Abstract
In human studies investigating factors that control cellular respiration in working skeletal muscle, pulmonary VO2 dynamics (VO2p) measured at the mouth by indirect calorimetry is typically used to represent muscle O2 consumption (UO2m). Furthermore, measurement of muscle oxygenation using near-infrared spectroscopy has provided information on the dynamic balance between oxygen delivery and oxygen consumption at the microvascular level. To relate these measurements and gain quantitative understanding of the regulation of VO2 at the cellular, tissue and whole-body level, a multiscale computational model of oxygen transport and metabolism during exercise was developed. The model incorporates mechanisms of oxygen transport from the airway opening to working muscle and other-organs cells, as well as the phosphagenic and oxidative pathways of ATP synthesis in these tissue cells. Model simulations of external (VO2p) and cellular (UO2m) respiration show that, during moderate exercise, their characteristic mean response times are similar even when a transit delay exists between tissue cells and the external environment for normal subjects.
Introduction
To distinguish mechanisms of impaired muscle oxygen delivery and oxidative metabolism in response to exercise, we need to evaluate how these factors affect muscle oxygen utilization (UO2m), which represents cellular respiration. During human or animal exercise experiments, direct in vivo measurement of UO2m is not feasible. Instead, pulmonary oxygen uptake (VO2p), which represents external respiration, is measured non-invasively at the mouth as an indirect indicator of metabolic processes that control cellular respiration in the working skeletal muscles. Factors that contribute to the differences between the dynamic responses of UO2m and VO2p are circulatory dynamics[14], ventilation, oxygen stores in blood and muscle[7], and oxygen exchange across membranes. Therefore, using VO2p as an indicator of metabolic processes may be misleading in the presence of various disease states. In chronic obstructive pulmonary disease[21], diabetes[3,12], or chronic heart failure[20], the dynamic response of VO2p to exercise is abnormally slow. In type 2 diabetes, low muscle blood flow may impair oxygen delivery to working muscle. Clinically, these diseases also may impair the mitochondrial oxidative metabolism[8].
In general, muscle power output and ATP utilization rate change in less than a second after the onset of exercise, but the VO2p response is much slower. Typically, the VO2p response has two phases whose slopes are discontinuous: a short (∼20s) cardiac-dependent rise characterized by a circulatory transit time delay (Phase I) followed by a longer exponential-type increase to a plateau (Phase II)[25]. The relationship between VO2p and UO2m dynamics has been studied under a variety of conditions[1,2,9,15]. From dynamic measurements of arterial and femoral venous blood and leg blood flow, Grassi et al. [9] evaluated muscle oxygen uptake (VO2m) dynamics, under the assumption its dynamics represents those of UO2m, and observed that during the transition from light to moderate intensity exercise, the dynamics of VO2p and VO2m did not differ significantly.
Barstow et al. [1,2] performed model simulations of VO2p in response to a moderate step increase in muscle work. Assuming no effects from muscle and pulmonary oxygen stores, simulated VO2m followed a monoexponential increase towards a steady state. Consistent with experimental findings of Grassi et al.[9], simulated VO2p dynamics during Phase II were similar to VO2m dynamics regardless of the Phase I dynamics. However, if oxygen stores are considered in a more general mathematical model of oxygen transport and utilization, then simulated exercise responses of VO2p in Phase II and VO2m may be different. Indeed, Lai et al. [15,17] used such a model to simulate VO2m, and UO2m dynamics in response to exercise. They estimated UO2m dynamics from muscle oxygen saturation (StO2m) measurements performed via near-infrared spectroscopy (NIRS). The UO2m and VO2m mean response times were much smaller than that of experimental VO2p. The dynamics of UO2m and VO2m are different during transient in exercise considering the O2 store in muscle tissue [16].
The Phase II characteristics of the VO2p exercise response depend on multiple factors, e.g., training status and cardiopulmonary disease. An overshoot may occur, as Koppo et al. [13] observed in the VO2p Phase II response of trained cyclists exercising at moderate intensity. Using VO2p as a proxy measurement for UO2m, these authors suggested that this phenomenon may be attributed to a higher ATP demand at the beginning of exercise in trained cyclists. Also, voluntary hyperventilation has been shown to slow the VO2p Phase II response to moderate intensity exercise[11].
Experiments conducted at a specific scale have showed that a perturbation in O2 transport can have different effects on oxygen uptake dynamics, viz, VO2p and VO2m. For instance, during moderate exercise, an increase in oxygen delivery due to an abrupt increase in heart rate –controlled by a cardiac pacemaker– resulted in a slower Phase II VO2p dynamic response than that obtained with the heart rate fixed [2]. In contrast, in an isolated dog muscle preparation, a faster O2 delivery due to a step increase in blood flow just before the onset of contraction did not affect VO2m significantly[10]. However, when these data were analyzed using a mathematical model, Lai et al.[18] found that increasing the rate of O2 delivery to contracting muscle did not change the dynamics of UO2m, but instead predicted a lag in VO2m response. From these and other experiments investigating the VO2 kinetic responses at various scales and characterizing them empirically using a linear combination of exponential functions, it can be inferred that the model parameters are not independent from each other and thus straightforward comparisons may not be possible. In general, the relationship between external and cellular respiration as indicated by VO2p and UO2m (or VO2m) depends on physiological processes and interconnections of oxygen uptake dynamics between whole body and local cellular-tissue responses to exercise. To gain quantitative understanding of the impact of oxygen transport limitations on the regulation of oxygen consumption at the cellular level, a multiscale computational model is needed that links O2 transport processes between the lungs and skeletal muscle cells and oxidative phosphorylation in skeletal muscle cells.
Methods
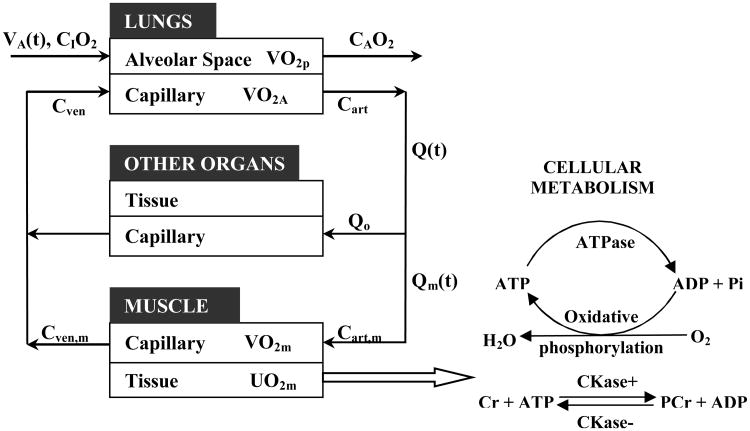
Simulation of oxygen uptake (VO2), at the tissue and whole-body levels and utilization (UO2) at the cellular level, in response to exercise can be accomplished with a multiscale model that incorporates a multi-organ, whole-body model [27] and a skeletal muscle model[17,18] of oxygen transport and metabolism. The output of this multiscale model can be combined with experimental measurements during exercise to evaluate the dynamic responses of muscle oxygenation (StO2m) and pulmonary oxygen uptake (VO2p). Such a multi-organ, whole-body model (Fig.1) distinguishes lungs, muscle, and other organ compartments, which are connected by the circulatory system (artery, vein, and capillaries).
Figure 1. Schematic representation of oxygen utilization in cell and transport between lungs and skeletal muscle.
Tissue transport and cellular metabolism
The blood O2 concentration in muscle, Cmb(v,t), changes with position in the capillary bed as indicated by cumulative volume (v)from the arterial input
| (1) |
where Vmb is the muscle capillary blood volume; Jm is the O2 transport rate between capillary blood and muscle tissue; and D is the O2 dispersion coefficient. The muscle blood flow in response to exercise is
| (2) |
where ΔQm is the muscle blood flow increase; t0 is the time at the onset of exercise; and τQ is the time constant of the blood flow response. The rate of muscle oxygen uptake is
| (3) |
where Cart,m(t)= Cmb(t,0) and Cven,m(t)= Cmb(t, Vmb) are the input and output O2 concentrations of the of muscle capillary bed.
The metabolic response of working skeletal muscle is described by bioenergetic systems associated with O2, ATP and phosphocreatine (PCr) metabolism (Fig.1). The change in tissue O2 concentration, Ctis(v,t) depends on mass transfer across the blood-tissue membrane and reaction processes (oxidative phosphorylation):
| (4) |
where fb = Vtis/Vmb is the volume ratio of muscle tissue to muscle capillary blood and ϕOxPhos is the oxidative phosphorylation rate:
| (5) |
where Vmax is the maximal flux of oxidative phosphorylation, CADP is the ADP concentration and is the free O2 concentration in muscle cells. The energy demand imposed by exercise is represented by the rate of ATP utilization, which is balanced by ATP production from oxidative phosphorylation and phosphocreatine (PCr) hydrolysis. [17] The P:O ratio in oxidative phosphorylation is assumed as 3. The reaction fluxes of creatine kinase are nonlinearly related to the coupled concentration of Cr, PCr, ADP and ATP, which must satisfies the conservation of adenosine and creatine [17] The contribution of glycolysis to ATP synthesis, however, is negligible when the imposed work rate is of moderate intensity. [22] The rate of oxygen utilization at the cellular level can be estimated as:
| (6) |
External respiration
In the lung compartment, we consider the alveolar gas as well-mixed with a constant breath-averaged volume. From the net input-output of oxygen in the alveolar gas[25], the rate of pulmonary oxygen uptake is computed as:
| (7) |
where CIO2 is the constant inspiratory O2 concentration and CAO2(t) is the alveolar O2 concentration. The alveolar ventilatory response, which is estimated from the ventilatory measurement during moderate exercise, is represented by an exponential function:
| (8) |
where VA0 is the ventilation at a warm-up steady state; ΔVA is the ventilatory increase in response to exercise; and τV is the time constant of the ventilatory response.
In the pulmonary capillary bed, O2 concentration in the blood from the arterial to venous sides is simulated by a one-dimensional convection-dispersion model with transport flux between blood and alveolar space[27]. The rate of oxygen uptake in alveolar blood is
| (9) |
where the dynamic response of cardiac output Q(t) has a similar form as Eq. 2. The increase in cardiac output and muscle blood flow in response to exercise are assumed to be the same.[28] The arterial-venous blood O2 concentration difference is given in the brackets. To simulate O2 transport between muscle and lungs, the O2 concentration in large blood vessels is represented by a one-dimensional convection-dispersion model[27]. The blood flow and oxygen uptake of the other organs compartment are assumed constant.
Model simulations for comparison with experimental data
Using this model, we can simulate and compare the quantitative relationship between cellular, tissue, alveolar blood and whole-body O2 uptake responses to moderate exercise indicated by UO2m, VO2m, VO2A, VO2p, respectively. We then characterize the dynamic responses of these outputs by the mean response time, MRT. Between the initial time, t0, and the time to reach the maximum response, t1, simulated step-up responses can be compared using the mean response time:
| (10) |
where Δy=ymax−y(t) represents the amplitude of the dynamic response of VO2p,VO2A, VO2m, or UO2m.
For comparison of model outputs to experimental data, we relate model variables to measurable variables at the muscle level. From the model, we evaluate muscle oxygenation as:
| (11) |
StO2m is intended to reflect the volume averaged signal from both hemoglobin in blood and myoglobin in tissue. The volume fractions of blood and tissue are fbl=1/(1+fb)=7% and ftis=fb/(1+fb)=93%. The concentrations of hemoglobin in blood and myoglobin in tissue are CHb and CMb. The averaged oxy-hemoglobin saturation is a weighted combination of hemoglobin in muscle arterioles, capillaries, and venules:
| (12) |
where ω art (=20%), ω cap (=15%), and ω ven (=65%) are blood volume fractions in muscle. The averaged oxy-myoglobin saturation in muscle tissue is SMb=〈 Stis 〉. The spatially averaged saturations Scap,m 〉and 〈 Stis 〉.are defined as:
| (13) |
The oxy-hemoglobin and oxy-myoglobin saturations are related to free O2 concentrations in blood and tissue [15].
Experimental data from a step change in work rate
Seven male African-American adolescents performed moderate intensity exercise (90% VT) on an electromagnetically-braked cycle ergometer. All investigational procedures were approved by the University Hospitals of Cleveland Institutional Review Board and written informed consent was obtained from both subjects and their parents. Local muscle oxygen saturation time profiles, StO2m(t), of the right quadriceps vastus lateralis muscle were obtained with near infrared spectrometry (NIRS). Minute ventilation VE and pulmonary oxygen uptake VO2p were continuously monitored with a commercially available metabolic cart system [17]. Values of most model parameters and exercise variables have been determined and published previously[17,27]. VA0, ΔVA and τV were estimated by least-squares fitting of VA(t)from the measured ventilatory response. Parameters Vmax and ΔQm for each subject were estimated by least-squares fitting of model output (StO2m(t)) to its corresponding experimental measurement. The time constant of the muscle blood flow τQ was computed using data from the first minute of heart rate response to exercise. Using the estimated parameters, the multiscale model was validated by comparing its VO2p simulation with experimental data. Model simulations are intended to show the effect of changing parameter values (e.g., Vmax) on oxygen uptake and utilization at various scales, i.e., UO2m, VO2m, VO2A, and VO2p.
Results
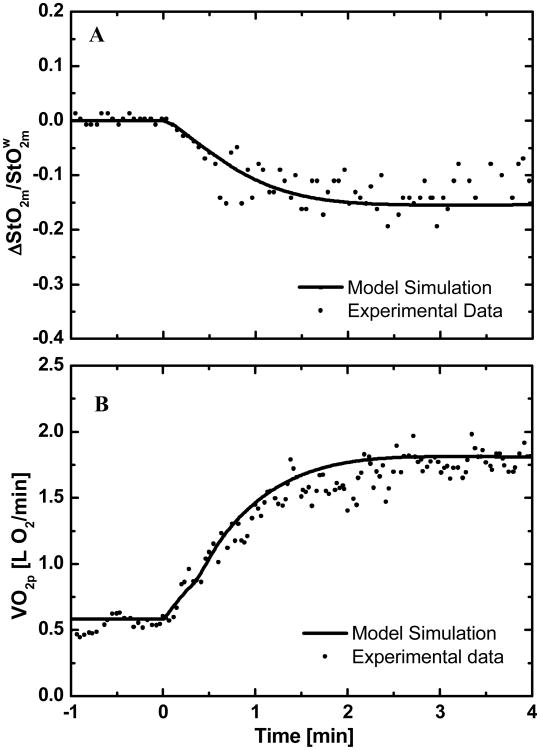
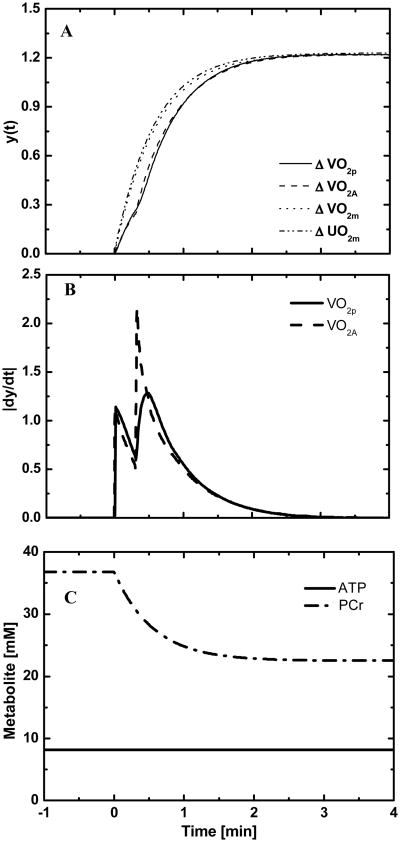
Simulations of the dynamic responses of StO2m and VO2p to a change in work rate (moderate intensity) from baseline are compared to experimental responses obtained in a typical normal human subject (Fig.2). For the same subject, model simulations of the dynamic responses of oxygen uptake (VO2p, VO2A, and VO2m) and oxygen utilization rate (UO2m) are compared (Fig. 3A). The dynamic responses of UO2m and VO2m, which represent the processes at the cellular-tissue level, are nearly the same. These responses at the tissue-cells level differ from the responses of VO2A and VO2p that represent processes at the whole-body level. The whole-body responses displayed two phases: Phase I reflects oxygen transport from muscle to lungs, whereas Phase II reflects cellular metabolism of exercising skeletal muscle. The biphasic behaviors are more evident in the derivatives of their dynamic responses (Fig. 3B). The area under the derivative curve represents the amplitude of the dynamics response. A comparison of the derivative responses shows that the amplitude and dynamics of the two phases of VO2p are different to those of VO2A. The model-predicted a ∼40% decrease in phosphocreatine concentration, while ATP concentration remained constant (Fig. 3C).
Figure 2.
Comparison of experimental and simulated responses of a representative subject from a warm-up steady-state to a moderate intensity exercise: (A) relative oxygen saturation in muscle and (B) pulmonary oxygen uptake.
Figure 3.
(A) Comparison of oxygen uptakes at different scales of the body (B) Derivatives of pulmonary and alveolar blood oxygen uptakes, VO2p and VO2A (C) Simulation of PCr break down and ATP concentration of a representative subject from warm-up to moderate intensity exercise
To characterize the metabolic dynamic response to exercise corresponding to seven human subjects, mean response times (MRT) of simulated oxygen uptake responses are compared in Table 1. The mean response times characterizing the pulmonary and alveolar oxygen uptake rates (VO2p, VO2A) were not significantly different (P>0.05). For VO2p and VO2A, the MRT was also computed for Phase II alone to eliminate the effect of the circulatory transport delay. These mean response times –calculated without including Phase I of the response– were consistently reduced by ∼10%, with their values not been significantly different from those characterizing the VO2m and UO2m dynamic responses (P>0.05).
Table 1.
Mean response time (MRT) of oxygen uptake and utilization responses to moderate exercise (n=7). The MRT within parentheses reflects Phase II only.
| MRT (s) | M1 | M2 | M3 | M4 | M5 | M6 | M7 | Mean±SD |
|---|---|---|---|---|---|---|---|---|
| VO2p | 25(19) | 34(31) | 27(23) | 28(23) | 27(23) | 33(29) | 24(19) | 28±3.8 (24±4.6) |
| VO2A | 24(19) | 35(33) | 28(25) | 29(25) | 28(26) | 34(31) | 24(20) | 29±4.3* (26±5.1) |
| VO2m | 20 | 34 | 26 | 25 | 26 | 33 | 24 | 27±4.6 |
| UO2m | 21 | 33 | 26 | 25 | 26 | 33 | 24 | 27±4.5 |
Difference is significant at P<0.05 when comparing with muscle O2 consumption (UO2m) using paired t-test.
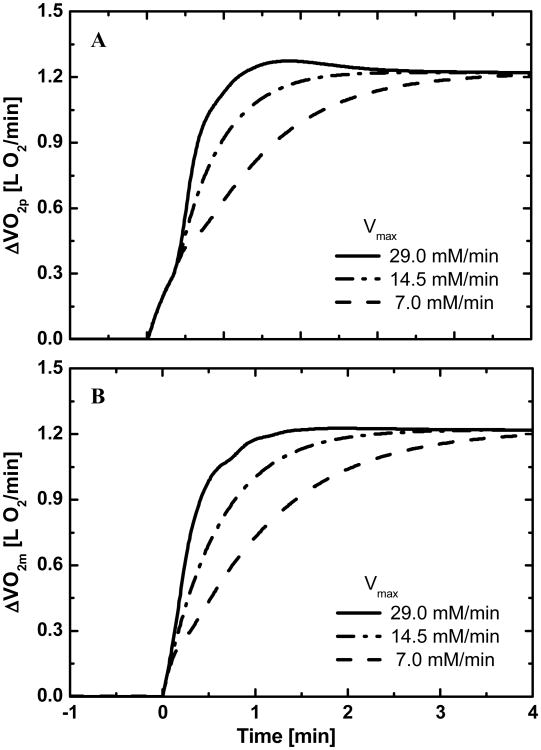
Model simulations show that different values of Vmax, the maximal flux rate of oxidative phosphorylation, affect the dynamic exercise responses of VO2p and VO2m (Fig.4). With high Vmax, an evident overshoot occurs in the VO2p response (Fig. 4A), but not in the VO2m response. Phase I does not change with Vmax . The effects of Vmax on MRT values for oxygen utilization and uptake responses are given in Table 2. With a higher Vmax, the MRT values are lower.
Figure 4.
Effects of the maximum oxidative phosphorylation flux rate Vmax on (A) Pulmonary oxygen uptake dynamics (B) Muscle oxygen uptake dynamics.
Table 2.
Effects of the maximum oxidative phosphorylation flux rate (Vmax) on mean response time (MRT) of oxygen uptake and utilization responses to moderate exercise (M2). The MRT within parentheses reflects Phase II only.
| Vmax (mM/min) | MRT (s) | |||
|---|---|---|---|---|
|
| ||||
| VO2p | VO2A | VO2m | UO2m | |
| 7.0 | 60 (57) | 60 (57) | 57 | 55 |
| 14.5 | 34 (31) | 35 (33) | 34 | 33 |
| 29 | 23(17) | 22 (19) | 18 | 18 |
Discussion
A mathematical multiscale model that couples pulmonary gas exchange[27] in a multi-organ whole-body model to a whole body-tissue model of O2 transport and cellular metabolism in skeletal muscle[15,17] was developed to study responses of oxygen uptake and cellular consumption to exercise. The corresponding energy demand due to exercise is represented by a step increase in ATP utilization. Cellular ATP homeostasis is maintained through oxidative phosphorylation and the reactions of ATPase and CK. The model also includes explicit relationships between free and bound forms of O2 that incorporate effects of hemoglobin and myoglobin in blood and skeletal muscle. To simulate experimental responses of human subjects, the maximal flux rate of oxidative phosphorylation Vmax and the blood flow increase ΔQm were estimated from measurements of muscle oxygenation during exercise[17]. The increase in cardiac output are assumed the same as ΔQm as Calbet et al.[28] showed that cardiac output and leg blood flow increased in parallel during incremental cycling exercise. Simulations with this model show the relationships between VO2p, VO2A, VO2m and UO2m and the effect of Vmax on oxygen uptake/utilization dynamics.
The cellular respiration is regulated by feedback control from ADP and tissue oxygen concentrations. In the literature, several feedback control models have been proposed for the regulation of cellular respiration: (1) feedback control using a Michaelis-Menten relationship between oxidative phosphorylation and [ADP][29]; (2) higher-order feedback control from [ADP][30,31]; (3) dependence of oxidative phosphorylation on the free energy of ATP hydrolysis.[32] However, the experimental data available in the present study are not sufficient to address this issue. In the measurement range of Ref. [30] ([ADP] from 0.018 to 0.084 mM), the experimental data were equally well described by the Michaelis-Menten relationship and higher-order model. Therefore, we chose the approach that was successfully applied previously to in vivo study obtained by NMR spectroscopy.[29]
Parameters estimates, especially for Vmax, are highly dependent on the type of compartmental model (lumped vs. distributed) used and on the value selected for working muscle volume. Lai et al. [17] used a lumped model and 49% of the whole body weight as the working skeletal muscle volume during cycling exercise. MRI measurements of body volumes show that the leg muscle volume is around 20% of the whole body weight[23]. In this study, we used a distributed model and a working muscle volume of 20% of the whole body weight. As a consequence, the estimated Vmax value in this study is 18.3±4 mM/min, which is much lower than the previously estimated value of 45±15 mM/min [17]. Model simulations of StO2m response to exercise corresponds closely with experimental data (Fig. 2A), but the predicted VO2p has a faster dynamics than the experimental data (Fig. 2B). The simulated VO2p response (Fig. 2B) indicates the presence of two phases, which are also evident for VO2A (Fig. 3B). Phase I reflects the effect of the circulatory transit time delay from skeletal muscle to lungs. The duration of Phase I in VO2p and VO2A simulations is similar to that (19±3 s) typically observed experimentally 25. During moderate-intensity exercise, the simulated PCr concentration decreased 14mM, which agrees well with the [PCr] decrease of 30% obtained experimentally by McCreary et al [19].
Since direct measurement of UO2m is not readily available, it was estimated by simulation with our model that includes the main elements of cellular metabolism and energetics. The UO2m and VO2m kinetic responses to exercise displayed a monophasic behavior without any delay after exercise onset; in contrast, the VO2p and VO2A kinetic responses displayed a biphasic behavior that includes a transport lag. When the effect of this circulatory transit time delay (Phase I) was eliminated from the responses of VO2p and VO2A, as is commonly done for the empirical analysis of the VO2 kinetic response to exercise[25], the MRTs of VO2p and VO2A during Phase II were only 3s smaller than the MRTs computed from the entire kinetic response (Table 1). However, even though these results are, in this case, consistent with previous experimental studies demonstrating that VO2m and VO2A have similar dynamic responses to moderate exercise [9], our predicted VO2p has a faster dynamics comparing to the experimental data (Fig. 2B). This discrepancy may due to the limitation in our NIRS or VO2p measurements. Firstly, considering the heterogeneity in structure and perfusion in the working skeletal muscle, using the NIRS signal obtained from a local region with an uncertain volume to represent the O2 saturation in the whole working muscle may be misleading. Secondly, our model assumed a constant volume of the alveolar space and didn't consider its change during exercise. But the VO2p experimental measurement included the alveolar gas store change due to the variation in its volume, which would slow down the VO2p dynamic response to exercise[5]. Finally, in this model, the permeability surface area coefficient (PS) in the working skeletal muscle was considered as a function of muscle blood flow[15]. PS coefficient was set to sufficiently high values to ensure enough oxygen supply to the working muscle. If the dynamics of PS change limits the blood-tissue O2 diffusion process at the onset of exercise, we would get a smaller Vmax estimated from the StO2m measurement. Specific experiments are needed to perform to quantify the muscle permeability surface area change during exercise. The advantage of our approach is that it provides a more general and mechanistic approach to investigating the dynamics of oxygen uptake at different biological scales. As a consequence, our mechanistic multiscale model –in contrast to empirical exponential fits– can be applied to many conditions under which the dissociation between pulmonary and muscle oxygen uptake may be significantly greater. For instance, when there is an oxygen transport limitation in the lungs compartment due to a less increase in alveolar permeability during exercise, which cause a decrease of the arterial O2 partial pressure to 65 to 70 mmHg, the dynamics of the simulated VO2p and VO2A can be slowed down with a 10s increase in their MRT but no significant changes occur in the simulated dynamics of VO2m and UO2m.
Model simulations quantify the relative changes of the oxygen uptake and utilization dynamic responses to exercise produced with different values of the maximal oxidative phosphorylation flux Vmax (Table 2). A 50% decrease in Vmax, which represents the disease condition with mitochondria dysfunction, both the cellular and external respirations have been slowed down even with sufficient ventilation and perfusion. An 100% increase in Vmax, which represents the fitness level of a subject, generates faster responses (i.e., MRT decreases) in VO2p, VO2A, VO2m and UO2m (Table 2). This is consistent with experimental studies that found trained subjects to have faster VO2p responses than untrained subjects during constant-load exercise[26]. Korzeniewski et al.[33,34] also proposed that an increase in the amount of mitochondrial proteins and an intensification of the parallel activation of ATP usage and ATP supply accelerate the oxygen-uptake kinetics at the onset of exercise. With higher Vmax, a Phase II overshoot can occur in the oxygen uptake response, which is most prominent for VO2p and least prominent for VO2m. No overshoot occurs in the UO2m response. Even with overshoot of the oxygen uptake response, the MRTs of VO2p and VO2A during Phase II are close to that of VO2m. Experimentally, Koppo et al.[13] reported an overshoot in the VO2p response to moderate-intensity cycle exercise. This overshoot was interpreted as an indication of a variable ATP demand that is higher at the beginning of exercise. From model simulations, however, this overshoot can occur in the oxygen uptake responses even with a constant ATP demand which is imposed by the ATP turnover rate through ATPase in the model. But the ATP turnover rate is very difficult to experimentally measure in vivo. When Vmax is increased, a temporary arterial hypoxemia happens at the onset of exercise with τQ and τV unchanged. The corresponding simulated alveolar PO2 is normal (>90 mmHg). If both τQ and τV decreased by 50%, the overshoot in the VO2p disappeared. Powers et al. found that highly trained subjects could have exercise-induced hypoxemia.[35] A further experiment for the arterial PO2 measurement is needed to indentify the origination of the overshoot signal (pulmonary vs. cellular level).
Conclusion
A multiscale mathematical model was developed to distinguish responses of external and cellular respiration to exercise of moderate intensity. Simulation shows that the characteristic response times (MRT) of external and cellular respiration are similar even when a transit delay exists between tissue cells and the lungs. The results of our model shows that the O2 transport processes from lungs to muscle are tightly coupled to provide enough O2 for working skeletal muscle during exercise in normal subjects. Under abnormal conditions, the effect of O2 transport limitation- occurring at different scale of the body - on internal to external respirations can be examined. Such results can be used for comparative quantitative analysis of the regulation of respiration in subjects suffering from abnormal function associated with disease states (e.g., chronic obstructive pulmonary disease, diabetes and congenital heart disease).
Acknowledgments
This work was supported by grants from the National Aeronautics and Space Administration (NASA – Johnson Space Center NNJ06HD81G) and the National Institute of General Medical Sciences of the National Institutes of Health (GM-66309).
References
- 1.Barstow TJ, Mole PA. Simulation of pulmonary O2 uptake during exercise transients in humans. J Appl Physiol. 1987;63:2253–2261. doi: 10.1152/jappl.1987.63.6.2253. [DOI] [PubMed] [Google Scholar]
- 2.Barstow TJ, Lamarra N, Whipp BJ. Modulation of muscle and pulmonary O2 uptakes by circulatory dynamics during exercise. J Appl Physiol. 1990;68:979–989. doi: 10.1152/jappl.1990.68.3.979. [DOI] [PubMed] [Google Scholar]
- 3.Bauer TA, Levi M, Reusch JEB, Regensteiner JG. Skeletal muscle Deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes care. 2007;30:2880–2885. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 4.Beaver WL, Lamarra N, Wasserman K. Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol. 1981;51:1662–1675. doi: 10.1152/jappl.1981.51.6.1662. [DOI] [PubMed] [Google Scholar]
- 5.Casaburi R, Weissman ML, Huntsman DJ, Whipp BJ, Wasserman K. Determinants of gas exchange kinetics during exercise in the dog. J Appl Physiol. 1979;46:1054–1060. doi: 10.1152/jappl.1979.46.6.1054. [DOI] [PubMed] [Google Scholar]
- 6.Casaburi R, Spitzer S, Haskell R, Wasserman K. Effect of altering heart rate on oxygen uptake at exercise onset. Chest. 1989;95:6–12. doi: 10.1378/chest.95.1.6. [DOI] [PubMed] [Google Scholar]
- 7.Di Prampero PE, Boutellier U, Pietsch P. Oxygen deficit and stores at onset of muscular exercise in humans. J Appl Physiol. 1983;55:146–153. doi: 10.1152/jappl.1983.55.1.146. [DOI] [PubMed] [Google Scholar]
- 8.Drexler H, Piede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 9.Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- 10.Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi N, Ishihara M, Tanaka A, Yoshida T. Impeding O2 unloading in muscle delays oxygen uptake response to exercise onset in humans. Am J Physiol. 1999;277:R1274–R1281. doi: 10.1152/ajpregu.1999.277.5.R1274. [DOI] [PubMed] [Google Scholar]
- 12.Kingwell BA, Bradley SJ, Formosa M, McConell GK, Muhlmann M. Type 2 diabetic individuals have impaired leg blood flow responses to exercise. Diabetes care. 2003;26:899–904. doi: 10.2337/diacare.26.3.899. [DOI] [PubMed] [Google Scholar]
- 13.Koppo K, Whipp BJ, Jones AM, Aeyels D, Bouckaert J. Overshoot in VO2 following the onset of moderate-intensity cycle exercise in trained cyclists. Eur J Appl Physiol. 2004;93:366–373. doi: 10.1007/s00421-004-1229-8. [DOI] [PubMed] [Google Scholar]
- 14.Lador F, zabji Kenfack M, Moia C, Cautero M, Morel DR, Capelli C, Ferretti G. Simultaneous determination of the kinetics of cardiac output, systemic O2 delivery, and lung O2 uptake at exercise onset in men. Am J Physiol. 2006;290:R1071–R1079. doi: 10.1152/ajpregu.00366.2005. [DOI] [PubMed] [Google Scholar]
- 15.Lai N, Dash RK, Nasca MM, Saidel GM, Cabrera ME. Relating pulmonary oxygen uptake to muscle oxygen consumption at exercise onset: in vivo and in silico studies. Eur J Appl Physiol. 2006;97:380–394. doi: 10.1007/s00421-006-0176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai N, Syed N, Saidel GM, Cabrera ME. Muscle oxygen uptake differs from consumption dynamics during transients in exercise. Advances in Experimental Medicine and Biology: ISOTT XXIX. 2006 doi: 10.1007/978-0-387-74911-2_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai N, Camesasca M, Saidel GM, Dash RK, Cabrera ME. Linking Pulmonary Oxygen Uptake, Muscle Oxygen Utilization and Cellular Metabolism during Exercise. Ann Biomed Eng. 2007;35:956–969. doi: 10.1007/s10439-007-9271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai N, Saidel GM, Grassi B, Gladden LB, Cabrera ME. Model of oxygen transport and metabolism predicts effect of hyperoxia on canine muscle oxygen uptake dynamics. J Appl Physiol. 2007;103:1366–1378. doi: 10.1152/japplphysiol.00489.2007. [DOI] [PubMed] [Google Scholar]
- 19.McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate-intensity calfexercise. J Appl Phsiol. 1996;81:1331–1338. doi: 10.1152/jappl.1996.81.3.1331. [DOI] [PubMed] [Google Scholar]
- 20.Mettauer B, Zhao Q, Epailly E, Charloux A, Lampert E, Heitz-Naegelen B, Piquard F, Di Prampero PE, Lonsdorfer J. VO2 kinetics reveal a central limitation at the onset of subthreshold exercise in heart transplant recipients. J Appl Physiol. 2000;88:1228–1238. doi: 10.1152/jappl.2000.88.4.1228. [DOI] [PubMed] [Google Scholar]
- 21.Nery LE, Wasserman K, Andrews JD, Huntsman DJ, Hansen JE, Whipp BJ. Ventilatory and gas exchange kinetics during exercise in chronic airways obstruction. J Appl Physiol. 1982;53:1594–1602. doi: 10.1152/jappl.1982.53.6.1594. [DOI] [PubMed] [Google Scholar]
- 22.Piiper J, Di Prampero PE, Cerretelli P. Oxygen debt and high-energy phosphates in gastrocnemius muscle of the dog. Am J Physiol. 1968;215:523–531. doi: 10.1152/ajplegacy.1968.215.3.523. [DOI] [PubMed] [Google Scholar]
- 23.Tolfrey K, Barker A, Thom JM, Morse CI, Narici MV, Batterham AM. Scaling of maximal oxygen uptake by lower leg muscle volume in boys and men. J Appl Physiol. 2006;100:1851–1856. doi: 10.1152/japplphysiol.01213.2005. [DOI] [PubMed] [Google Scholar]
- 24.Wasserman K. Coupling of external to cellular respiration during exercise: the wisdom of the body revisited. Am J Physiol. 1994;266:E519–E539. doi: 10.1152/ajpendo.1994.266.4.E519. [DOI] [PubMed] [Google Scholar]
- 25.Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K. Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol. 1982;52:1506–1513. doi: 10.1152/jappl.1982.52.6.1506. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Johnson MC, Chow N, Wasserman K. The role of fitness on VO2 and VCO2 kinetics in response to proportional step increases in work rate. Eur J Appl Physiol. 1991;63:94–100. doi: 10.1007/BF00235176. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Saidel GM, Cabrera ME. Multi-organ system model of O2 and CO2 transport during isocapnic and poikilocapnic hypoxia. Respir Physiol Neurobiol. 2007;156:320–330. doi: 10.1016/j.resp.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Calbet JA, Gonzalez-Alonso J, Helge JW, Søndergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol. 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- 29.Vicini P, Kushmerick MJ. Cellular energetic analysis by a mathematical model of energy balance: estimation of parameters in human skeletal muscle. Am J Physio Cell Physiol. 2000;279:213–224. doi: 10.1152/ajpcell.2000.279.1.C213. [DOI] [PubMed] [Google Scholar]
- 30.Jeneson JA, Wisemant RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem. 1996;271:27995–27998. doi: 10.1074/jbc.271.45.27995. [DOI] [PubMed] [Google Scholar]
- 31.Cieslar JH, Dobson GP. Free [ADP] and aerobic muscle work follow at least second order kinetics in rat gastrocnemius in vivo. J Biol Chem. 2000;275:6129–6134. doi: 10.1074/jbc.275.9.6129. [DOI] [PubMed] [Google Scholar]
- 32.Jeneson JA, Westerhoff HV, Brown TR, Van Echteld CJ, Berger R. Quasi-linear relationship between Gibbs free energy of ATP hydrolysis and power output in human forearm muscle. Am J Physio Cell Physiol. 1995;268:1474–1484. doi: 10.1152/ajpcell.1995.268.6.C1474. [DOI] [PubMed] [Google Scholar]
- 33.Korzeniewski B, Zoladz JA. Factors determining the oxygen consumption rate (VO2) on-kinetics in skeletal muscles. Biochem J. 2004;379:703–710. doi: 10.1042/BJ20031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korzeniewski B, Zoladz JA. Training-induced adaptation of oxidative phosphorylation in skeletal muscles. Biochem J. 2003;374:37–40. doi: 10.1042/BJ20030526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powers SK, Dodd S, Lawler J, Landry G, Kirtley M, Mcknight T, Grinton S. Incidence of exercise induced hypoxemia in elite endurance athletes at sea level. Eur J Appl Physiol. 1988;58:298–302. doi: 10.1007/BF00417266. [DOI] [PubMed] [Google Scholar]