ± 0.31 |
4.42 ± 0.12 |
<0.0001* |
| HDL cholesterol (mmol/l) |
1.63 ± 0.13 |
1.58 ± 0.17 |
0.137 |
| LDL cholesterol (mmol/l) |
2.94 ± 0.24 |
3.12 ± 0.08 |
0.036* |
| TG (mmol/l) |
1.02 ± 0.65 |
1.13 ± 0.87 |
0.236 |
| HbA1c (%) |
4.38 ± 1.43 |
7.52 ± 1.05 |
<0.0001* |
| Serum creatinine (μmol/l) |
47.82 ± 12.5 |
50.31 ± 26.7 |
0.098 |
| Uric acid (μmol/l) | 221.43 ± 25.96 | 229.57 ± 23.2 | 0.569 |
Notes: Data are presented as means ± standard deviation. *Value between controls and patients with diabetes P < 0.05.
Abbreviations:BMI body mass index; BSA body surface area; SBP systolic blood pressure; DBP diastolic blood; TC total cholesterol; HDL high density lipoprotein; LDL low density lipoprotein; TG triglycerides.
Arterial structure and stiffness detection
Carotid arterial plaque was found in 16% of the control group and 37% of the patient group, which was not significantly different between patients group and control group. No significant alteration in IMT was seen in the patient group compared to controls (P = 0.1506). The results of QAS analysis in Table 3 shows that PWV and β arterial stiffness parameters were much higher in the patient group, while CC was significantly lower (P = 0.0430, P = 0.0463, and P = 0.0414 respectively). The stiffness index αshowed a little higher in patients group, while no significant difference was found.
Table 3.
Comparison between the two groups by QIMT and QAS measurements
| Variables | Patients | Controls | P value |
|---|---|---|---|
| IMT (μm) |
626.5 ± 169.1 |
568.5 ± 122.6 |
0.1506 |
| PWV (m/s) |
8.388 ± 3.254 |
7.269 ± 1.332 |
0.0430* |
| α |
5.481 ± 7.208 |
4.987 ± 1.749 |
0.1429 |
| β |
12.51 ± 14.16 |
9.279 ± 2.871 |
0.0463* |
| CC (mm2/Kpa) | 0.802 ± 0.3094 | 0.968 ± 0.3992 | 0.0414* |
Notes: *Value between controls and patients with diabetes P < 0.05. Data are presented as means ± standard deviation.
Abbreviations:IMT intima-media thickness; PWV pulse wave velocity; CC compliance coefficient.
X-strain analysis
The results of the X-strain analysis indicate that radial displacement difference (RD-D) and longitudinal displacement difference (LD-D) were higher in diabetes patients compared to the control group, while rotation displacement difference (ROT-D) decreased in the patient group (P = 0.0212, P = 0.0235 and P = 0.0072, respectively) (Table 4). In addition, the time difference of radial peak displacement (RD-TD) and the time of rotation peak displacement difference (ROT-DT) in the patient group presented earlier than in the healthy controls. No significant differences were found between the two groups as far as other variables were concerned.
Table 4.
Comparison of displacement variables between the two groups by X-strain analysis
| Variables | Patients group | Control group | P value |
|---|---|---|---|
|
LD-D (mm) |
0.0884 ± 0.053 |
0.0638 ± 0.028 |
0.0212* |
|
LD-DT (ms) |
390.0 ± 163.1 |
365.1 ± 205.1 |
0.2262 |
|
LD-TD (ms) |
35.96 ± 27.78 |
65.14 ± 67.36 |
0.0420* |
|
RD-D (mm) |
0.8188 ± 0.5993 |
0.6000 ± 0.2290 |
0.0235* |
|
RD-DT (ms) |
314.7 ± 87.15 |
337.3 ± 79.16 |
0.1074 |
|
RD-TD (ms) |
137.8 ± 49.7 |
130.8 ± 43.89 |
0.5080 |
|
ROT-D (mm) |
0.4981 ± 0.2225 |
0.6876 ± 0.3874 |
0.0161* |
|
ROT-DT (ms) |
316.4 ± 79.9 |
394.3 ± 147.1 |
0.0072* |
| ROT-TD (ms) | 123.7 ± 59.81 | 122.2 ± 77.56 | 0.2568 |
Notes: *Value between controls and patients with diabetes P < 0.05. Data are presented as means ± standard deviation.
Abbreviations:RD-D radial displacement difference; RD-DT the time of radial peak displacement difference; RD-TD the time difference of radial peak displacement; LD-D longitudinal displacement difference; LD-DT the time of longitudinal peak displacement difference; LD-TD the time difference of longitudinal peak displacement; ROT-D difference of rotation displacement; ROT-DT the time of rotation peak displacement difference; ROT-TD the time difference of rotation peak displacement.
With respect to the type of strain and strain rate variables, only the time of circumferential peak strain difference (CS-DT) and the time of radial peak strain rate (RSR-T) were found to be significantly different between the two groups (341.9 ± 77.56 ms vs. 369.0 ± 78.26 ms, P = 0.0494; 142.7 ± 22.43 ms vs. 136.2 ± 30.70 ms, P = 0.0474) (Table 5 and Table 6). Other variables showed no obviously different between two groups.
Table 5.
Comparison of strain variables between the two groups by X-strain analysis
| Variables | Patients group | Control group | P value |
|---|---|---|---|
|
LS-D (%) |
1.936 ± 0.9507 |
1.601 ± 0.5719 |
0.1136 |
|
LS-DT (ms) |
359.5 ± 78.11 |
379.2 ± 138.7 |
0.7695 |
|
LS-TD (ms) |
65.86 ± 49.97 |
61.64 ± 36.65 |
0.9615 |
|
CS-D (%) |
22.51 ± 7.056 |
21.72 ± 7.576 |
0.5789 |
|
CS-DT (ms) |
341.9 ± 77.56 |
369.0 ± 78.26 |
0.0494* |
|
CS-TD (ms) |
132.6 ± 52.33 |
151.3 ± 72.17 |
0.2715 |
|
RS (%) |
3.191 ± 1.212 |
3.318 ± 1.586 |
0.6541 |
| RS-T (ms) | 276.6 ± 45.37 | 268.4 ± 62.3 | 0.3850 |
Notes: *Value between controls and patients with diabetes P < 0.05. Data are presented as means ± standard deviation.
Abbreviations:CS-D circumferential strain difference; CS-DT the time of circumferential peak strain difference; CS-TD the time difference of circumferential peak strain; LS-D longitudinal strain difference; LS-DT the time of longitudinal peak strain difference; LS-TD the time difference of longitudinal peak strain; RS radial strain; RS-T the time of radial peak strain.
Table 6.
Comparison of strain rate variables between the two groups by X-strain analysis
| Variables | Patients group | Control group | P value |
|---|---|---|---|
|
CSR-D (1/s) |
0.2433 ± 0.7987 |
0.2517 ± 0.0968 |
0.7458 |
|
CSR-DT (ms) |
264.3 ± 115.9 |
279.7 ± 104.5 |
0.3555 |
|
CSR-TD (ms) |
71.72 ± 78.20 |
62.17 ± 76.90 |
0.1732 |
|
LSR-D (1/s) |
5.761 ± 4.456 |
6.099 ± 3.517 |
0.5579 |
|
LSR-DT (ms) |
339.1 ± 147.2 |
317.6 ± 110.7 |
0.7933 |
|
LSR-TD (ms) |
155.9 ± 71.13 |
162.0 ± 78.33 |
0.9588 |
|
RSR (1/s) |
0.4126 ± 0.1615 |
1.804 ± 6.997 |
0.7827 |
| RSR-T (ms) | 142.7 ± 22.43 | 136.2 ± 30.70 | 0.0474* |
Notes: *Value between controls and patients with diabetes P < 0.05.
Abbreviations:CSR-D circumferential strain rate difference; CSR-DT the time of circumferential peak strain rate difference; CS-TD the time difference of circumferential peak strain rate; LSR-D longitudinal strain rate difference; LSR-DT the time of longitudinal peak strain rate difference; LSR-TD the time difference of longitudinal peak strain rate; RSR radial strain rate; RSR-T the time of radial peak strain rate.
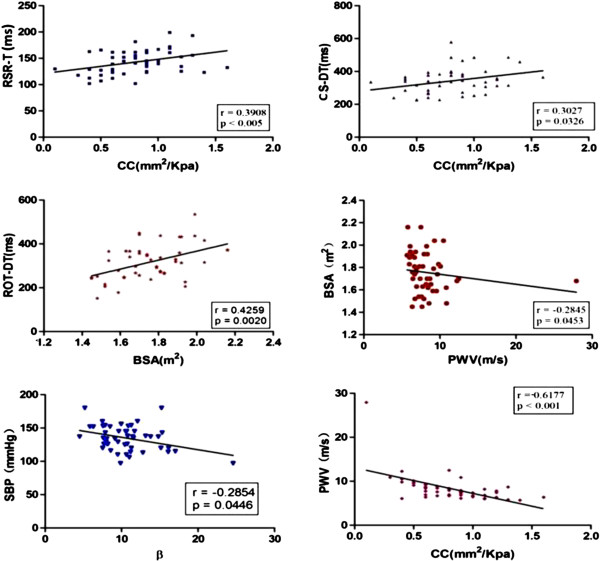
Correlation of arterial stiffness parameters with X-strain parameters
Among the significantly different parameters by X-strain analysis (Figure 4), RSR-T and CS-DT were found to be positively correlated with CC value (r = 0.3098, P < 0.005 and r = 0.3027, P = 0.0326, respectively). ROT-DT showed a positive relationship with BSA (r = 0.4259, P = 0.0020). With respect to the other stiffness and clinical variables, BSA and PWV (r = –0.2845, P = 0.0453), SBP and β (r = –0.2854, P = 0.0446), and CC and PWV (r = –0.6177, P < 0.001) were also found to be negatively associated with each other. No more statistical significance was found among other evaluated variables. Conversely, the significant relationships identified in the patient group were not observed in the control group.
Figure 4.
Correlations of significantly different parameters in the patient group. Among them, the correlations of BSA and ROT-DT, BSA and β, BSA and PWV, CS-DT and CC, CC and RSR-T, and CC and PWV had statistical significance as shown.
Repeatability comparison
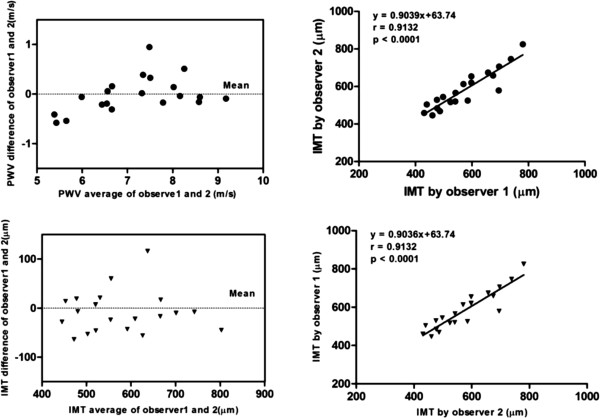
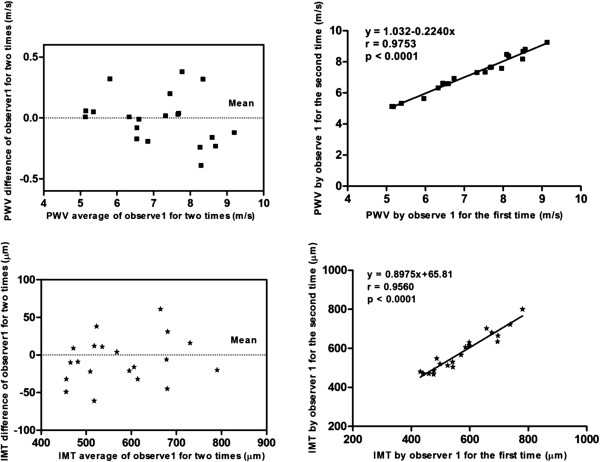
Good agreement was found between intragroup and intergroup comparisons for IMT and PWV values (intragroup: a mean bias of 8.45 ± 43.13 μm; intergroup: a mean bias of 7.05 ± 30.05 μm for IMT; intragroup: a mean bias of 0.013 ± 0.364 m/s; intergroup: a mean bias of 0.007 ± 0.201 m/s for PWV). Bland-Altman analysis showed a consistent trend in the difference and mean values of IMT and PWV by repeated measurement (Figures 5 and 6).
Figure 5.
Inter-observer variability of PWV and IMT measurements. Linear regression analysis shows good agreement between measurements for PWV and IMT by two independent observers.
Figure 6.
Intra-observer repeatability of PWV and IMT measurements. Linear regression analysis shows good agreement between measurements for PWV and IMT by the same observer.
Discussion
Changes in both structural and functional aspects of arteries have been a research interest for several years as they are considered risk factors for cardiovascular events [12-14]. As diabetes is one of the most common causes of cardiovascular disease, aortic elasticity was impaired in those with impaired fasting glucose [15], in the present study we focused on carotid arterial wall changes induced by type 2 diabetes by evaluating artery lesions using a new method called ultrasound RF data and X-strain technology. By using QIMT and QAS techniques, high resolution ultrasound acquisitions based on RF signals allow us to assess local IMT and stiffness in a rapid and specific manner.
The structural indicators IMT and plaque have been endpoint markers in clinical trials [16-18]. We discovered that IMT of the RCCA showed no obvious changes in patients with diabetes, whereas some of the elastic parameters showed significant differences in these patients relative to controls. Although our findings differed from other reports [4,19], it may be one that supports the notion that functional impairment of the arterial wall may occur early in the atherosclerotic process [20], and arterial stiffening may be a process independent of arterial thickening [21].
Many researches showed to us that arterial stiffness is correlated with the presence and severity of arterial atherosclerosis, and also associated with myocardial dysfunction [22-24]. For functional changes, we assessed both the performance of QAS measurements of vascular stiffness, such as PWV, CC, α, β, and the performance of ultrasonographic speckle tracking-based X-strain measurements. As the most useful and robust index of arterial stiffness [25-27], PWV measured by QAS likely provides accurate local characteristics of vascular alterations. The local vascular stiffness values PWV and β we measured increased in the patient group, while CC decreased, which validates the hypothesis that arterial remodeling occurring in local and elastic arteries increases the risk for future cardiovascular disease.
Besides the stiffness values acquired by QAS, X-strain techniques, a novel method based on the two-dimensional information obtained directly from the arterial wall itself both for the intima and adventitia at the same time, can give us more information about local arterial stiffness, rather than depending on luminal changes. The quantitative assessment of the regional function of the arterial wall may be based on the mobility of the intima, of the adventitia and on wall thickening. Strain and strain rate imaging provides a quantitative method for assessing the regional function of the arterial wall. The variables include the displacement, strain, and strain rate of the arterial wall movement for circumferential, longitudinal, radial, and rotation movements. Like myocardial movement, the intima and adventitial wall stretch in different sectors at the same time can be showed to us by the real-time X-strain analysis. The final arterial wall movement at that moment is decided by the multi-directional forces on both intima and adventitial wall. Unlike other strain analysis technology, X-strain analysis allows us to evaluate all of the forces at the same time and helps us to comprehensively understand the movement of the arterial wall. To our knowledge, this is the first report in the literature to assess arterial stiffness from multiple angles using the speckle tracking method.
Among all these parameters, circumferential, radial, and rotation variables (CS-DT, RSR-T, and ROT-DT) appeared to be sensitive to CCA stiffness changes compared to the longitudinal variables. In mechanics, Laplace’s law and Poisson’s ratio emphasize that when the force on a circumferential or radial section is two times the force on the longitudinal section, the vascular stretching longitudinally can be neglected, suggesting that the movement of an elastic artery is mainly a type of stretching in the circumferential and radial directions and, as such, the movement of the vascular wall is a vascular diameter change, rather than the vascular length change. Therefore, circumferential and radial strain analysis is capable of accurately reflecting local vascular flexibility. In the study, we introduced time differences for displacement, strain, and strain rate changes as indicators of arterial stiffness because of the specificity of this technology. Neither the intima nor adventitia variables were capable of unilateral reflecting the difference in arterial wall movement between the two groups in the present study. In this study, we, for the first time, validated rotation variables, which was not previously possible to assess because only the elasticity average of the intima and media of the carotid artery wall are calculated using other modalities. Interestingly, the time of rotation peak displacement was much earlier in the patient group than in the control group.
Our study has several limitations, including the small number of patients; patients with hypertension, hyperlipidemia, coronary heart disease, and nephropathy were excluded from the study, which limited the number of patients we could enroll. Secondly, although some clinical measurements were different between two groups’ comparisons in the present study, no obvious relationship was found with the arterial stiffness changes by the statistical correlation analysis, further analyze the data with adjustment was not performed as other studies did [28,29]. Thirdly, considering the fact that the number of subjects with the presence of plaque was not sufficient for quantitative statistical analysis, the value of plaque score [30] was not evaluated in this study. Although the patients have significant higher TC, LDL, SBP and DBP than controls, but all these laboratory parameters are still in the normal range. Further research with larger populations should be carried out to verify the present results and validate the changes in arterial stiffness in patients with advanced stage diabetes.
Conclusions
In conclusion, the present study demonstrated that patients with diabetes have significantly increased arterial stiffness as assessed by QAS and X-strain assessment. QIMT and QAS techniques can be used to noninvasively and comprehensively assess local elastic arterial remodeling in patients with early stage diabetes.
Abbreviations
CCA: Carotid arterial wall; QIMT: Quality intima-media thickness; QAS: Quality arterial stiffness; PWV: Pulse wave velocity; CC: Compliance coefficient; CVD: Cardiovascular disease; CHD: Coronary heart disease; RF: Radiofrequency; TC: Total cholesterol; TG: Triglycerides; LDL: Low density lipoprotein; HDL: High density lipoprotein; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; SD: Standard deviation; RCCA: Right common carotid artery; BSA: Body surface area; BMI: Body mass index; RD-D: Radial displacement difference; LD-D: Longitudinal displacement difference; ROT-D: Rotation displacement difference; RD-TD: Radial peak displacement; ROT-DT: Time of rotation peak displacement difference; CS-DT: Time of circumferential peak strain difference; RSR-T: Time of radial peak strain rate.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LZ devised the study, designed the protocol, participated in fund raising, interpretation of results and prepared the manuscript draft. JKY performed all the statistical analysis. YYD participated in the study design, fund raising and corrected the final version of the manuscript. XL participated in the study design, analytical methods, interpretation of results, and data collection. JW and LX participated in data collection and interpretation of results. YLY participated in the protocol design, fund raising, analysis of results. LJY and TSC participated in the final review of the manuscript. Finally, all authors reviewed and approved the final version of the manuscript.
Contributor Information
Li Zhang, Email: lilyzhang319_20@hotmail.com.
Ji-Kai Yin, Email: yjkfmmuu@hotmail.com.
Yun-You Duan, Email: duanyy@fmmu.edu.cn.
Xi Liu, Email: liuxi916@126.com.
Lei Xu, Email: zhuangshengxiaomeng@126.com.
Jia Wang, Email: 364878788@qq.com.
Yi-Lin Yang, Email: yangyl66@126.com.
Li-Jun Yuan, Email: yuanlj@fmmu.edu.cn.
Tie-Sheng Cao, Email: caots@fmmu.edu.cn.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China, NSFC 81101092.
References
- Gordon T, Kannel WB. Predisposition to atherosclerosis in the head, heart. JAMA. 1972;221:661–666. doi: 10.1001/jama.1972.03200200011003. [DOI] [PubMed] [Google Scholar]
- Sharrett AR, Sorlie PD, Chambless LE, Folsom AR, Hutchinson RG, Heiss G, Szklo M. Relative importance of various risk factors for asymptomatic carotid atherosclerosis versus coronary heart disease incidence the ARIC Study. Am J Epidemiol. 1999;149:843–852. doi: 10.1093/oxfordjournals.aje.a009900. [DOI] [PubMed] [Google Scholar]
- O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- Nomura K, Hamamoto Y, Takahara S, Kikuchi O, Honjo S, Ikeda H, Wada Y, Nabe K, Okumra R, Koshiyama H. Relationship between carotid intimamedia thickness and silent cerebral infarction in Japanese subjects with type 2 diabetes. Diabetes Care. 2010;33:168–170. doi: 10.2337/dc09-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- Sharma K, Blaha MJ, Blumenthal RS, Musunuru K. Clinical and research applications of carotid intima-media thickness. Am J Cardiol. 2009;103:1316–1320. doi: 10.1016/j.amjcard.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Larsson M, Kremer F, Claus P, Kuznetsova T, Brodin LA, D’hooge J. Ultrasound-based radial and longitudinal strain estimation of the carotid artery: a feasibility study. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:2244–2251. doi: 10.1109/TUFFC.2011.2074. [DOI] [PubMed] [Google Scholar]
- Sivesgaard K, Christensen SD, Nygaard H, Hasenkam JM, Sloth E. Speckle tracking ultrasound is independent of insonation angle and gain: an in vitro investigation of agreement with sonomicrometry. J Am Soc Echocardiogr. 2009;22:852–858. doi: 10.1016/j.echo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Cho GY, Chan J, Leano R, Strudwick M, Marwick TH. Comparison of two dimensional speckle and tissue velocity based strain and validation with harmonic phase magnetic resonance imaging. Am J Cardiol. 2006;97:1661–1666. doi: 10.1016/j.amjcard.2005.12.063. [DOI] [PubMed] [Google Scholar]
- Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, Lecompte T, Lacolley P, Benetos A, Zannad F. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40:1229e36. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, Marmot MG, Deanfield JE. Endothelial function predicts progression of carotid intimaemedia thickness. Circulation. 2009;119:1005e12. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- Barra S, Gaeta G, Cuomo S, Guarini P, Foglia MC, Capozzi G, Materazzi C, Trevisan M. Early increase of carotid intima-media thickness in children with parental history of premature myocardial infarction. Heart. 2009;95:642e5. doi: 10.1136/hrt.2008.142836. [DOI] [PubMed] [Google Scholar]
- Dogan SM, Aktop Z, Aydin M, Karabag T, Sayin MR, Bilici HM, Atmaca H. Effects of impaired fasting glucose on aortic elasticity. Exp Clin Endocrinol Diabetes. 2012;120:424–427. doi: 10.1055/s-0032-1306295. [DOI] [PubMed] [Google Scholar]
- Shinoda-Tagawa T, Yamasaki Y, Yoshida S, Kajimoto Y, Tsujino T, Hakui N, Matsumoto M, Hori M. A phosphodiesterase inhibitor, cilostazol, prevents the onset of silent brain infarction in Japanese subjects with type II diabetes. Diabetologia. 2002;45:188–194. doi: 10.1007/s00125-001-0740-2. [DOI] [PubMed] [Google Scholar]
- Bernard S, Sérusclat A, Targe F, Charrière S, Roth O, Beaune J, Berthezène F, Moulin P. Incremental predictive value of carotid ultrasonography in the assessment of coronary risk in a cohort of asymptomatic type 2 diabetic subjects. Diabetes Care. 2005;28:1158–1162. doi: 10.2337/diacare.28.5.1158. [DOI] [PubMed] [Google Scholar]
- Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, O’Leary DH. The value of carotid artery plaque and intima-media thichness foe incident cardiovascular diseasae: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Marcos MA, Recio-Rodríguez JI, Patino-Alonso MC, Agudo-Conde C, Gómez-Sánchez L, Rodríguez-Sánchez E, Martín-Cantera C, García-Ortiz L. Relationship between intima-media thickness of the common carotid artery and arterial stiffness in subjects with and without type 2 diabetes: a case report. Cardiovasc Diabetol. 2011;10:3. doi: 10.1186/1475-2840-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvat J, Chlumsky J, Zakovicova E, Kvapil M. Common carotid artery intima-media thickness is not increased but distensibility is reduced in normotensive patients with type 2 diabetes compared with control subjects. J Int Med Res. 2010;38:860–869. doi: 10.1177/147323001003800312. [DOI] [PubMed] [Google Scholar]
- Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: the ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol. 1997;23:157–164. doi: 10.1016/S0301-5629(96)00211-6. [DOI] [PubMed] [Google Scholar]
- Myung Y, Seo HS, Jung IH, Lee NH, Suh J, Choi JH, Cho YH. The correlation of carotid artery stiffness with heart function in hypertensive patients. J Cardiovasc Ultrasound. 2012;20:134–139. doi: 10.4250/jcu.2012.20.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu KH, Zhao CT, Chen Y, Siu CW, Chan YH, Lau KK, Liu S, Lau CP, Tse HF. Association of subclinical myocardial injury with arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:94. doi: 10.1186/1475-2840-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EY, Chambless L, Sharrett AR, Virani SS, Liu X, Tang Z, Boerwinkle E, Ballantyne CM, Nambi V. Carotid arterial wall characteristics are associated with incident ischemic stroke but not coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2012;43:103–108. doi: 10.1161/STROKEAHA.111.626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for non-invasive investigation of large arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- Lau KK, Wong YK, Chan YH, Yiu KH, Teo KC, Li LS, Ho SL, Chan KH, Siu CW, Tse HF. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with type 2 diabetes. Cardiovasc Diabetol. 2012;11:101. doi: 10.1186/1475-2840-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén EM, Bjarnegård N, Länne T, Nystrom FH, Ostgren CJ. Sagittal abdominal diameter is a more independent measure compared with waist circumference to predict arterial stiffness in subjects with type 2 diabetes–a prospective observational cohort study. Cardiovasc Diabetol. 2013;12:55. doi: 10.1186/1475-2840-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto H, Ishigaki Y, Koiwa Y, Hinoko Y, Ogihara T, Suzuki S, Katagiri H, Ohkubo T, Hasegawa H, Kanai H, Oka Y. A novel method for evaluating human carotid artery elasticity: possible detection of early stage atherosclerosis in subjects with type 2 diabetes. Atherosclerosis. 2008;196:391–397. doi: 10.1016/j.atherosclerosis.2006.11.020. [DOI] [PubMed] [Google Scholar]