Abstract
Objective:
Prior literature has identified inconsistent longitudinal associations between total cholesterol and cognitive decline. Here we examined prospective nonlinear relations of coincident trajectories of total cholesterol and cognitive function among persons free of stroke, dementia, and other neurological disease.
Method:
Up to 1,601 participants from the Baltimore Longitudinal Study of Aging (aged 19 to 93, 51% male, 75% white) underwent fasting cholesterol measurement and neuropsychological assessment on up to 12 occasions (M = 3.2, SD = 2.1) over up to 19 years (M = 6.4, SD = 5.3) of follow-up. Mixed-effects regression analyses were adjusted for age, sex, race, education, systolic blood pressure, body mass index, cardiovascular disease, lipid-lowering medication use, smoking, alcohol use, and depressive symptoms.
Results:
Analyses revealed significant longitudinal associations between quadratic total cholesterol and performance on measures of global mental status, verbal learning, executive function, and language (all p’s < .05). In general, higher total cholesterol was associated with poorer middle-aged or “young-old” (60-69 years) cognitive performance, but better “old-old” (80-89 years) cognitive performance. Linear models also revealed an association between lower total cholesterol and accelerated decline in visual memory performance.
Conclusions:
Overall, results indicate nonlinear longitudinal relations of total cholesterol to cognitive decline. Whereas higher cholesterol levels were associated with cognitive decline in the middle-aged or young-old, lower cholesterol levels were related to cognitive decline among old-old participants.
Keywords: cholesterol, neuropsychology, cognitive decline, longitudinal
Nonlinear Longitudinal Trajectories of Cholesterol and Cognitive Function
A sparse prior literature has identified equivocal longitudinal associations between total cholesterol and prospective cognitive decline. In separate studies, higher total cholesterol has predicted both more and less prospective decline in neurocognition. For example, a comprehensive meta-analysis of 18 studies indicated an association between high midlife total cholesterol and late-life cognitive impairment (Anstey, Lipnicki, & Low, 2008). Conversely, longitudinal data from both the Framingham Heart Study (Elias, Elias, D’Agostino, Sullivan, & Wolf, 2005) and National Heart, Lung, and Blood Institute Twin Study (Swan, LaRue, Carmelli, Reed, & Fabsitz, 1992) have indicated associations between low total cholesterol and prospective cognitive decline. Cross-sectional studies are similarly conflicting (Teunissen et al., 2003; West et al., 2008). Multiple factors may contribute to such divergent findings, including the impact of effect modifiers such as statin use (Benito-Leon, Louis, Vega, & Bermejo-Pareja, 2010) and pathologic declines in cholesterol often described in premorbid dementia (Mathew, Yoshida, Maekawa, & Kumar, 2011; Mielke et al., 2005). Recent reviews have underscored this growing confusion (Schreurs, 2010).
Other cardiovascular risk factors, such as blood pressure and body mass index, demonstrate quadratic, longitudinal associations with neurocognition, such that both high and low levels are associated with decrements in cognitive performance (Sturman et al., 2008; Waldstein, Giggey, Thayer, & Zonderman, 2005). However, little or no research has directly examined nonlinearity in relations of total cholesterol to cognitive function. Consideration of nonlinear longitudinal associations between total cholesterol and cognitive functions is important in light of nonlinear patterns across other cardiovascular risk factors, as well as the current complexity of the cholesterol-cognition literature. Additionally, few, if any, studies have examined relations between concurrent changes in cholesterol and cognitive function across the life span. Accordingly, in the present study, we examined nonlinear relations of coincident trajectories of total cholesterol and cognitive function among participants in the Baltimore Longitudinal Study of Aging (BLSA).
Method
Participants
Participants were enrolled in the BLSA, a prospective study of community-dwelling volunteers initiated by the National Institute on Aging in 1958. Approximately every 2 years, participants visit the National Institute on Aging in Baltimore for medical, psychological, and cognitive testing. Beginning in 1986, a more extensive neuropsychological battery was implemented predominantly among participants aged 60 years and older. The present analyses were limited to visits occurring on or after January 1, 1986 through December 31, 2006, resulting in 1,851 participants for potential inclusion. We excluded persons with dementia (Kawas, Gray, Brookmeyer, Fozard, & Zonderman, 2000) cerebrovascular disease including stroke (n=86), and other neurologic disease (i.e., Parkinson’s disease, Huntington’s disease, epilepsy, multiple sclerosis; n=11). A total of 1,601 participants were thus available for data analysis. Because the BLSA uses continuous enrollment procedures, participants have differential start times and ages, numbers of visits, and follow-up times in the project (see Table 1). Participants had an average of 3.2 visits (SD = 2.1; range = 1-12), and the average time between visits was 2.2 years (SD = 0.8; range = 0.5-7.0). Participants were followed for up to 19 years (mean = 6.4; SD = 5.3; median = 6;). Institutional Review Board approval was obtained from the Johns Hopkins Bayview Medical Center before 2002 and the MedStar Research Institute afterwards. All participants provided written informed consent, and all of the procedures followed were in accordance with institutional guidelines.
Table 1.
Sample Size by Number of Visits
| Number of Visits | n (% of Sample) |
|---|---|
| 1 | 1,601 (100.0) |
| 2 | 1176 (73.5) |
| 3 | 864 (54.0) |
| 4 | 636 (39.7) |
| 5 | 427 (26.7) |
| 6 | 258 (16.1) |
| 7 | 134 (8.4) |
| 8 | 52 (3.2) |
| 9 | 19 (1.2) |
| 10 | 9 (0.6) |
| 11 | 3 (0.2) |
| 12 | 1 (0.01) |
Cholesterol Assessment
Total cholesterol was assessed at each BLSA visit. After an overnight fast, blood for lipid assay was drawn from an antecubital vein between 7:00 and 8:00 a.m. while the participant was supine. Concentrations of total cholesterol were determined enzymatically (ABA-200 ATC Biochromatic Analyzer; Abbott Laboratories).
Neuropsychological Assessment
At each BLSA visit, standard neuropsychological tests (Lezak, Howieson, & Loring, 2004) were administered by highly trained psychometricians. The numbers that follow each test indicate respective sample sizes because of test-specific missing data. The Blessed Information-Memory-Concentration (I-M-C) Test (n = 1,601) and the Mini Mental State Examination (MMSE; n = 850) are cognitive screening measures and assessed global cognitive status. The Digits Forward (n = 1,268) and Digits Backward (n = 1,269) portions of the Digit Span subtest of the Wechsler Adult Intelligence Scale-Revised assessed attention and concentration. The California Verbal Learning Test (CVLT; n = 1,190) measured verbal learning and memory, including immediate free recall, learning slope (average number of new words gained per learning trial), and free recall following short and long delays. The Benton Visual Retention Test (BVRT; n = 1,350) evaluated immediate visual memory. The Trail Making Test Part A (n = 836) and Part B (n = 824) assessed attention, perceptuomotor speed, visuomotor scanning, and mental flexibility, an executive function. Letter Fluency (n = 838) and Category Fluency (n = 837) examined phonemic and semantic association fluency, respectively, and executive function. The Boston Naming Test (n = 721) assessed confrontation naming, a language ability. The Card Rotations Test (n = 1,186) measured mental spatial rotation, a visuospatial function. Due to the aforementioned expansion of the test battery in 1986, the MMSE, Trail Making Test, Fluency tests, and Boston Naming Test were predominantly administered to participants aged 60 and older.
Covariates
Covariate selection was predicated upon theoretical potential for confounding of analyses, given known associations of certain risk factors and conditions with total cholesterol and neuropsychological function. Age and education were assessed in years. Binary covariates included sex (male = 1), race (white = 1, non-white = 0), smoking (ever = 1/never = 0), and use of lipid lowering medications (yes = 1/no = 0). Resting brachial systolic and diastolic blood pressure (SBP, DBP) values were obtained three times bilaterally following a 5-minute quiet resting period. SBP and DBP were defined by Korotkoff phases I and V, respectively. SBP was used as a covariate in the present analyses. Body mass index (BMI) was calculated as the ratio of weight (in kilograms) to height (in meters) squared, both measured with a clinical calibrated scale. A cardiovascular and metabolic disease comorbidity index was defined as presence/absence of one or more (range=0-5) of the following conditions: coronary artery disease, heart failure, peripheral arterial disease, transient ischemic attack, and diabetes mellitus. Alcohol use was defined as average consumption of ≥2 drinks per day versus less than that amount over the past 12 months. One drink was defined as 12 oz of beer, 4 to 5 oz of wine, or 2 oz of spirits. The Center for Epidemiological Studies Depression Scale assessed self-reported depressive symptomatology (Radloff, 1977).
Statistical Analyses
All statistical analyses were performed using SAS version 9.2 (Cary, NC). Mixed-effects regression analyses were conducted to examine longitudinal relations of total cholesterol to cognitive function. This statistical approach handles inconsistent measurement intervals both within and across BLSA participants, remains unaffected by randomly missing data, and accounts for the correlation among repeated measurements on the same participants (Singer, 1998). Both total cholesterol and cognitive function were assessed at each BLSA visit; analyses thus examine coincident trajectories of total cholesterol and cognitive function over time.
Each cognitive measure was entered as a single outcome variable in separate sequential mixed-effects regression models. Covariates included age, sex, race, education, SBP, BMI, lipid-lowering medication use, cardiovascular disease, smoking, and alcohol use. All covariates were coded time-dependently except for sex, race, education, and alcohol use, for which a mean of all available measurements was used. Age was transformed linearly to reflect decade (i.e., divided by 10) for model estimation purposes. Age was also modeled as a random effect to index time. The practice of using age as a metric for time is recommended by McArdle and Bell (2000) for instances in which a study lacks a natural baseline due to randomness of time of study entry, which is the case in the BLSA. The distribution of participants over time is regarded as a sample of performance opportunities across ages. With sufficient numbers of participants over a sufficiently broad range of ages, we can examine the overall differences in rates of change in time-varying effects.
Models included both linear and quadratic terms of interest, all of which were treated as fixed effects. Linear effects included the main effect of total cholesterol (representing overall differences in total cholesterol, regardless of longitudinal changes) and a 2-way interaction of total cholesterol × age (representing longitudinal change in cognitive outcomes associated with total cholesterol over time). Similarly, quadratic effects included a total cholesterol2 term (main effect) and total cholesterol2 × age term (longitudinal effect). We applied backward elimination of quadratic terms for cognitive tests without significant quadratic effects. In all models, main effects of total cholesterol and total cholesterol2 were presumed to be qualified by significant higher order longitudinal effects. Cohen’s f2 local effect sizes were calculated according to guidelines specific to mixed-effects models that include random effects (Selya, Rose, Dierker, Hedeker, & Mermelstein, 2012).
Results
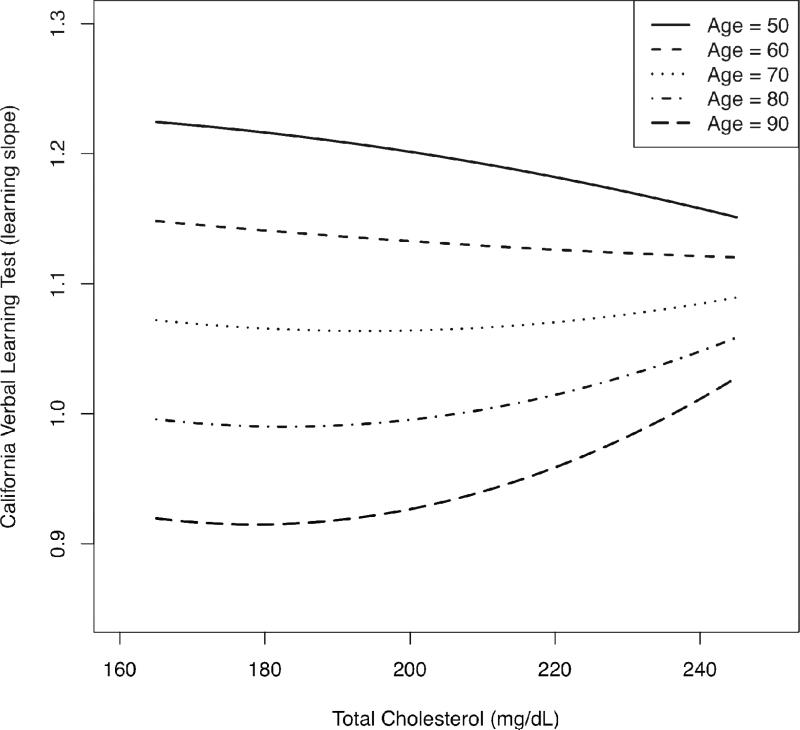
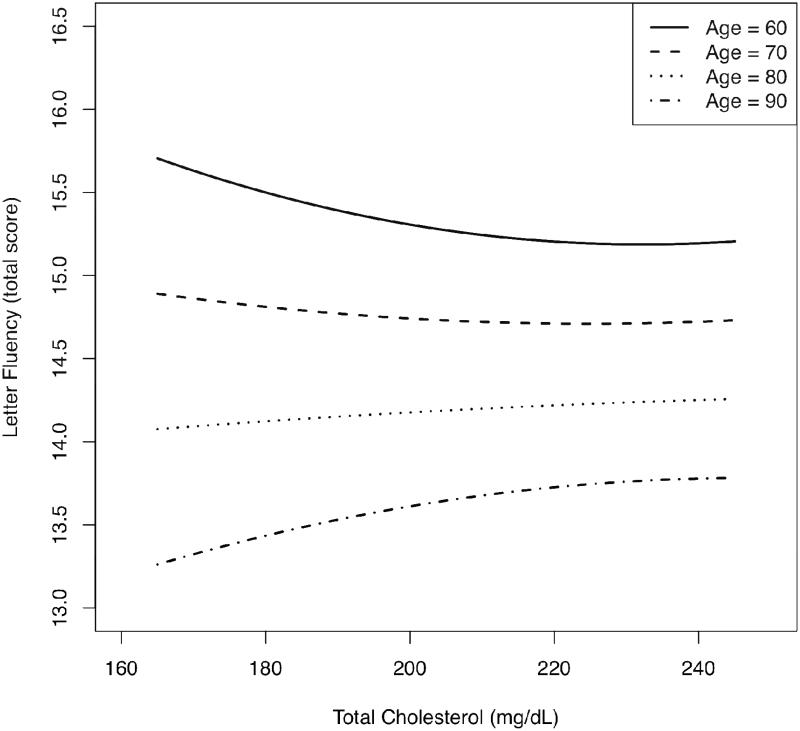
Table 2 shows sample characteristics at first assessment. Principal findings from mixed-effects regression analyses are shown in Table 3; for a comprehensive results table please see Table S1 in the online version of this article. Regarding nonlinear effects, all significant main effects (total cholesterol2) were qualified by significant longitudinal effects (total cholesterol2 × age). Significant longitudinal, quadratic effects were identified for Boston Naming Test (b = −.000130, p = .004), CVLT learning slope (b = .000008, p = .021), Letter Fluency (b = −.000060, p = .027), and MMSE (b = −.000050, p = .001). Figures 1 and 2 depict the significant quadratic, longitudinal effects of total cholesterol for CVLT learning slope and Letter Fluency, respectively; other cognitive outcomes displayed similar patterns. To summarize the effects most clearly, plots were generated using the predicted cognitive test scores associated with total cholesterol across representative age points. Ages 50-90 are shown for CVLT learning slope, and ages 60-90 are shown for Letter Fluency due to the predominance of 60+ year-old individuals in the Letter Fluency sample. Each graph depicts age-related change in cognitive performance as a function of total cholesterol using all information in the analyses, regardless of the number of repeated assessments. In general, the plots show that higher total cholesterol was associated with poorer middle-aged (ages 40-60) or young-old (ages 60-69) cognitive performance, but better old-old (ages 80-89) cognitive performance. The age-range labels represent widely used subcategories first described by Burnside and colleagues (1979).
Table 2.
Characteristics of Study Sample at First Assessment
| Variable | Mean (SD) or % | Range |
|---|---|---|
| Age (years) | 54.4 (16.4) | 19-93 |
| Sex (% male) | 50.8 | |
| Race (% white) | 74.6 | |
| Education (years) | 16.7 (2.5) | 4-24 |
| Body mass index (kg/m2) | 25.9 (4.3) | 17.4-48.8 |
| Systolic blood pressure (mm Hg) | 127.4 (20.2) | 85-210 |
| Total cholesterol (mg/dL) | 201.0 (36.2) | 95-338 |
| Cardiovascular disease* (% with ≥1 condition) | 17.9 | |
| Lipid-lowering medication use (%) | 4.2 | |
| Smoking (% ever) | 49.6 | |
| Alcohol use† (%) | 27.0 | |
| CES-D (total score) | 6.6 (6.8) | 0-50 |
Includes coronary artery disease, heart failure, peripheral arterial disease, transient ischemic attack, and diabetes mellitus.
Percentage reporting consumption of ≥2 alcoholic drinks per day over past 12 months on ≥1 BLSA visit.
Abbreviation: CES-D = Center for Epidemiological Studies - Depression scale.
Table 3.
Results from Mixed-Effects Regression Models Predicting Neuropsychological Test Performance from Total Cholesterol and Covariatesa
| Neuropsychological Test | TC × Age | TC × TC × Age | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| b | SE | P | f2 b | b | SE | P | f2 c | f2 d | |
| Benton Visual Retention Test | −.001910 | .000783 | .015 | .004 | .005 | ||||
| Blessed I-M-C Test | −.000360 | .000348 | .307 | .001 | .003 | ||||
| Boston Naming Test | .060990 | .019110 | .002 | .003 | −.000130 | .000046 | .004 | .004 | .007 |
| Card Rotation Test | .001452 | .008906 | .871 | .001 | <.001 | ||||
| CVLT, Immediate Free Recall | .000351 | .003271 | .915 | <.001 | <.001 | ||||
| CVLT, Learning Slope | −.002650 | .001399 | .058 | .002 | .000008 | .000003 | .021 | .002 | .004 |
| CVLT, Short Delay Free Recall | .001031 | .000910 | .257 | <.001 | <.001 | ||||
| CVLT, Long Delay Free Recall | .001124 | .000907 | .215 | <.001 | <.001 | ||||
| Digits Forward | .000790 | .000625 | .206 | <.001 | <.001 | ||||
| Digits Backward | .001361 | .000768 | .077 | <.001 | <.001 | ||||
| Category Fluency | .002683 | .001602 | .094 | <.001 | <.001 | ||||
| Letter Fluency | .030500 | .012270 | .013 | .001 | −.000060 | .000029 | .027 | .003 | .004 |
| Mini Mental State Examination | .023650 | .006219 | <.001 | .002 | −.000050 | .000015 | .001 | .005 | .008 |
| Trail Making Test, Part A | .005975 | .008969 | .505 | <.001 | <.001 | ||||
| Trail Making Test, Part B | −.017800 | .024510 | .468 | <.001 | <.001 | ||||
Coefficients in bold are significant at p<.05.
Models adjusted for age, sex, race, education, systolic blood pressure, body mass index, cardiovascular disease, lipid-lowering medication use, smoking, alcohol use, and depressive symptoms. Blank cells appear due to backward elimination of non-significant quadratic effects. Main effects of TC and TC × TC not shown for space reasons and are available in Table S1 in the online version of this article.
f2 = local effect size for linear cholesterol terms only.
f2 = local effect size for quadratic cholesterol terms (above and beyond linear cholesterol).
f2 = local effect size for all cholesterol terms combined.
Abbreviations: TC = Total cholesterol; I-M-C = Information-Memory-Concentration; CVLT = California Verbal Learning Test
Figure 1.
Nonlinear longitudinal change in performance on the California Verbal Learning Test (learning slope) as a function of quadratic total cholesterol, across five age groups. Higher test scores indicate better performance.
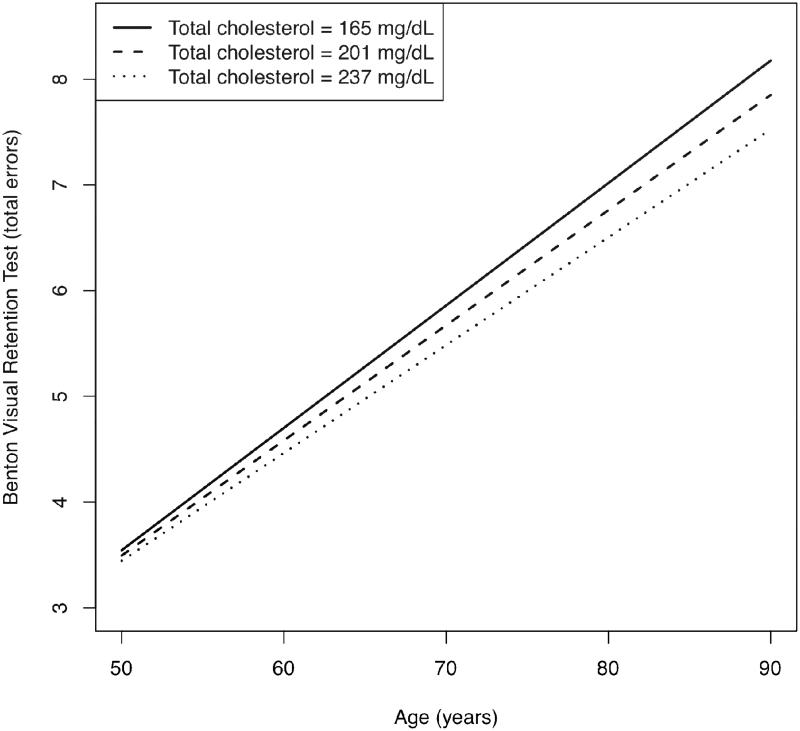
For the remainder of the cognitive tests, for which no significant quadratic effects arose, quadratic terms were removed by backward elimination. Resultant models revealed a significant linear longitudinal effect for BVRT (b = −.001910, p = .015). Figure 3 demonstrates that lower total cholesterol (represented graphically as -1 SD from mean) was associated with accelerated decline in visual memory performance over time, when compared with mean and higher (+1SD from mean) total cholesterol. There were no significant main effects of total cholesterol except for BVRT, for which the effect was qualified by the aforementioned significant longitudinal effect.
Figure 3.
Linear longitudinal change in performance on the Benton Visual Retention Test as a function of total cholesterol. Higher test scores indicate worse performance (number of errors).
Discussion
In this prospective study of up to 1,601 community-dwelling, neurologically intact participants studied on up to 12 occasions, we identified nonlinear associations between total cholesterol and multiple cognitive functions, including global mental status, verbal learning, executive function, and language. In general, higher total cholesterol was associated with poorer cognitive performance at earlier ages, but better old-old cognitive performance. Linear models also revealed lower total cholesterol to be associated with accelerated longitudinal decline in visual memory performance.
The present findings imply a possible synthesis of ostensibly conflicting cross-sectional and longitudinal findings to date. Consistent with the conclusions of a recent review (van Vliet, 2012), the current study provides direct evidence (i.e., within the same participant sample) of differential associations of total cholesterol with cognitive function at different points across the life span. More specifically, among middle-aged or young-old individuals, lower total cholesterol was associated with less prospective decline in global mental status, verbal memory, executive function, and language function. In contrast, among the old-old, higher total cholesterol was associated with less prospective decline in cognitive performance. At least two studies have shown a similar pattern of findings, though without direct examination of nonlinear effects (Reynolds, Gatz, Prince, Berg, & Pedersen, 2010; Solomon et al., 2009). Solomon and colleagues (2007) have also noted bidirectionality in the association between cholesterol and prospective dementia risk. It is therefore conceivable that studies that (a) examine a more limited age range, (b) do not assess coincident change in cholesterol and cognitive function, and/or (c) omit nonlinear analysis may inevitably produce conflicting results.
Several additional factors bear mention relevant to associations between total cholesterol and cognitive function. First, it is possible that findings at older ages may, to some degree, reflect selective survival. That is, at advanced ages, individuals most vulnerable to the pathobiological effects of cholesterol may not have survived. Further, though beyond the scope of the current study, multiple effect modifiers may affect these associations. For instance, sex differences may occur secondary to cholesterol’s role as a precursor of steroid hormones (e.g., estrogen) or the biological changes during and after menopause. There is also debate regarding the impact of statins on cognitive function in the absence (Wagstaff, Mitton, Arvik, & Doraiswamy, 2003) and presence (Biondi, 2011) of dementia, as well as prospective risk of mild cognitive impairment and dementia (Beydoun et al., 2011; Shepardson, Shankar, & Selkoe, 2011). Declines in total cholesterol in premorbid dementia further cloud this literature. Though cases of prevalent and incident dementia were excluded from the present analyses, future onset of dementia in included individuals cannot be ruled out. Lastly, frailty may act as an effect modifier. Among older adults, lipid levels may have opposite effects on health risk in normal versus frail individuals (Corti et al., 1997). That is, high total cholesterol serves as a good prognostic factor in frail individuals, whereas the converse is true among healthy older adults. Based on this premise, the present findings could be in part driven by frailty among our older participants.
Different mechanistic hypotheses may explain relations of both high and low total cholesterol with cognitive function. Brain and peripheral lipid levels were once thought to be entirely isolated from one another by the blood-brain barrier, but recent evidence indicates they interact continuously via oxysterol molecules (e.g., 24S- and 27 hydroxycholesterol) (Bjorkhem, 2006). Within the brain, cholesterol is a crucial component of myelin, neuronal membranes, and glial membranes and is involved in both cerebral structure (e.g., structural integrity of neurons, membrane fluidity) and function (e.g., neurotransmission, nutrient transport to the brain) (Muldoon, Flory, & Ryan, 2001). Accordingly, lower levels of peripheral cholesterol may be deleterious to the brain’s microstructure and function, thereby negatively affecting cognitive function and trajectories. Conversely, higher levels of cholesterol are strongly tied to atherogenesis. Atherosclerosis leads to macro- and micro-vascular disease that in turn can impact cognitive function negatively on both subclinical (e.g., carotid artery intimal medial thickness, white matter disease) and clinical (e.g., stroke) levels (de Haan, Nys, & Van Zandvoort, 2006; Wendell, Zonderman, Metter, Najjar, & Waldstein, 2009; Wright et al., 2008). Additionally, apolipoprotein E genotype may act as a third variable, given its association with both cholesterol function and risk of Alzheimer’s disease (Hauser, Narayanaswami, & Ryan, 2011). Elevated cholesterol levels are also associated with increased formation of beta-amyloid from amyloid-precursor proteins (Kojro, Gimpl, Lammich, Marz, & Fahrenholz, 2001), which is independently associated with cognitive function in the absence of dementia (Resnick et al., 2010).
Strengths of this investigation include its longitudinal design, examination of coincident cholesterol and cognitive function trajectories, analysis of nonlinear effects, length of follow-up, use of an extensive neuropsychological battery, and incorporation of time-dependent covariates. A limitation of this investigation is its use of a convenience sample of typically highly educated participants. The homogeneity and nonrepresentative nature of the sample may limit the study’s generalizability, though the sample’s homogeneity may also restrict the effect of confounding demographic factors. Additionally, analysis of select neuropsychological tests was limited by predominately 60+ year-old participant samples. Future research should consider nonlinear relations of subcomponents of cholesterol (e.g., low- and high-density lipoprotein) with cognitive trajectories, as well as higher-order nonlinear effects.
In conclusion, results from this investigation indicate quadratic associations between coincident trajectories of total cholesterol and cognitive functions, including global mental status, verbal learning, executive function, and language function. Higher total cholesterol was associated with poorer cognitive performance among the middle-aged or young-old, but better cognitive performance among the old-old. For visual memory, lower cholesterol was associated with more pronounced linear decline in performance over time. These results indicate differential longitudinal relations of total cholesterol to cognitive decline across different ages and domains of function, which could in part explain existing inconsistencies in relevant literature. Both high and low total cholesterol deserve continued consideration as risk factors for detrimental cognitive changes across the life span.
Supplementary Material
Figure 2.
Nonlinear longitudinal change in performance on Letter Fluency as a function of quadratic total cholesterol, across four age groups. Higher test scores indicate better performance.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute on Aging (NIA).
References
- Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. American Journal of Geriatric Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Louis ED, Vega S, Bermejo-Pareja F. Statins and cognitive functioning in the elderly: A population-based study. Journal of Alzheimers Disease. 2010;21:95–102. doi: 10.3233/JAD-2010-100180. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Beason-Held LL, Kitner-Triolo MH, Beydoun HA, Ferrucci L, Resnick SM, et al. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. Journal of Epidemiology and Community Health. 2011;65:949–957. doi: 10.1136/jech.2009.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi E. Prescription of lipophilic statins to Alzheimer’s disease patients: Some controversies to consider. Neurological Science. 2011;32:195–201. doi: 10.1007/s10072-010-0440-0. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- Burnside IM, Ebersole P, Monea HE. Psychosocial caring throughout the life span. McGraw Hill; New York: 1979. [Google Scholar]
- Corti MC, Guralnik JM, Salive ME, Harris T, Ferrucci L, Glynn RJ, et al. Clarifying the direct relation between total cholesterol levels and death from coronary heart disease in older persons. Annals of Internal Medicine. 1997;126:753–760. doi: 10.7326/0003-4819-126-10-199705150-00001. [DOI] [PubMed] [Google Scholar]
- de Haan EH, Nys GM, Van Zandvoort MJ. Cognitive function following stroke and vascular cognitive impairment. Current Opinions in Neurology. 2006;19:559–564. doi: 10.1097/01.wco.0000247612.21235.d9. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D’Agostino RB, Sullivan LM, Wolf PA. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosomatic Medicine. 2005;67:24–30. doi: 10.1097/01.psy.0000151745.67285.c2. [DOI] [PubMed] [Google Scholar]
- Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: From lipid transport to neurobiology. Progress in Lipid Research. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proceedings of the National Academy of Sciences U S A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, editors. Neuropsychological assessment. 4th Oxford University Press; New York: 2004. [Google Scholar]
- Mathew A, Yoshida Y, Maekawa T, Kumar DS. Alzheimer’s disease: Cholesterol a menace? Brain Research Bulletin. 2011;86:1–12. doi: 10.1016/j.brainresbull.2011.06.006. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Bell RQ. An introduction to latent growth models for developmental data analysis. In: Little TD, Schnabel KU, Baumert J, editors. Modeling longitudinal and multilevel data: practical issues, applied approaches, and specific examples. Erlbaum; Mahwah, NJ: 2000. pp. 69–107. [Google Scholar]
- Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Flory JD, Ryan CM. Serum cholesterol, the brain, and cognitive functioning. In: Waldstein SR, Elias MF, editors. Neuropsychology of cardiovascular disease. Erlbaum; Mahwah, NJ: 2001. pp. 37–59. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Gatz M, Prince JA, Berg S, Pedersen NL. Serum lipid levels and cognitive change in late life. Journal of the American Geriatrics Society. 2010;58:501–509. doi: 10.1111/j.1532-5415.2010.02739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG. The effects of cholesterol on learning and memory. Neuroscience and Biobehavioral Reviews. 2010;34:1366–1379. doi: 10.1016/j.neubiorev.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Frontiers in Psychology. 2012;3:111. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Archives of Neurology. 2011;68:1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24:323–355. [Google Scholar]
- Solomon A, Kareholt I, Ngandu T, Winblad B, Nissinen A, Tuomilehto J, et al. Serum cholesterol changes after midlife and late-life cognition: Twenty-one-year follow-up study. Neurology. 2007;68:751–756. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- Solomon A, Kareholt I, Ngandu T, Wolozin B, Macdonald SW, Winblad B, et al. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiology of Aging. 2009;30:1006–1009. doi: 10.1016/j.neurobiolaging.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70:360–367. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- Swan GE, LaRue A, Carmelli D, Reed TE, Fabsitz RR. Decline in cognitive performance in aging twins. Heritability and biobehavioral predictors from the National Heart, Lung, and Blood Institute Twin Study. Archives of Neurology. 1992;49:476–481. doi: 10.1001/archneur.1992.00530290058012. [DOI] [PubMed] [Google Scholar]
- Teunissen CE, De Vente J, von Bergmann K, Bosma H, van Boxtel MP, De Bruijn C, et al. Serum cholesterol, precursors and metabolites and cognitive performance in an aging population. Neurobiology of Aging. 2003;24:147–155. doi: 10.1016/s0197-4580(02)00061-1. [DOI] [PubMed] [Google Scholar]
- van Vliet P. Cholesterol and late-life cognitive decline. Journal of Alzheimers Disease. 2012;30:S147–162. doi: 10.3233/JAD-2011-111028. [DOI] [PubMed] [Google Scholar]
- Wagstaff LR, Mitton MW, Arvik BM, Doraiswamy PM. Statin-associated memory loss: Analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–880. doi: 10.1592/phco.23.7.871.32720. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: The Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke. 2009;40:3180–3185. doi: 10.1161/STROKEAHA.109.557280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Beeri MS, Schmeidler J, Hannigan CM, Angelo G, Grossman HT, et al. Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. American Journal of Geriatric Psychiatry. 2008;16:781–785. doi: 10.1097/JGP.0b013e3181812790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Festa JR, Paik MC, Schmiedigen A, Brown TR, Yoshita M, et al. White matter hyperintensities and subclinical infarction: Associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–805. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.