Abstract
Objective
We aimed to evaluate the impact of a novel noninvasive oral pressure therapy (OPT) (Winx®, ApniCure) system on polysomnographic measures of sleep-disordered breathing, sleep architecture, and sleep stability in obstructive sleep apnea (OSA).
Subjects and methods
A 4-week, multicenter, prospective, open-label, randomized, crossover, first-night order of control vs treatment, single-arm trial was conducted in five American Academy of Sleep Medicine (AASM) – accredited sleep clinics and one research laboratory. Sixty-three subjects (analysis cohort) were studied from a screening cohort of 367 subjects. The analysis cohort was 69.8% men, ages 53.6 ± 8.9 years (mean ± SD), body mass index of 32.3 ± 4.5 kg/m2, with mild to severe OSA. At treatment initiation, subjects received random assignment to one night with and one without (control) treatment, and they were assessed again following 28 nights of treatment. Breathing and sleep architecture were assessed each night based on blind scoring by a single centralized scorer using AASM criteria.
Results
Average nightly usage across the take-home period was 6.0 ± 1.4 h. There were no severe or serious device-related adverse events (AEs). Median apnea–hypopnea index (AHI) was 27.5 events per hour on the control night, 13.4 events per hour on the first treatment night, and 14.8 events per hour after 28 days of treatment. A clinically significant response (treatment AHI ≤10/h and ≤50% of control values) was seen in 20 of the 63 subjects evaluated. Rapid eye movement percentage (REM%) was significantly increased, and N1%, stage shifts to N1 sleep, overall stage shifts, total awakenings, and arousals per hour were all significantly reduced at both treatment nights compared to controls. Mean Epworth sleepiness scale (ESS) was significantly reduced from 12.1 to 8.6 (Cohen d effect size, 0.68) in those untreated for two or more weeks prior to OPT study participation and remained unchanged in subjects who directly switched from continuous positive airway pressure (CPAP) therapy to OPT.
Conclusion
Clinically significant improvements in sleep quality and continuity, AHI, ODI, ESS, and overall clinical status were achieved in an easily identified subgroup. OPT was safe and well-tolerated and nightly usage was high.
Keywords: Oral pressure therapy, Obstructive sleep apnea, AHI, ODI, Compliance, Epworth sleepiness scale
1. Introduction
Obstructive sleep apnea (OSA) is a common medical condition [1,2] which causes substantial morbidity and possibly mortality [3]. The process of repetitive airflow obstruction during sleep and the consequent need to arouse to breathe precipitates a reduction in the restorative effect of sleep, often leading to daytime sleepiness [4]. OSA patients also are at a significantly increased risk for negative impact on measures of attention and vigilance and on mood disturbance [5]. Moreover, chronic untreated OSA yields a significantly increased risk for cardiovascular diseases over a period of years, including hypertension, cardiac arrhythmias, atherosclerotic heart disease, myocardial infarction, stroke, death [6], and possibly type 2 diabetes mellitus [7].
No treatment exists for OSA that is both completely effective and fully tolerated by all patients; many partially efficacious or partially tolerated treatments currently are in use. Although nasal continuous positive airway pressure (CPAP) is the recommended therapy for reducing apneas and hypopneas [8], adherence to CPAP often is low [9,10]. Given the seriousness of the risks associated with untreated or ineffectively treated OSA, it is important for physicians and patients to have a wide variety of treatment options to increase the likelihood of a successful option [11]. Our study describes a novel treatment modality, oral pressure therapy (OPT), which applies a vacuum to the mouth to stabilize upper airway tissue in patients with OSA.
2. Methods
The ATLAST study was a 4-week, multicenter, prospective, open-label, randomized, crossover, first-night order of control vs treatment, single-arm trial of the Winx Sleep Therapy System for the treatment of OSA. The objective of the study was to evaluate safety and efficacy of the Winx Sleep Therapy System. The study was approved by Western Institutional Review Board, Olympia, Washington, was conducted in compliance with US Food and Drug Administration Good Clinical Practice guidelines, and was registered as a clinical trial with clinicaltrials.gov (NCT01146782).
2.1. Subject population
Subjects with mild (apnea–hypopnea index [AHI] ≥5/h and <15/h), moderate (AHI ≥15/h and <30/h), or severe (AHI ≥30/h) OSA were recruited at six clinical research sites. The major inclusion criteria were (1) subjects aged between 18 and 80 years; (2) subjects with an oxygen desaturation index (ODI) (based on 4% decrease in oxygen saturation [SaO2]) between 10 and 60 events per hour, as assessed during a home-screening night; (3) subjects with a body mass index less than 40 kg/m2; (4) subjects with at least one molar in each of the four quadrants of the mouth; and (5) subjects with a proper mouthpiece fit, as assessed by a home-screening night. The major exclusion criteria were (1) subjects with poor nasal patency, as evidenced by peak nasal inspiratory flow (PNIF) of less than 75 L per minute; (2) subjects with a clinical history of oral cavity infection, loose teeth, temporomandibular joint conditions, prior OSA surgical treatment, use of medications that may affect sleep or polysomnography (PSG), concomitant diagnosed sleep or chronic neurologic disorder other than OSA, central sleep apnea, or severe cardiovascular or pulmonary disease; (3) subjects working nights or rotating shifts; and (4) female subjects who were pregnant or intended to become pregnant during the study.
2.2. Screening
Control tests and procedures performed during the initial screening phase (S1) included: Epworth sleepiness scale (ESS), a modified version of the Functional Outcome of Sleep Questionnaire (mFOSQ) in which we removed the section on intimate and sexual relations, PNIF measure, mouthpiece measurement by bite wax, and system training.
Following initial screening, OSA eligibility screening criteria were assessed (S2) with a 1-night level-4 home sleep test utilizing an oximeter and actigraph to confirm ODI between 10 and 60 events per hour. If the subject met the ODI criteria, he or she continued with the next home screen (S3). The purpose of the S3 screen was to exclude subjects in whom PSG data were unlikely to be evaluable due to inadequate duration of sleep, to exclude subjects with inadequate mouthpiece fit, and to eliminate subjects meeting exclusion criteria that could only be functionally detected (i.e., nighttime mouth breathing, inadequate nasal patency). One-night home use of the Winx System with actigraphy to screen for proper fit including maintenance of oral vacuum was conducted. Subjects needed to have a total of at least 4 h of sleep time.
2.3. Treatment phase
For subjects meeting the requirements of the three screening phases, the sequence of control and treatment night PSGs were randomized. Subjects were required to stop any OSA treatment for at least 2 weeks prior to the first PSG night. After completing the control and first treatment night PSGs, the subjects started the 28-day take-home period. There was telephone contact on day three and clinic visits for console data download and adverse event (AE) assessment on days 7, 14, 21, and 28. The final office visit on day 28 included ESS, mFOSQ, and Clinical Global Impression Change (CGI-C) and was followed with the day 28 treatment night PSG. Subjects’ opinion of therapy was scored on a Likert scale.
2.4. Device description
The system (Winx Sleep Therapy System, ApniCure, Inc., Redwood City, CA) is comprised of three components: an oral interface, a pump, and tubing (Fig. 1). The oral interface is a polymer mouthpiece which incorporates a lip seal and a connector. In our study, 10 different mouthpiece sizes were available. The pump consisted of a vacuum pump, pump controller, and pressure measurement component. The pump applied continuous negative pressure to the oral interface using feedback control. Tubing connected the oral interface to the pump and allowed for withdrawal of saliva from the mouth. The console included a reservoir for the collection of excess saliva. The negative pressure generated by the console and conveyed via tubing through the mouthpiece into the oral cavity was intended to create a pressure gradient to draw the soft palate anteriorly into stable contact with the tongue to permit improved airflow during sleep. During system operation, the user breathes normally through the nose. The negative pressure in the oral cavity is isolated from the nasopharyngeal airway by the natural seal that occurs between the soft palate and tongue. The user can choose to insert or remove the mouthpiece at any time.
Fig. 1.
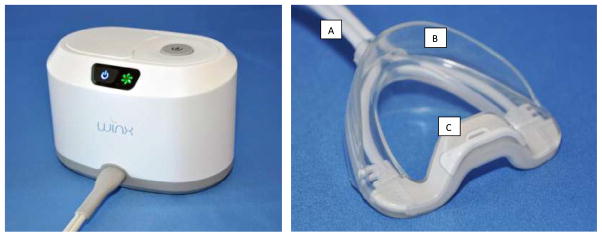
Oral pressure therapy (OPT) system (WinxTM). The left panel shows the nightstand console unit containing the vacuum pump and saliva reservoir. The right panel shows the mouthpiece. Letter A indicates the vacuum pressure and sensor tubing, B shows the lip seal, and C shows the vacuum aspiration port.
2.5. PSG data
Data from the control PSG, first treatment night PSG, and day 28 treatment night PSG were scored by a single independent scorer using American Academy of Sleep Medicine (AASM) scoring criteria (AASM Manual for Scoring Sleep, 2007) while blinded to control or treatment status. Specifically, respiratory events were scored using the AASM recommended criteria, and thus hypopneas were required to have at least a 30% airflow reduction and a 4% SaO2 desaturation. Mixed events were scored as obstructive events. Study investigators were blinded to PSG results and instructed not to score the subjects’ PSG data.
2.6. Safety data
All AEs, regardless of seriousness, severity, or relationship to study device, were recorded. The evaluation included a determination of the seriousness and severity of the event, whether or not the event or the severity of the event was anticipated or unanticipated, and the relationship of the event to the study device. Severity was categorized as mild (awareness of sign or symptom but easily tolerated), moderate (discomfort enough to cause interference with usual activity), or severe (incapacitating with inability to work or do usual activity).
Dental casts from dental impressions taken at control and after 28 days of home use were evaluated by a dentist to assess any evidence of tooth movement or occlusal changes.
2.7. Analysis and responder cohorts
The analysis cohort included all evaluable subjects, defined as those who completed both PSG nights at the beginning of the study with total sleep time of at least 4 h each night, and who had an AHI of ≥5 events per hour on the control PSG night. The safety cohort included all subjects who used the Winx Sleep Therapy System at least one night during any phase of the study. The screening cohort included all subjects who signed informed consent. For analysis of the relationship of changes in sleep architecture in relation to changes in AHI, a first-night responder cohort was defined as those subjects meeting the combination criteria of AHI ≤10 events per hour combined with reduction of at least 50% compared to control.
2.8. Statistical analysis
Data summaries and listings were performed using Statistical Analysis Software (SAS®), version 9.1 (SAS Institute, Cary, NC). Variables meeting criteria for the use of parametric statistics were reported as mean ± standard deviation, with differences between control and treatment nights assessed with paired t tests. For variables not meeting parametric criteria, statistics are reported as medians and interquartile ranges and differences between control and treatment nights assessed with Wilcoxon signed rank tests. Correlations between treatment-related changes in sleep and breathing variables were assessed using Spearman rho.
3. Results
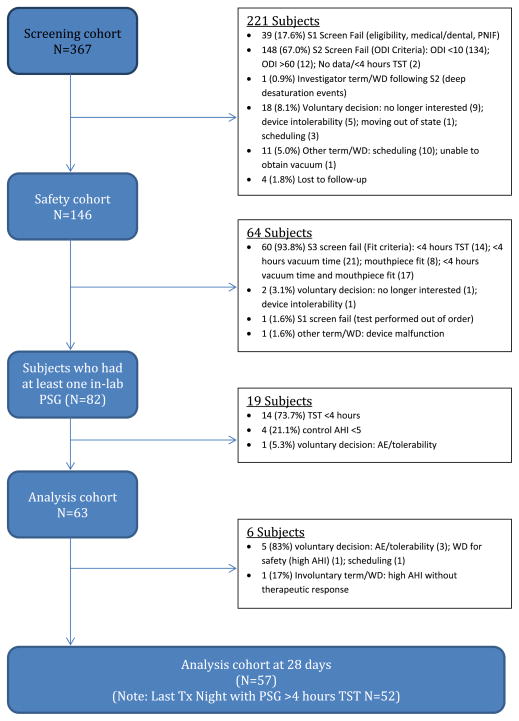
3.1. Subject disposition
Subject disposition is summarized in Fig. 2. The analysis cohort of 63 subjects comprised 33% without prior OSA treatment, 24% with prior CPAP experience who had abandoned CPAP therapy, and 43% who were active CPAP users when they elected to participate in our study.
Fig. 2.
Shows the flowchart of subject disposition from initial enrolment through to the end of the 28-day take-home portion of the protocol.
3.2. AHI and ODI
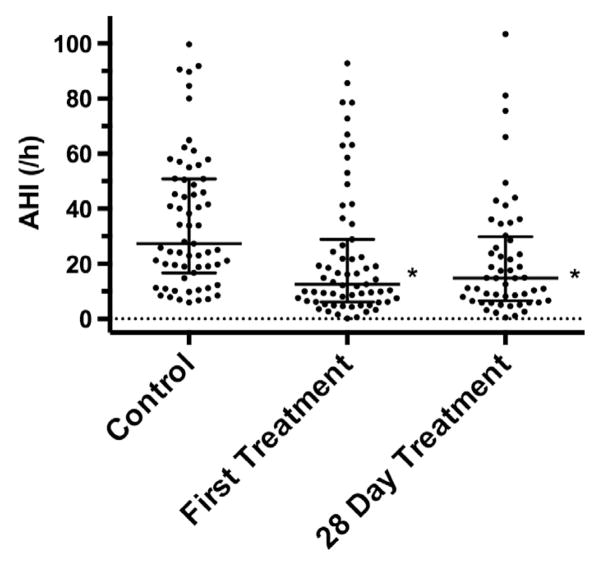
Across the whole analysis cohort, the AHI reduction was statistically significant compared to controls at the first treatment night (Wilcoxon signed rank, P < .0001) and the treatment night following day 28 (Wilcoxon signed rank, P = .0002), as shown in Fig. 3 and Table 1. The responder cohort consisted of 32% (20/63) of subjects displaying an AHI at first treatment night of ≤10 events per hour and reduction in excess of 50% comparing first treatment night to controls. In this subset of 20 subjects, median (interquartile range) AHI was 4.9 (3.0–7.2 events/h) at first treatment night and 8.7 (4.4–14.3 events/h) at the last treatment night.
Fig. 3.
Apnea–hypopnea index at control, first treatment night, and after 28 days of treatment. Points represent individual subjects and lines denote median and interquartile range. *P < .001 vs control by Wilcoxon signed rank test.
Table 1.
First treatment night and day 28 treatment night apnea–hypopnea index and oxygen desaturation index (analysis cohort).
| Control night median (Q1–Q3) | Treatment night median (Q1–Q3) | Median change (Q1–Q3) | Median of % change (Q1–Q3) | ||
|---|---|---|---|---|---|
| Control night vs first treatment night (N = 63) | AHI | 27.5 (16.7, 50.9) | 13.4 (6.2, 28.9) | −10.4 (−21.8, 1.0) | −46.2 (−74.7, 3.3) |
| P value | <.0001 | <.0001 | |||
| ODI | 22.7 (11.7, 41.6) | 12.0 (5.7, 23.9) | −6.5 (−18.3, 2.2) | −41.9 (−63.5, 9.5) | |
| P value | <.0001 | .0001 | |||
| Control night vs day 28 treatment night (N = 52) | AHI | 24.6 (11.8, 48.4) | 14.8 (6.6, 29.4) | −7.9 (−26.0, 4.2) | −43.2 (−71.1, 15.9) |
| P value | .0002 | .0050 | |||
| ODI | 20.2 (10.8, 39.5) | 12.5 (6.6, 23.9) | −6.3 (−24.1, 4.0) | −43.1 (−69.5, 20.0) | |
| P value | .0012 | .0151 |
Abbreviations: AHI, apnea–hypopnea index; ODI, oxygen desaturation index.
P value = Wilcoxon signed rank test.
Changes in AHI were considered in relation to the severity of OSA observed in the control PSG, and the results are shown in Fig. 4. A clinically significant response in AHI was seen in four out of 15 subjects with mild OSA, nine out of 18 subjects with moderate OSA, and seven out of 30 subjects with severe OSA. ODI also was significantly improved with treatment in the analysis cohort (Table 1), with significant reductions at both first treatment night (Wilcoxon signed rank, P < .0001) and the treatment night following day 28 (Wilcoxon signed rank, P = .0012).
Fig. 4.
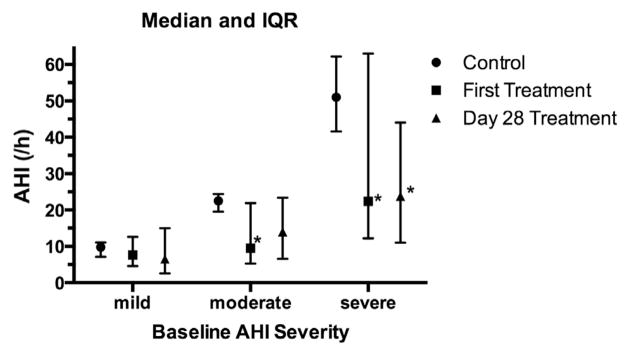
Apnea–hypopnea index (AHI) at control, first treatment night, and after 28 days of treatment in relation to control obstructive sleep apnea severity. Control AHI severity was classified as mild (5–15/h), moderate (15–30/h), or severe (>30/h). Lines denote median and interquartile range. *P < .02 vs control by Wilcoxon signed rank test.
3.3. Symptomatic measures
Forty subjects who completed the ESS were treatment naïve or stopped using their OSA treatment at least 2 weeks prior to their first visit, and therefore had untreated control ESS scores (naïve). Seventeen subjects completed the ESS before stopping their OSA treatment for 2 weeks, and therefore their control ESS scores reflected the results of prior treatment (pretreated). With treatment, improvement in ESS was seen in the naïve subjects (paired t test, P < .0001) with an effect size (Cohen d) of 0.68 and maintenance of ESS was observed in pretreated subjects. (Table 2). Similar results were seen in the mFOSQ as shown in Table 2, with improvement in the naïve subgroup (paired t test, P = .0009; Cohen d = 0.63). When we compared clinical conditions after 28 nights of treatment to the control condition, significant improvement was noted in the CGI-C, as shown in Table 3 (P < .0001, χ2 test). At the conclusion of participation, 76% of subjects indicated that they agreed with the statement, “I would use the system to treat my sleep apnea,” on a Likert scale.
Table 2.
Epworth sleepiness scale and modified Function Outcome of Sleep Questionnaire (analysis cohort).
| Naïvea | Pretreatedb | ||
|---|---|---|---|
| No. of subjects | 40 | 17 | |
| ESS | Control | 12.1 ± 5.1 | 9.4 ± 7.0 |
| Day 28 | 8.6 ± 5.2 | 8.5 ± 5.9 | |
| Change from control | −3.5 ± 4.6 | −0.9 ± 2.9 | |
| P value (t test) | <.0001 | .1938 | |
| Cohen d | 0.68 | 0.14 | |
| mFOSQ | Control | 15.5 ± 3.1 | 17.2 ± 2.9 |
| Day 28 | 17.2 ± 2.2 | 17.6 ± 2.5 | |
| Change from control | 1.7 ± 3.1 | 0.4 ± 1.4 | |
| P value (t-test) | .0009 | 0.63 | |
| Cohen’s d | .2716 | 0.15 |
Abbreviations: EES, Epworth sleepiness scale; mFOSQ, modified Function Outcome of Sleep Questionnaire.
Values are expressed as mean ± standard deviation.
Subgroup 1: subjects were treatment naïve or off obstructive sleep apnea (OSA) treatment 2 weeks prior to completing survey.
Subgroup 2: subjects were on OSA treatment time of survey.
Table 3.
Clinical global impression scale changes (analysis cohort).
| Very much improved | 12.3% |
| Much improved | 45.6% |
| Minimally improved | 28.1% |
| No change | 12.3% |
| Minimally worse | 1.8% |
| Much worse | 0.0% |
| Very much worse | 0.0% |
| P value (χ2 test) | <.0001 |
3.4. Sleep architecture
PSG results are shown in Table 4 for the analysis cohort. The difference in AHI between controls and first treatment night was significantly correlated with differences in shifts to stage 1 (rho = 0.482), arousal index (rho = 0.613), rapid eye movement percentage (REM%) (rho = −0.293), and stage 1% (rho = 0.387) (all P < .01). The same pattern of correlations was seen for differences between control and day 28 treatment night (shifts to stage 1, rho = 0.376; arousal index, rho = 0.548; stage 1%, rho = 0.413) (all P < .01), with the exception of REM% (rho = −0.200).
Table 4.
Sleep architecture (analysis cohort).
| Control | First treatment night | Day 28 treatment night | |
|---|---|---|---|
| No. of subjects | 63 | 63 | 52 |
| Stage N1 shifts | 12.0 ± 6.2 | 9.5 ± 4.5* | 8.7 ± 4.6* |
| Stage shifts | 33.1 ± 12.1 | 27.6 ± 9.1* | 26.3 ± 10.7* |
| Awakenings | 43.5 ± 23.5 | 37.7 ± 16.1* | 33.9 ± 16.7* |
| Arousal index/h | 42.8 ± 18.1 | 32.4 ± 15.8* | 31.4 ± 13.1* |
| Sleep efficiency (% TST) | 79.7 ± 9.1 | 78.6 ± 8.7 | 83.2 ± 8.1* |
| WASO (min) | 74.3 ± 41.5 | 78.5 ± 39.5 | 61.7 ± 37.7* |
| % TST in N1 | 27.1 ± 18.0 | 21.6 ± 12.4* | 21.4 ± 13.3* |
| %TST in N2 | 49.3 ± 12.7 | 51.3 ± 11.6 | 52.8 ± 12.5* |
| %TST in N3 | 7.1 ± 7.0 | 8.6 ± 8.4 | 7.6 ± 6.5 |
| %TST in REM | 16.5 ± 7.5 | 18.4 ± 6.5* | 18.2 ± 6.9 |
| %TST supine | 62.2 ± 35.2 | 69.3 ± 31.4* | 69.2 ± 32.2* |
Abbreviations: h, hour; TST, total sleep time; WASO, wake after sleep onset; min, minutes; N1, stage 1 sleep; N2, stage 2 sleep; N3 stage 3 sleep; REM, rapid eye movement. Values are expressed as mean ± standard deviation.
P < .05 vs control (t test).
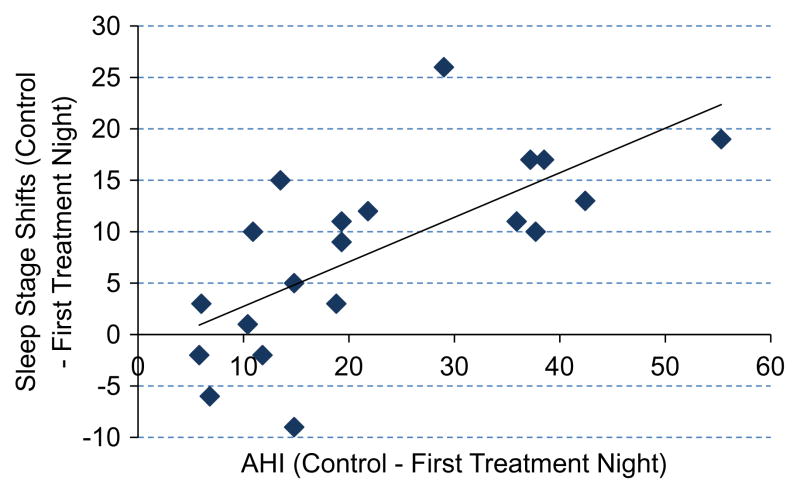
The PSG results for the responder cohort are shown in Table 5, and Fig. 5 shows the correlation of the difference in AHI between control and first treatment night with differences in stage shifts in the responder cohort.
Table 5.
Sleep architecture (responder cohort).
| Control | First treatment night | Day 28 treatment night | |
|---|---|---|---|
| No. of subjects | 20 | 20 | 20 |
| Stage N1 shifts | 11.0 ± 4.6 | 7.2 ± 2.6* | 6.2 ± 2.7* |
| Stage shifts | 31.9 ± 10.2 | 23.7 ± 5.1* | 22.1 ± 7.0* |
| Awakenings | 39.0 ± 17.1 | 31.5 ± 12.1* | 22.3 ± 8.3* |
| Arousal index/h | 41.3 ± 17.5 | 19.7 ± 7.9* | 23.4 ± 9.4* |
| Sleep efficiency (% TST) | 82.1 ± 8.7 | 80.7 ± 9.6 | 82.9 ± 15.7 |
| WASO (min) | 64.5 ± 38.5 | 71.0 ± 43.6 | 63.5 ± 67.7 |
| % TST in N1 | 23.5 ± 11.2 | 16.9 ± 11.3* | 14.9 ± 9.2* |
| %TST in N2 | 52.6 ± 7.9 | 52.2 ± 10.7 | 54.2 ± 8.1 |
| %TST in N3 | 7.7 ± 6.2 | 11.0 ± 8.08* | 10.0 ± 5.9* |
| %TST in REM | 16.2 ± 6.4 | 19.9 ± 6.4* | 21.0 ± 7.8* |
| % TST supine | 52.9 ± 38.0 | 50.5 ± 36.8 | 55.7 ± 36.1 |
Abbreviations: h, hour; TST, total sleep time; WASO, wake after sleep onset; min, minutes; N1, stage 1 sleep; N2, stage 2 sleep; N3 stage 3 sleep; REM, rapid eye movement. Values are expressed as mean ± standard deviation.
P < .05 vs control (t test).
Fig. 5.
Relationship between change in apnea–hypopnea index and change in number of sleep-stage shifts between control and first treatment night in the responder cohort.
3.5. Safety
Safety data were obtained from 146 subjects with 2496 nights of device exposure. There were no serious device-related AEs. Two serious AEs were reported, both of which were not related to the device and included a subject who was hit by a car while on a motorcycle (deemed that the other driver was at fault) and a subject who had an elective surgical removal of a benign tumor on his thigh. Of the device-related AEs, the majority (81%) were categorized as mild, 19% as moderate, and zero as severe. Typical AEs were oral tissue discomfort or irritation (73%), dental discomfort (9%), and dry mouth (6%). Device-related AEs were reported on an average of 50% of nights of usage. Of the subjects reporting device-related AEs, 41% were not recurring, 20% recurred with each use of the device and resolved less than 2 h after device removal, 3% recurred with each use of the device and resolved 2–4 h after device removal, 19% recurred with each use of the device and resolved before the next night’s use, and 17% recurred for the first few days of device use and then resolved.
There were no reports of device-related oral infection, tooth damage, temporomandibular joint dysfunction, pain, or discomfort. None of the device-related AEs were rated as severe or progressed in severity with time or continued use. All AEs resolved without consequence. Three subjects with mild AEs and one subject with a moderate AE (throat irritation) were treated with over-the-counter medication, and the remaining subjects required no medical intervention.
Three subjects in the analysis cohort terminated participation in our study due to an AE. One subject had mild oral tissue and dental discomfort, one subject had moderate dental discomfort, and one subject had moderate oral tissue irritation and oral tissue discomfort. None of these subjects required medical intervention to treat these issues.
Dental cast evaluation demonstrated no clinically significant tooth movement or clinically significant occlusal changes in any of the evaluated subjects between control and the final (day 28) study visit.
3.6. Adherence
Analysis of device usage adherence was performed using vacuum pressure data recorded electronically by the console on the analysis cohort, which completed their 28-day take-home evaluation. Thus adherence data reflect the time that the device was being used, rather than just being turned on. Any night in which the system was not turned on was counted as zero hours of use. In these 57 subjects, the usage per night was 6.0 ± 1.4 h (mean ± standard deviation). The usage per night is shown for individual subjects in Fig. 6. For 84% of subjects, their median usage per night significantly exceeded 4 h (Wilcoxon signed rank, P < .05). The usage results did not vary for subjects who experienced AEs compared with subjects who did not experience AEs.
Fig. 6.
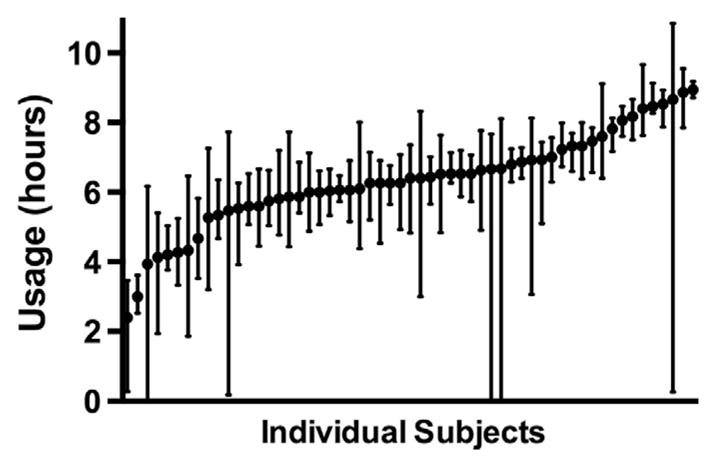
Nightly usage (median ± interquartile range) for each subject in the analysis cohort who completed the take-home period.
4. Discussion
Data from the analysis cohort of 63 subjects indicated that OPT led to statistically significant improvements in AHI and ODI on the first treatment night and following 28 days of use. In the same cohort, there were statistically significant alterations in sleep architecture on treatment nights with reduced stage 1 shifts, reduced awakenings, a smaller arousal index, and reduced time spent in stage 1 sleep on both treatment nights relative to baseline; increased sleep efficiency, decreased wake after sleep onset, and increased time in REM sleep were seen after 28 days of treatment. Significant changes in subjective sleepiness on the ESS and the perceived benefits of sleep on the mFOSQ were seen at 28 days in those subjects who were not on treatment immediately prior to the baseline assessment.
Administration of positive airway pressure (PAP) therapy can be extremely efficacious in the reduction of OSA and works well for many patients [12]. However, the effectiveness of PAP therapy is limited if usage is low [13]. The OPT used in our study represents a new therapeutic approach to OSA, which was found to be effective in a substantial number of subjects in its ability to reduce measured indices of sleep-disordered breathing, to improve objectively measured sleep parameters, and to reduce subjective daytime sleepiness. Clinical success was seen irrespective of disease severity, with classification of patients as mild, moderate, or severe not being predictive of success. Although there was a statistically significant improvement in AHI across the whole group, OPT was not effective in all participants in improving breathing during sleep or improving sleep itself. However, in those who showed a clinically significant effect, the benefits were seen on the first night of treatment, highlighting the capacity to rapidly identify those likely to have meaningful AHI improvement with OPT through PSG evaluation.
There was no direct assessment of the mechanism of action of OPT in our study, but preliminary data collected using magnetic resonance imaging [14] suggest that OPT acts via stabilization and anterior placement of the soft palate. Therefore, it is likely to be in the class of focused therapies, targeting retropalatal collapse without the risk, side effects, and irreversibility associated with uvulopalatopharyngoplasty.
The 28-day take-home portion of our study demonstrated high levels of adherence and low incidence of AEs; however, our data, as with those of all research studies, are drawn from a population of highly motivated individuals who are actively engaged in finding an effective therapy for their disorder. Further studies will be required to assess adherence over a longer time frame, and a sample that is more reflective of the general clinical population should be used. The evaluation of improvement in sleepiness was complicated by 17 of the subjects, with 28 days of use having initially been assessed for sleepiness before discontinuing previous PAP therapy. In the remaining 40 subjects who were either treatment naïve or assessed after at least a 2-week washout, the average level of sleepiness on the ESS was not severe prior to treatment. Nonetheless, these subjects showed a 3.5-point improvement on the ESS, similar to the change reported in a meta-analyses of CPAP [15]. The effect size of the change in ESS in these subjects (Cohen d = 0.68) favorably compares with that reported for CPAP in two meta-analyses reporting average effect sizes between 0.17 and 0.27 [16, 17] and a large scale clinical trial of CPAP [18] reporting an effect size of 0.57 after 2 months of treatment.
The improvement in subjective sleepiness is accompanied by changes of sleep parameters in a manner consistent with those expected in effective OSA therapy. For all measures derived from the PSG, changes that were observed on the first treatment night persisted and typically were further impacted after 28 days of treatment. Reduction in AHI was associated with reduction in the number of frank awakenings, brief arousals, and sleep-stage shifts.
Our study has a number of limitations common to early evaluations of minimal-risk medical devices. Treatment efficacy is limited to two single-night studies, one at the beginning and the other at the end of a 28-day take-home period. Future studies will need to extend the period of use for both safety and long-term efficacy evaluation; additionally, the studies preferably should have multiple measurement points, if not nightly monitoring of oxygen saturation in the home. The study also was conducted on a highly selected study population. Medical inclusion criteria included the necessity of nasal patency, as demonstrated with a PNIF of at least 75 L per minute. The requirement for nasal patency was a consideration, given the need to breathe nasally while on the device, and is similar to the requirements used in studies evaluating nasal CPAP [19–21] or expiratory PAP via a nasal valve device [22–25].
Of the 146 subjects in the safety cohort, 60 were screened out based on a priori criteria of not being able to demonstrate more than 4 h of sleep with an adequate mouth vacuum in an at-home screening night. In some ways, this criterion is analogous to the need for participants to have undergone a successful CPAP titration night in studies of CPAP efficacy [26–29]. The decision to conduct this night in the subjects’ homes rather than as an in-laboratory study probably contributed to the large number of subjects screened out, as no explicit instructions were provided to the subjects regarding the number of hours of sleep required on the device. This protocol design decision was based on cost and efficiency of execution and was not validated for accuracy in predicting therapeutic response.
An additional limitation is the lack of a sham-placebo controlled condition. Several attempts were made to produce a sham device to be used as a control. The nature of the OPT device made this task extremely challenging from an engineering perspective, and it proved impractical to develop a device that had minimal vacuum suction but would still remain comfortably in the mouth for a complete night of study. The use of a simple mouthpiece also was considered problematic, as anything that could keep the mouth closed and the jaw advanced might provide therapeutic benefit, and anything that predisposed to mouth opening could increase AHI [30].
In our initial study of a new therapeutic intervention for the treatment of OSAs and hypopneas, OPT was found to be safe and well-tolerated. In the analysis cohort, there was a 46% median change on the first treatment night and a 43% median change after 28 days of treatment. In addition our data met US Food and Drug Administration criteria for clinical effectiveness in 41% of the subjects evaluated. Beneficial effects on sleep quality were observed as well as improvements in subjective daytime sleepiness. Coupled with the high levels of observed adherence and the low level of reported AEs, these findings suggest that OPT may provide useful therapy for a subset of OSA patients who do not tolerate nasal CPAP.
Supplementary Material
Acknowledgments
This work was supported by ApniCure Inc.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.sleep.2013.05.009.
Footnotes
Conflicts of interest
Dr Black is a consultant to ApniCure and Dr Siegel is employed by ApniCure.
References
- 1.Redline S, Young T. Epidemiology and natural history of obstructive sleep apnea. Ear Nose Throat J. 1993;72(20–1):4–6. [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. published online ahead of print August 18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilleminault C, Eldridge FL, Tilkian A, Simmons FB, Dement WC. Sleep apnea syndrome due to upper airway obstruction: a review of 25 cases. Arch Intern Med. 1977;137:296–300. [PubMed] [Google Scholar]
- 5.Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13:437–44. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol. 2011;26:541–7. doi: 10.1097/HCO.0b013e32834b806a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3:126. doi: 10.3389/fneur.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kushida CA, Littner MR, Hirshkowitz M, Morgenthaler TI, Alessi CA, Bailey D, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 9.Powell ED, Gay PC, Ojile JM, Litinski M, Malhotra A. A pilot study assessing adherence to auto-bilevel following a poor initial encounter with CPAP. J Clin Sleep Med. 2012;8:43–7. doi: 10.5664/jcsm.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, Brown T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031–5. [PubMed] [Google Scholar]
- 12.Sanchez AI, Martinez P, Miro E, Bardwell WA, Buela-Casal G. CPAP and behavioral therapies in patients with obstructive sleep apnea: effects on daytime sleepiness, mood, and cognitive function. Sleep Med Rev. 2009;13:223–33. doi: 10.1016/j.smrv.2008.07.002. published online ahead of print February 7, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwab RJ, Kim C, Siegel LC, Black J, Farid-Moayer M, Podmore JL, et al. Mechanism of action of a novel device using oral pressure therapy (OPT) for the treatment of OSA. Am J Respir Crit Care Med. 2012 http://dx.doi.org/10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A6811.
- 15.Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–71. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 16.Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver TE, Mancini C, Maislin G, Cater J, Staley B, Landis JR, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186:677–83. doi: 10.1164/rccm.201202-0200OC. published online ahead of print July 26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the apnea positive pressure long-term efficacy study (APPLES) Sleep. 2012;35:1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teo M, Amis T, Lee S, Falland K, Lambert S, Wheatley J. Equivalence of nasal and oronasal masks during initial CPAP titration for obstructive sleep apnea syndrome. Sleep. 2011;34:951–5. doi: 10.5665/SLEEP.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dungan GC, 2nd, Marshall NS, Hoyos CM, Yee BJ, Grunstein RR. A randomized crossover trial of the effect of a novel method of pressure control (SensAwake) in automatic continuous positive airway pressure therapy to treat sleep disordered breathing. J Clin Sleep Med. 2011;7:261–7. doi: 10.5664/JCSM.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan S, Garvey JF, Swan V, Behan R, McNicholas WT. Nasal pillows as an alternative interface in patients with obstructive sleep apnoea syndrome initiating continuous positive airway pressure therapy. J Sleep Res. 2011;20:367–73. doi: 10.1111/j.1365-2869.2010.00873.x. [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34:479–85. doi: 10.1093/sleep/34.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel AV, Hwang D, Masdeu MJ, Chen GM, Rapoport DM, Ayappa I. Predictors of response to a nasal expiratory resistor device and its potential mechanisms of action for treatment of obstructive sleep apnea. J Clin Sleep Med. 2011;7:13–22. [PMC free article] [PubMed] [Google Scholar]
- 24.Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2008;4:426–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh JK, Griffin KS, Forst EH, Ahmed HH, Eisenstein RD, Curry DT, et al. A convenient expiratory positive airway pressure nasal device for the treatment of sleep apnea in patients non-adherent with continuous positive airway pressure. Sleep Med. 2011;12:147–52. doi: 10.1016/j.sleep.2010.06.011. published online ahead of print January 21, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Blau A, Minx M, Peter JG, Glos M, Penzel T, Baumann G, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients-a pilot study. Sleep Breath. 2012;16:773–9. doi: 10.1007/s11325-011-0574-1. published online ahead of print August 27, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Vennelle M, White S, Riha RL, Mackay TW, Engleman HM, Douglas NJ. Randomized controlled trial of variable-pressure versus fixed-pressure continuous positive airway pressure (CPAP) treatment for patients with obstructive sleep apnea/hypopnea syndrome (OSAHS) Sleep. 2010;33:267–71. doi: 10.1093/sleep/33.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushida CA, Berry RB, Blau A, Crabtree T, Fietze I, Kryger MH, et al. Positive airway pressure initiation: a randomized controlled trial to assess the impact of therapy mode and titration process on efficacy, adherence, and outcomes. Sleep. 2011;34:1083–92. doi: 10.5665/SLEEP.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javaheri S, Goetting MG, Khayat R, Wylie PE, Goodwin JL, Parthasarathy S. The performance of two automatic servo-ventilation devices in the treatment of central sleep apnea. Sleep. 2011;34:1693–8. doi: 10.5665/sleep.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meurice JC, Marc I, Carrier G, Series F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153:255–9. doi: 10.1164/ajrccm.153.1.8542125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.