Abstract
Background
Carbon sources for biofuel production are wide-ranging and their availability depends on the climate and soil conditions of the land where the production chain is located. Henequen (Agave fourcroydes Lem.) is cultivated in Yucatán, Mexico to produce natural fibers from the leaves, and a juice containing fructans is produced during this process. Fructans can be hydrolyzed to fructose and glucose and metabolized into ethanol by appropriate yeasts. In Mexico, different Agave species provide the carbon source for (distilled and non-distilled) alcoholic beverage production using the stem of the plant, whilst the leaves are discarded. In this work, we investigated the effect of thermal acid and enzymatic hydrolysis of the juice on the amount of reducing sugars released. Growth curves were generated with the yeasts Saccharomyces cerevisiae and Kluyveromyces marxianus and fermentations were then carried out with Kluyveromyces marxianus to determine alcohol yields.
Results
With thermal acid hydrolysis, the greatest increase in reducing sugars (82.6%) was obtained using 5% H2SO4 at 100°C with a 30 min reaction time. Statistically similar results can be obtained using the same acid concentration at a lower temperature and with a shorter reaction time (60°C, 15 min), or by using 1% H2SO4 at 100°C with a 30 min reaction time. In the case of enzymatic hydrolysis, the use of 5.75, 11.47 and 22.82 U of enzyme did not produce significant differences in the increase in reducing sugars. Although both hydrolysis processes obtained similar results, the difference was observed after fermentation. Ethanol yields were 50.3 ± 4 and 80.04 ± 5.29% of the theoretical yield respectively.
Conclusions
Final reducing sugars concentrations obtained with both thermal acid and enzymatic hydrolysis were similar. Saccharomyces cerevisiae, a good ethanol producer, did not grow in the hydrolysates. Only Kluyveromyces marxianus was able to grow in them, giving a higher ethanol yield with the enzymatic hydrolysate. The leaves account for a non-negligible weight of the total agave plant biomass, so this work complements the knowledge already developed on agave fermentations by making it possible to produce ethanol from almost the entire plant (stem and leaves).
Keywords: Biofuel, Sugars, Oligofructans, Hydrolysis, Pretreatments
Background
The use of biofuels such as bioethanol, biodiesel and biogas in the transport sector offers a valuable alternative in order to minimize greenhouse gas emissions and fossil-fuel dependence, and is being developed in many countries. Bioethanol is currently produced at competitive prices from two main raw materials: sugar and starch [1]. However, in order to avoid the conflict between using land for food or energy production, the use of forestry and/or agricultural residues for bioethanol production is attracting the interest of scientists with a view to establishing economical and sustainable processes. The availability of local raw materials is therefore an important issue that has to be addressed when attempting to assess their viability. Carbon sources for bioethanol production are wide-ranging [2] and their availability depends on the climate and soil conditions of the land where the production chain is located. The north of the Yucatán peninsula, Mexico, consists mainly of semi-arid lands with poor soils that do not support the cultivation of sugar cane. Corn is cultivated on a small scale, but cannot be used for fuel purposes because this crop forms the basis of the traditional Mexican diet. Nevertheless, agaves can grow well in these soils due to the fact that their metabolism is adapted to arid conditions [3]. Although biomass productivity of henequen (Agave fourcroydes Lem.) is not as high as sugar cane or sweet sorghum, it is similar to a variety of other energy crops, as shown in Table 1[4,5]. Henequen is cultivated in this region to produce natural fibers from the leaves, and a juice containing fructans is produced during this process [6]. These fructans can be hydrolyzed to fructose and glucose and metabolized into ethanol by appropriate yeasts [7]. In Mexico, different Agave species provide the carbon source for (distilled and non-distilled) alcoholic beverage production and both traditional and modern processes have been discussed at length in the literature [8]. They all use the stem of the plant as the source of sugars, whilst the leaves are discarded. The stems are cooked to hydrolyze the fructan chains into their monomers, and pressed to extract a fermentable sugar-rich juice. In the case of henequen, the older leaves at the base of the plant are harvested every 3-4 months and decorticated to obtain the fibers. During processing, water is used to wash the fibers, resulting in the dilution of the components present in the juice.
Table 1.
Comparison of Agave fourcroydes L. biomass productivity with other energy crops
| Crop | Dry biomass productivity (t∙ha-1∙year-1) |
|---|---|
|
Agave fourcroydes |
15 |
|
Agave tequilana |
25 |
| Sugarcane |
50-67 |
| Corn |
17-18 |
| Sugar beet |
11-17 |
| Sweet sorghum |
24-47 |
| Switchgrass |
10-15 |
| Miscanthus | 10-13 |
Although producing ethanol from the juice (stem and/or leaves) of agaves is not a direct fermentation of simple sugars (as with sugar cane or sweet sorghum), it is not as difficult as producing it from lignocellulosic materials. There is no need for solid material pretreatments, but a hydrolysis step is needed because fermentable sugars are not directly available. Unlike fructans from chicory, which are mainly composed of fructose molecules joined by β(2-1) linkages, agave fructans contain an important amount of β(2-6) linkages that result in branched molecules [7,9,10]. This can affect, for example, the choice of enzyme for the hydrolysis of the fructan chains. Commercial enzyme preparations that contain endo- and exo-inulinases can be used to achieve good yields in agave fructans hydrolysis. Inulinases can mainly be produced by microorganisms such as yeast strains (Candida sp., Sporotrichum sp., Pichia sp., and Kluyveromyces sp.) or fungi (Aspergillus and Penicillium species) [11]. Thermal acid hydrolysis can also be used, but this process is associated with the production of unwanted by-products (hydroxymethyl furfural and fructose dianhydride) that can affect further biological steps or the purity of final products [12].
The juice from the leaves of the henequen plant has previously been studied for ethanol production [6]. In that work, the juice was diluted to simulate the concentrations obtained from the mills. The low sugar content was compensated for by the addition of molasses, another industrial residue. However, technical proposals exist to obtain pure juice from the mills, meaning that sugar concentrations could be higher and there would be no need to add other carbon sources. The use of a mixture of yeast strains, Saccharomyces cerevisiae and Kluyveromyces marxianus, helped to improve ethanol yields, although they were still low. The aim of this work was to investigate the effect of thermal acid and enzymatic hydrolysis of the juice on the amount of reducing sugars released from these processes. Temperature, time and sulfuric acid concentrations were varied for thermal acid hydrolysis. Temperature and enzyme concentration were varied for enzymatic hydrolysis. The treatment that gave the highest reducing sugars yields was used to continue the study. Growth curves were generated with the yeasts Saccharomyces cerevisiae and Kluyveromyces marxianus and fermentations were then carried out with Kluyveromyces marxianus to determine alcohol yields.
Results and discussion
Thermal acid hydrolysis
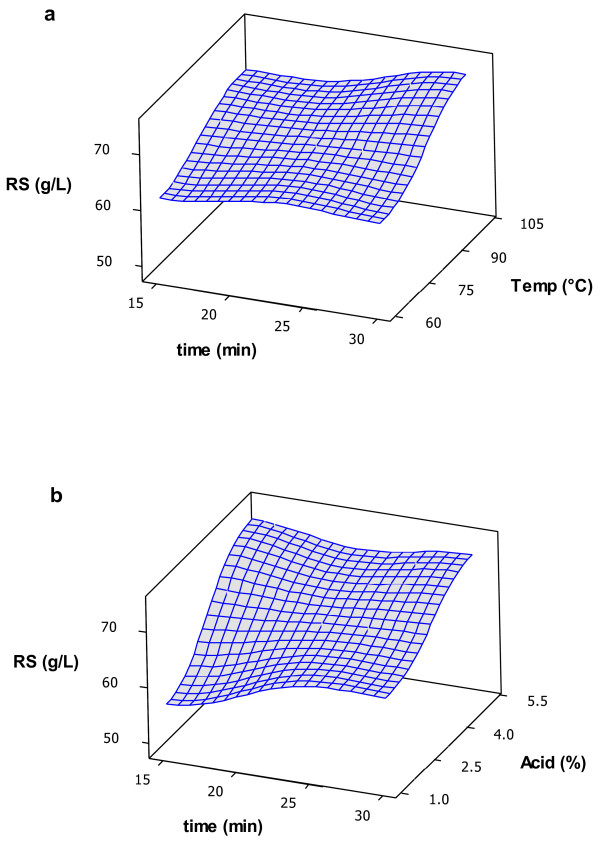
The juice obtained from henequen leaves had a soluble solids concentration of 10°Bx, which was similar to that obtained previously [6], and an initial reducing sugars concentration of 40.2 ± 0.64 g∙L-1. Time had little effect on thermal acid hydrolysis of the juice, as can be seen in the surface response graphs in Figure 1. The reducing sugars concentrations remained almost the same when reaction temperature (Figure 1a) or acid concentration (Figure 1b) were kept the same and only time was changed. Acid concentration showed a greater effect on reducing sugars release than reaction temperature within the ranges employed in this work. As expected, a lower release of reducing sugars was obtained when lower values were used for acid concentration and reaction temperature, as shown in Figure 2. Good yields were also obtained at low acid concentrations and high temperatures. Reducing sugars values after the thermo-acid treatments are shown in Table 2. The greatest increases in reducing sugars concentrations were obtained when 5% H2SO4 was added to the juice. The only experiment that produced a statistically similar increase in reducing sugars using 1% H2SO4 was treatment 7, carried out at 100°C for 30 min. Although it requires more energy and a longer reaction time, the amount of H2SO4 is 5 times lower and neutralization requires less NaOH. Treatment 7 conditions were used in further experiments in this work. The selection of parameters and their ranges depends not only on better results, but also on economic, environmental and practical factors [13]. For larger-scale production, procedures requiring smaller energy inputs are preferred, and even Treatment 4 conditions could be used without a detrimental effect on reducing sugars production. To what extent the use of less H2SO4 or lower temperature and reaction times produce greater savings is beyond the scope of this work, but should be studied. There are few reports on agave fructan hydrolysis, and most of them are on the stems of Agave tequilana W. because this is used for tequila production [9,10]. Waleckx et al. recently reported that 98% hydrolysis efficiency was obtained after 25.5 h of stem cooking [7]. However, it was possible to use shorter heating times in this work due to the liquid nature of the juice. Avila et al. [14] reported a hydrolysis efficiency of greater than 90% at 60°C with a 10 minute reaction time after fructans from A. tequilana stems were extracted and then thermally hydrolyzed using 0.54 N HCl.
Figure 1.
Response surfaces for the effect of a) reaction temperature and b) H 2 SO 4 concentration on reducing sugars concentration.
Figure 2.
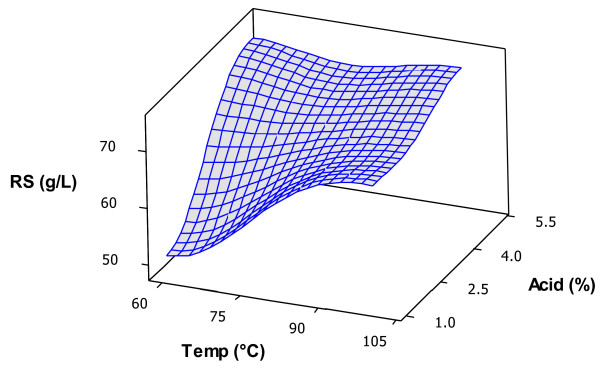
Response surface for the combined effect of reaction temperature and H 2 SO 4 concentration on reducing sugars concentration.
Table 2.
Increase in reducing sugars after thermal acid hydrolysis of henequen juice
| Treatment | Tempeature (°C) | Heating time (min) | H2SO4 conc. (%v/v) | Reducing sugars2 (g L-1) | Reducing sugars increase (%) |
|---|---|---|---|---|---|
| Reference1 |
-- |
-- |
-- |
40.25 ± 0.64a |
-- |
| 1 |
60 |
15 |
1 |
48.84 ± 2.58b |
21.3 |
| 2 |
60 |
15 |
5 |
74.59 ± 1.05c |
85.3 |
| 3 |
60 |
30 |
1 |
53.55 ± 1.83d |
33 |
| 4 |
60 |
30 |
5 |
73.36 ± 1.7c |
82.3 |
| 5 |
100 |
15 |
1 |
64.38 ± 2.3e |
60 |
| 6 |
100 |
15 |
5 |
74.01 ± 1.49c |
83.9 |
| 7 |
100 |
30 |
1 |
74.4 ± 3.29c |
84.8 |
| 8 | 100 | 30 | 5 | 74.95 ± 1.41c | 86.2 |
1Raw henequen leaf juice was used as a reference.
2Different letters indicate statistically significant differences.
Enzymatic hydrolysis
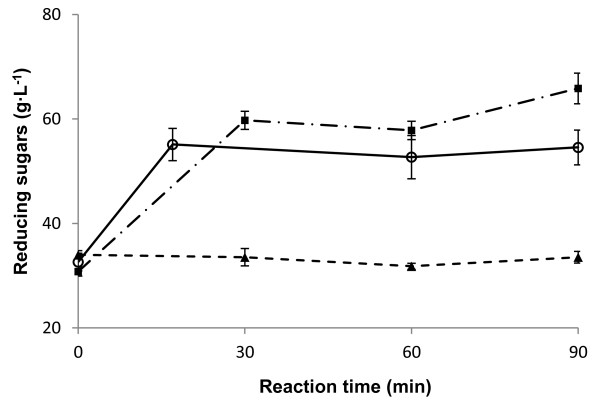
The effect of temperature on reducing sugars concentration during enzymatic hydrolysis of henequen juice is shown in Figure 3. As can be seen, the maximum release of reducing sugars is obtained after a 30 min reaction at both temperatures (50 and 60°C) using 22.82 U of enzyme as indicated by the manufacturer. A slightly higher concentration of reducing sugars was obtained at 60°C. The reference (henequen juice without enzyme) was also conducted at 50 and 60°C and the results were the same for both temperatures, showing that no increase in reducing sugars concentrations occurred without enzyme. It has been reported [11] that inulinase from the marine yeast Pichia guilliermondii is most active at 60°C. However, Ricca et al. [15] reported that higher inulinase activity is obtained at 60°C, but 30% activity loss occurred after 5 h. This must be considered when scaling-up the process, as procedures are longer than at laboratory level, meaning that lower temperatures may give better results. In order to obtain the maximum concentration of reducing sugars, experiments on enzymatic hydrolysis of henequen juice were conducted at 60°C in this study.
Figure 3.
Effect of temperature on enzymatic hydrolysis of henequen juice. (■) 60°C; (○) 50°C; (▲) without enzyme at both 50°C and 60°C.
To determine the effect of enzyme concentration on reducing sugars release, lower concentrations than 22.82 U were tested. As can be seen in Figure 4, hydrolysis of the fructans present in the juice occurs during the first 10 min of the reaction. This experiment also showed that the use of 4 times less enzyme (5.75 U) is enough to attain the same levels of reducing sugars release as using 22.82 U. This suggests that there is no saturation of the enzyme at lower levels. There were no significant differences in the increase in reducing sugars using 5.75, 11.47 and 22.82 U of enzyme. The reference was henequen juice incubated under the same conditions without enzyme; in this case, no change in reducing sugars concentrations was observed. The concentrations of reducing sugars obtained by enzymatic hydrolysis are similar to those obtained by thermal acid hydrolysis.
Figure 4.
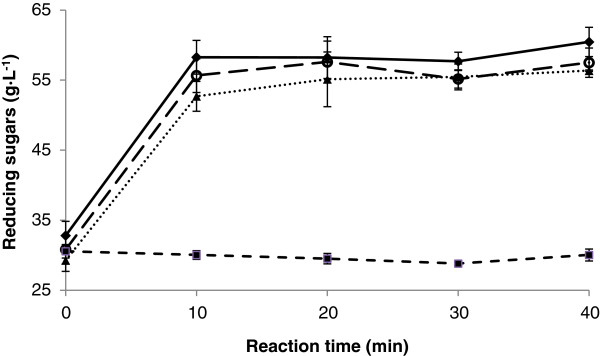
Effect of enzyme concentration on enzymatic hydrolysis of henequen juice. (♦) 22.82 U; (○) 11.47 U; (▲) 5.75 U; (■) without enzyme.
Fermentation
A growth curve was first generated for the yeast Saccharomyces cerevisiae with both thermo-acid and enzymatic henequen juice hydrolysates. The yeast did not grow in either of the hydrolysates. This behavior could be due to the presence of inhibitory compounds in the henequen juice. It is well documented that agave plants produce saponins with inhibitory activity [16,17]. To corroborate this, Cira et al. [18] produced a modified strain of S. cerevisiae which resists the saponins present in the musts of Agave tequilana Weber var. azul and Agave salmiana, and succeeded in fermenting them into ethanol. Saponins can be hydrolyzed to their corresponding sapogenins, but higher acid concentrations and longer reaction times than the ones used in this work are needed [19]. This lack of performance has been reported in the literature [20], where lower ethanol yields were obtained with Saccaromyces cerevisiae (70-76%) compared to a Kluyveromyces marxianus strain (92-96%) when fermenting Agave tequilana musts. On the contrary, the yeast Kluyveromyces marxianus was able to grow in the henequen juice hydrolysates, as shown in Figure 5. The yeast could also grow in raw juice (the juice was only heated to 80°C and cooled immediately to lower microbial contamination). This can be explained by the fact that the strain used in this work was isolated from the henequen plant and is adapted to the metabolites present in the juice or has the enzymes to hydrolyze them. In a spontaneous fermentation of A. fourcroydes stem must, a great diversity of yeasts was present and K. marxianus and S. cerevisiae were the dominant species by the end of the fermentation [Dr. Patricia Lappe, personal communications]. K. marxianus presented a lag phase of approximately 6 hours. In Figure 5, it can be seen that the yeast performed better in the enzymatic hydrolysate and in the raw juice than in the thermal acid hydrolysate at the beginning of the growth curves. It has been reported that materials subjected to acid hydrolysis are harder to ferment [21]. After 18 hours of growth, cell concentrations were the same in both hydrolysates.
Figure 5.
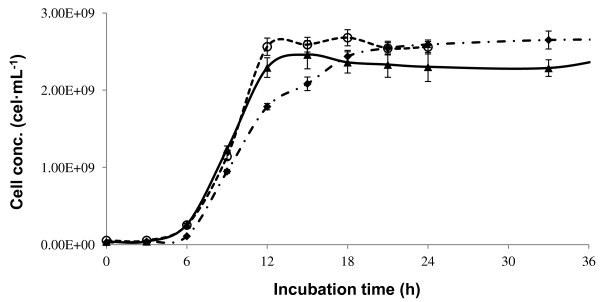
Growth curves of Kluyveromyces marxianus. Enzymatic (▲), thermal acid (♦) and non-hydrolyzed (○) henequen juice hydrolysates.
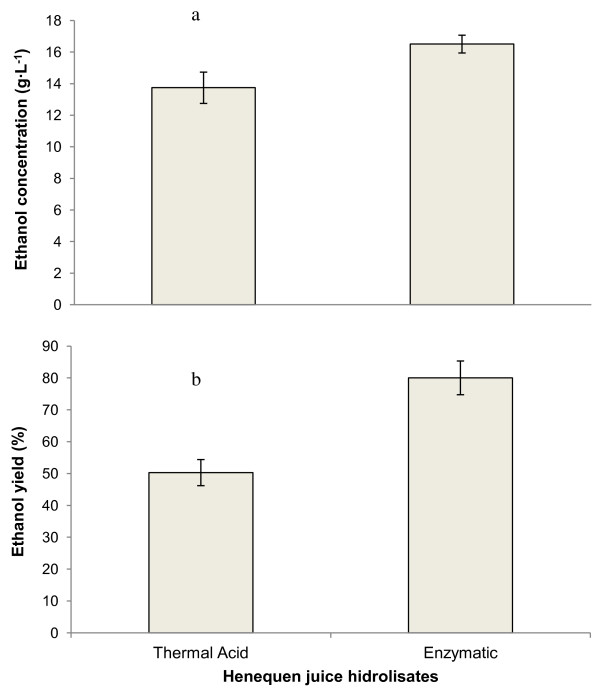
Alcoholic fermentations were carried out with enzyme and thermal acid hydrolyzed henequen juice. Reducing sugars were consumed during the first 24 hours of fermentation as shown in Figure 6. After 48 hours of fermentation, ethanol concentrations were 13.74 ± 0.99 g · L-1 for the thermal acid hydrolyzed juice and 16.5 ± 0.56 g · L-1 for the enzyme-hydrolyzed juice. As shown in Figure 7a, the difference between the two results was found to be statistically significant. Based on the amount of reducing sugars consumed during the fermentations, ethanol yields of 50.3 ± 4 and 80.04 ± 5.29% were obtained respectively. As show in Figure 7b, the difference between the two results was statistically significant. The yeast was able to consume the reducing sugars, but did not efficiently transform them into ethanol in the case of the thermal acid hydrolysate. Ethanol yields depend on fermentation conditions. When inulinase-hydrolyzed artichoke tubers were fermented using a strain of S. cerevisiae, yields of 80-84% of the theoretical value were obtained [22] which are similar to those obtained in this work. This indicates that S. cerevisiae can assimilate the sugars released by an enzymatic hydrolysis of oligofructans, so henequen leaves contain compounds that inhibit growth of this yeast. Ethanol yields of up to 90% of the theoretical value were obtained when S. cerevisiae was used to ferment YM broth with nitrogen supplementation [23]. In another study carried out in our laboratories, the same S. cerevisiae strain used in this work gave an ethanol yield of 98% of the theoretical value when a thermal acid hydrolysate of jatropha kernel fruit was fermented. The yeast S. cerevisiae is known for its higher tolerance to ethanol concentrations [24], whilst K. marxianus is known for its thermotolerance [25,26].
Figure 6.
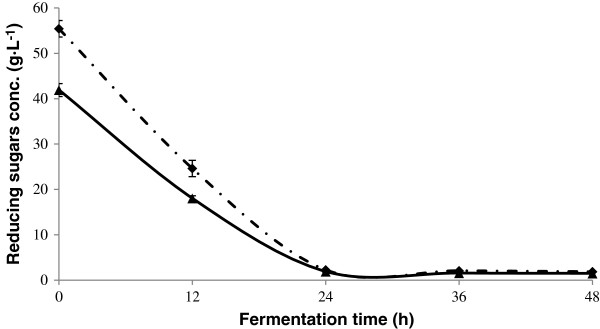
Reducing sugars consumption during fermentation of henequen juice hydrolysates. Enzymatic (▲) and thermal acid (♦) hydrolysates.
Figure 7.
Comparison of a) ethanol concentrations and b) ethanol yields obtained after fermentation of thermal acid and enzymatic hydrolysates of henequen juice.
The selection of one yeast over the other depends on the nature of the must and fermentation conditions. The use of a mixture of both yeasts can even be envisaged [6].
An ethanol production curve was generated using an enzymatic hydrolysate. This juice was obtained during the dry season and was more concentrated. It has been documented that the juice of henequen stems is more concentrated in the dry season [27] and we found the same effect with the leaf juice. It was decided not to dilute the juice to find out if there was any effect on the hydrolysis and fermentation processes. A higher concentration of reducing sugars was obtained after enzymatic hydrolysis, indicating that the amount of enzyme chosen previously can perform well with any juice concentration obtained during the year. Reducing sugars uptake and ethanol production are shown in Figure 8. It can be seen that after 30 hours of fermentation, sugar consumption is already complete with no statistically significant differences in ethanol concentrations until 48 h. An ethanol yield of 77 ± 5.86% of the theoretical yield was obtained.
Figure 8.
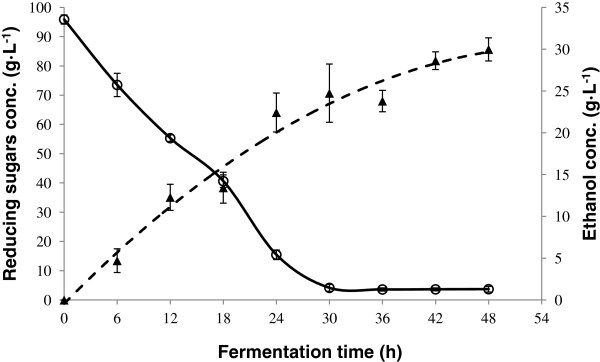
Reducing sugars consumption (○) and ethanol production (▲) during fermentation of an enzymatic hydrolysate of henequen juice.
With the higher yields obtained in this work, it is possible to produce 0.038 L of ethanol per liter of leaf juice. Taking into account that a henequen leaf contains 0.78-0.89 L of juice and approximately 250 million leaves are processed per year, 7.125 million liters of ethanol could therefore be produced with this residue. As for production costs, they will be higher than for processes employing direct fermentation of simple sugars, such as sugar cane or sweet sorghum juice, due to the inclusion of the enzymatic hydrolysis step. The amount of enzyme used, however, is minimal. Production costs will be lower than for solid lignocellulosic residues, because there is no pre-treatment step before saccharification. It is easier and cheaper to process liquid materials.
To our knowledge, no data has previously been reported on hydrolyzed agave leaf juice fermentations to produce ethanol.
Conclusions
Final reducing sugars concentrations obtained with both thermal acid and enzymatic hydrolysis were similar, but enzymatic hydrolysis gave better ethanol yields after fermentation. Saccharomyces cerevisiae, a good ethanol producer and one used in agave stem must fermentations, could not grow in either hydrolysate. The development of strains resistant to inhibitory compounds present in agave leaves is important for commercial exploitation of this part of the plant. Fermentation of thermal acid and enzymatic hydrolysates with Kluyveromyces marxianus resulted in theoretical ethanol yields of 50.3 ± 4 and 80.04 ± 5.29% respectively. This yeast is capable of producing inulinase, but attempts to ferment the leaf juice without hydrolysis failed, meaning that preliminary hydrolysis of the fructans present in the juice appears to be necessary. K. marxianus can also be manipulated in order to attain higher ethanol yields. These studies, together with the isolation of new strains from henequen leaf juice, are being carried out by our group. This work complements the knowledge already developed on agave juice fermentation. Agave leaves (fourcroydes or tequilana) account for a non-negligible weight of total plant biomass and comprise an important carbohydrate source that can be used to produce ethanol as biofuel.
Methods
Reagents
All reagents were analytical grade. Inulinase enzyme (Sigma-Aldrich) was obtained from Aspergillus niger with a declared activity of 2000 U · mL-1 and a density of 1.13 g · cm-3. One enzymatic unit is defined as the amount of enzyme necessary to release one g of reducing sugars per minute at 50°C.
Plant material
Henequen leaves were obtained from healthy plants grown in the gardens of Centro de Investigación Científica de Yucatán AC. They were rinsed with tap water and passed through a three-roller mill to extract the juice, which was filtered to eliminate solid leaf residues and stored at -20°C until use.
Thermal acid hydrolysis
The variables considered important in this process were temperature, heating time and H2SO4 concentration. A 32 full factorial design leading to eight sets of experiments was used to determine the effect of the above mentioned variables on the release of reducing sugars. The levels for each variable were determined on the basis of previous experience with henequen juice hydrolysis and were decided as follows: temperature = 60 and 100°C, heating time = 15 and 30 min, H2SO4 concentration = 1 and 5% (v/v). These levels were decided not only based on reaction system characteristics, but also based on economic and practical factors. Thermal acid hydrolysis experiments were carried out in 250 mL Erlenmeyer flasks with 80 mL of henequen leaf juice. Fresh juice was used as a reference for the initial reducing sugars concentration.
Enzymatic hydrolysis
To study enzymatic hydrolysis, a more classical (one variable at a time) approach was used due to the lack of previous data. Conditions of preliminary assays were based on enzyme data provided by the manufacturer. They were carried out in 25 mL Erlenmeyer flasks with 5 mL of raw henequen juice at pH 4.5. Two temperatures, 55 and 60°C, were tested using 22.82 U of enzyme and 90 min incubation time. Subsequently, three enzyme concentrations were tested at 60°C: 5.75, 11.47 and 22.82 U of enzyme and 40 min incubation time.
For fermentation assays, enzymatic hydrolysis was carried out in 250 mL Erlenmeyer flasks with 180 mL of raw henequen juice. An enzyme concentration of 5.75 U was used. Incubation was carried out at 60°C for 30 min and pH 4.5. All enzymatic hydrolysis incubations were performed at 150 rpm.
Yeast strains
Kluyveromyces marxianus was isolated from the base of henequen leaves following classical isolation procedures. It was characterized by phenotypical and molecular tests [28]. Commercial Saccharomyces cerevisiae was obtained from Safmex S.A. de C.V. (Mexico). Both yeasts were maintained in Petri dishes on YPGA medium containing glucose (20 g∙L-1), yeast extract (5 g∙L-1), peptone (10 g∙L-1) and agar (20 g∙L-1), and incubated at 30 ± 2°C in darkness. The cultures were collected in sterile water and kept at 4°C until use. Viability of the cell suspensions was measured by staining with methylene blue. Cell suspension viabilities ≥ 95% of both strains were used in all experiments.
Growth curves
Growth curves were generated in 250 mL Erlenmeyer flasks with 100 mL of thermal acid or enzyme hydrolyzed henequen juice adjusted to pH 4.5. Prior to hydrolysis, soluble solids of the raw henequen juice were adjusted to 6°Bx. Ammonium sulfate (1.5 g∙L-1) was used as the nitrogen source [23] and a cell concentration of 3×107 cell∙mL-1 was employed in all cases. The flasks were incubated at 30 ± 2°C with 150 rpm agitation for 33 h. Samples were harvested every 3 h. Non-hydrolyzed henequen juice was used as a reference. In this case, to avoid contamination, the juice was heated to 80°C and immediately cooled to room temperature after adjusting soluble solids to 6°Bx.
Fermentations
Inocula for fermentations were prepared with thermal acid or enzymatically hydrolyzed henequen juice with a soluble solids concentration of 6°Bx and an initial cell concentration of 3×107 cell∙mL-1. pH was adjusted to 4.5 and 1.5 g∙L-1 ammonium sulfate was added. Growth was maintained at 30 ± 2°C for 18 hours. The amount of the inoculum was 10% of total fermentation volume. Fermentations were carried out in 250 mL Erlenmeyer flasks with 180 mL of non-diluted thermal acid or enzymatically hydrolyzed henequen juice. pH was adjusted to 4.5 and 1.5 g∙L-1 ammonium sulfate was added. After inoculation, the flasks were kept at 30 ± 2°C for 48 hours without agitation.
Analytical methods
Soluble solids concentration was measured with a portable refractometer (Cole-Parmer FG103/113) and expressed as °Bx. Reducing sugars concentrations were determined in the hydrolysates and during fermentations by the 3, 5-dinitrosalicilic acid method [29]. The absorbance of the samples was read at 550 nm. For ethanol quantification, 25 mL of fermented sample was diluted with 25 mL of distilled water and distilled at 100°C until 25 mL of distillate was recovered. Ethanol concentration was determined by the dichromate method [30]. The absorbance of the samples was read at 585 nm.
Statistical analysis
All experiments were performed in triplicate (n = 3). For statistical analysis, the Statgraphics Centurion XV (Statpoint technologies Inc., Warrenton, VA, USA) software was employed. Significant differences among treatments were determined using the Tukey HSD test with p ≤ 0.05. For response surface analysis, the MINITAB 16 (Minitab Inc., State College, PA, USA) software was used.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PAVS carried out the experimental work and participated in the design of the study. TTT participated in the reducing sugar and statistical analysis. BBCC participated in the design and analysis of the enzymatic hydrolysis experiments. ALS participated in the design of the study and commented on the manuscript. LFBP conceived the study, participated in its design and drafted the manuscript. All authors read and approved the final manuscript.
Authors’ information
PAVS received his M.Sc. in Renewable Energy working on this project. TTT is a laboratory technician at CICY working in the Renewable Energy Department. BBCC is head of the Biotechnology Department at CICY. Her research interests are on enzymatic hydrolysis. ALS is a researcher in the Natural Resources Department at CICY. His main area of expertise is Biomass and Productivity. LFBP is head of the Renewable Energy Department. His research area involves biofuel production.
Contributor Information
Pablo A Villegas-Silva, Email: pablovillegasib@gmail.com.
Tanit Toledano-Thompson, Email: tanit@cicy.mx.
Blondy B Canto-Canché, Email: cantocanche@cicy.mx.
Alfonso Larqué-Saavedra, Email: larque@cicy.mx.
Luis F Barahona-Pérez, Email: barahona@cicy.mx.
Acknowledgements
The authors would like to thank Gerardo Rivera for his advice and María Guadalupe Serrano for her support during the experimental work.
References
- Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Lidén G, Zacchi G. Bio-ethanol – the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006;24(12):549–556. doi: 10.1016/j.tibtech.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lin Y, Tanaka S. Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol. 2006;69:627–642. doi: 10.1007/s00253-005-0229-x. [DOI] [PubMed] [Google Scholar]
- García-Moya E, Romero-Manzanares A, Nobel PS. Highlights for Agave productivity. Glob Change Biol Bioenergy. 2011;3:4–14. doi: 10.1111/j.1757-1707.2010.01078.x. [DOI] [Google Scholar]
- Escamilla-Treviño LL. Potential of plants from the genus Agave as bioenergy crops. Bioenerg Res. 2012;5:1–9. doi: 10.1007/s12155-011-9159-x. [DOI] [Google Scholar]
- Günnur K, Nilgün C. An overview of biofuels from energy crops: current status and future prospects. Renew Sust Energ Rev. 2013;28:900–916. [Google Scholar]
- Cáceres-Farfán M, Lappe P, Larqué-Saavedra A, Magdub-Méndez A, Barahona-Pérez L. Ethanol production from henequen (Agave fourcroydes Lem.) juice and molasses by a mixture of two yeasts. Bioresour Technol. 2008;99:9036–9039. doi: 10.1016/j.biortech.2008.04.063. [DOI] [PubMed] [Google Scholar]
- Waleckx E, Gschaedler A, Colonna-Ceccaldi B, Monsan P. Hydrolysis of fructans from Agave tequilana Weber var. azul during the cooking step in a traditional tequila elaboration process. Food Chem. 2008;108:40–48. doi: 10.1016/j.foodchem.2007.10.028. [DOI] [Google Scholar]
- Lappe-Oliveras P, Moreno-Terrazas R, Arrizón-Gaviño J, Herrera-Suárez T, García-Mendoza A, Gschaedler-Mathis A. Yeasts associated with the production of Mexican alcoholic nondistilled and distilled Agave beverages. FEMS Yeast Res. 2008;6:1037–1052. doi: 10.1111/j.1567-1364.2008.00430.x. [DOI] [PubMed] [Google Scholar]
- López MG, Mancilla-Margalli NA, Mendoza-Díaz G. Molecular structures of fructans from Agave tequilana Weber var. azul. J Agric Food Chem. 2003;51(27):7835–7840. doi: 10.1021/jf030383v. [DOI] [PubMed] [Google Scholar]
- Mancilla-Margalli NA, López MG. Generation of Maillard compounds from inulin during the thermal processing of Agave tequilana Weber Var. azul. J Agric Food Chem. 2002;50(4):806–812. doi: 10.1021/jf0110295. [DOI] [PubMed] [Google Scholar]
- Gong F, Zhang T, Chi Z, Sheng J, Li J, Wang X. Purification and characterization of extracellular inulinase from a marine yeast Pichia guilliermondii and inulin hydrolysis by the purified inulinase. Biotechnol Bioprocess Eng. 2008;13:533–539. doi: 10.1007/s12257-007-0177-7. [DOI] [Google Scholar]
- Singh RS, Dhaliwal R, Puri M. Production of high fructose syrup from asparagus inulin using immobilized exoinulinase from Kluyveromyces marxianus YS-1. J Ind Microbiol Biotechnol. 2007;34:649–655. doi: 10.1007/s10295-007-0237-1. [DOI] [PubMed] [Google Scholar]
- Dequan Zhou D, Xu X, Mu H, Høy CE, Adler-Nissen J. Synthesis of structured triacylglycerols containing caproic acid by lipase-catalyzed acidolysis: optimization by response surface methodology. J Agric Food Chem. 2001;49:5771–5777. doi: 10.1021/jf0103020. [DOI] [PubMed] [Google Scholar]
- Avila-Fernández A, Galicia-Lagunas N, Rodríguez-Alegría ME, Olvera C, López-Munguía A. Production of functional oligosaccharides through limited acid hydrolysis of agave fructans. Food Chem. 2011;129:380–386. doi: 10.1016/j.foodchem.2011.04.088. [DOI] [PubMed] [Google Scholar]
- Ricca E, Calabrò V, Curcio S, Iorio G. Optimization of inulin hydrolysis by inulinase accounting for enzyme time- and temperature-dependent deactivation. Biochem Eng J. 2009;48:81–86. doi: 10.1016/j.bej.2009.08.009. [DOI] [Google Scholar]
- Ohtsuki T, Koyano T, Kowithayakorn T, Sakai S, Kawahara N, Goda Y, Yamaguchi N, Ishibashi M. New chlorogenin hexasaccharide isolated from Agave fourcroydes with cytotoxic and cell cycle inhibitory activities. Bioorg Med Chem. 2004;12(14):3841–3845. doi: 10.1016/j.bmc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Yokosuka A, Mimaki Y. Steroidal saponins from the whole plants of Agave utahensis and their cytotoxic activity. Phytochemistry. 2009;70:807–815. doi: 10.1016/j.phytochem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Cira L, González G, Torres J, Pelayo C, Gutiérrez M, Ramírez J. Heterologous expression of Fusarium oxysporum tomatinase in Saccharomyces cerevisiae increases its resistance to saponins and improves ethanol production during the fermentation of Agave tequilana Weber var. azul and Agave salmiana must. Antonie Van Leeuwenhoek. 2008;93:259–266. doi: 10.1007/s10482-007-9200-4. [DOI] [PubMed] [Google Scholar]
- Eskander J, Lavaud C. Steroidal saponins from the leaves of Agave macroacantha. Fitoterapia. 2010;81:371–374. doi: 10.1016/j.fitote.2009.11.002. [DOI] [PubMed] [Google Scholar]
- López-Alvarez A, Díaz-Pérez AL, Sosa-Aguirre C, Macías-Rodríguez L, Campos-García J. Ethanol yield and volatile compound content in fermentation of agave must by Kluyveromyces marxianus UMPe-1 comparing with Saccharomyces cerevisiae baker’s yeast used in tequila production. J Biosci Bioeng. 2012;113(5):614–618. doi: 10.1016/j.jbiosc.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Galbe M, Zacchi G. Pretreatment of lignocellulosic materials for efficient bioethanol production. Adv Biochem Eng Biotechnol. 2007;108:41–65. doi: 10.1007/10_2007_070. [DOI] [PubMed] [Google Scholar]
- Szambelan K, Nowak J, Czarnecki Z. Use of Zymomonas mobilis and Saccharomyces cerevisiae mixed with Kluyveromyces fragilis for improved ethanol production from Jerusalem artichoke tubers. Biotechnol Lett. 2004;26:845–848. doi: 10.1023/b:bile.0000025889.25364.4b. [DOI] [PubMed] [Google Scholar]
- Breisha GZ. Production of 16% ethanol from 35% sucrose. Biomass Bioenergy. 2010;34:1243–1249. doi: 10.1016/j.biombioe.2010.03.017. [DOI] [Google Scholar]
- Bai FW, Anderson WA, Moo-Young M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol Adv. 2008;26:89–105. doi: 10.1016/j.biotechadv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Hacking AJ, Taylor IWF, Hanas CM. Selection of yeast able to produce ethanol from glucose at 40°C. Appl Microbiol Biotechnol. 1984;19:361–363. [Google Scholar]
- Banat IM, Nigam P, Singh D, Marchant R, McHale AP. Review: ethanol production at elevated temperatures and alcohol concentrations: part I - yeasts in general. World J Microbiol Biotechnol. 1998;14:809–821. doi: 10.1023/A:1008802704374. [DOI] [Google Scholar]
- Rendón-Salcido LA, Colunga-García P, Barahona-Pérez LF, Pimienta-Barrios E, Magdub-Méndez A, Larqué-Saavedra A. Sugars and alcoholic byproducts from henequen (Agave fourcroydes) as influenced by plant age and climate. Rev Fitotec Mex. 2009;32(1):39–44. [Google Scholar]
- Pérez-Brito D, Tapia-Tussell R, Quijano-Ramayo A, Larqué-Saavedra A, Lappe P. Molecular characterization of Kluyveromyces marxianus strains isolated from Agave fourcroydes Lem. in Yucatan, Mexico. Mol Biotechnol. 2007;37:181–186. doi: 10.1007/s12033-007-0036-y. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Williams MB, Reese HD. Colorimetric determination of ethyl alcohol. Anal Chem. 1950;22:1556–1561. doi: 10.1021/ac60048a025. [DOI] [Google Scholar]