Abstract
BACKGROUND: In the last decade, significant improvements have been achieved in maternal-fetal and diabetic care which make pregnancy possible in an increasing number of type 1 diabetic women with end-organ damage. Optimal counseling is important to make the advancements available to the relevant patients and to ensure the safety of mother and child. A systematic review will help to provide a survey of the available methods and to promote optimal counseling. OBJECTIVES: To review the literature on diabetic nephropathy and pregnancy in type 1 diabetes. METHODS: Medline, Embase, and the Cochrane Library were scanned in November 2012 (MESH, Emtree, and free terms on pregnancy and diabetic nephropathy). Studies were selected that report on pregnancy outcomes in type 1 diabetic patients with diabetic nephropathy in 1980-2012 (i.e. since the detection of microalbuminuria). Case reports with less than 5 cases and reports on kidney grafts were excluded. Paper selection and data extraction were performed in duplicate and matched for consistency. As the relevant reports were highly heterogeneous, we decided to perform a narrative review, with discussions oriented towards the period of publication. RESULTS: Of the 1058 references considered, 34 fulfilled the selection criteria, and one was added from reference lists. The number of cases considered in the reports, which generally involved single-center studies, ranged from 5 to 311. The following issues were significant: (i) the evidence is scattered over many reports of differing format and involving small series (only 2 included over 100 patients), (ii) definitions are non-homogeneous, (iii) risks for pregnancy-related adverse events are increased (preterm delivery, caesarean section, perinatal death, and stillbirth) and do not substantially change over time, except for stillbirth (from over 10% to about 5%), (iv) the increase in risks with nephropathy progression needs confirmation in large homogeneous series, (v) the newly reported increase in malformations in diabetic nephropathy underlines the need for further studies. CONCLUSIONS: The heterogeneous evidence from studies on diabetic nephropathy in pregnancy emphasizes the need for further perspective studies on this issue.
Keywords: type 1 diabetes, nephropathy, pregnancy, preeclampsia, chronic kidney disease, preterm delivery
Abbreviations: DCCT - Diabetes Control and Complications Trial; GFR - glomerular filtration rate; HELLP - hemolysis elevated liver enzymes, low platelet; IUGR - intrauterine growth restriction; KDOQI - Kidney Disease Outcomes Quality Initiative; MESH - medical subject headings; NICU - Neonatal Intensive Care Unit; PE - pre-eclampsia; SGA - small for gestational age; SPSS - statistical package for social sciences
Background
Diabetic nephropathy is the main cause of end-stage kidney disease worldwide [1-3]. Despite major improvements in diabetic care, its overall incidence remains considerable, and it is increasing in type 2 diabetes because of life span prolongation in diabetic patients among other factors [1-4]. While both the presentation and onset of diabetic nephropathy in type 1 diabetes have changed over time in developed countries, with delayed onset and a reduced number of cases with nephrotic syndrome, the prognosis in developing countries is still poor. The increase in patients with suboptimal diabetes control and the presence of severe diabetic nephropathy in young type 1 patients present a challenge to both nephrologists and obstetricians [5-7].
The prevalence of type 1 diabetes during pregnancy is variable world-wide and there are differences in the definition of diabetic nephropathy [8-9], which makes it difficult to combine epidemiological data. When diabetic nephropathy is broadly defined as the presence of any sign of renal disease including microalbuminuria, its prevalence ranges from 5% to over 25% in type 1 diabetic pregnant women. The highest prevalence is recorded in tertiary care centers owing to the obvious selection of referred patients [10-12].
Diabetes during pregnancy has been associated with a variety of complications, including congenital malformations, fetal growth retardation, stillbirth, early mortality, and preterm delivery [13-18]. In this context, the presence of diabetic nephropathy adds further risks. These include progression of chronic kidney disease during or after pregnancy, worsening of microvascular or macrovascular disease and increased incidence of pregnancy-related hypertensive disorders such as pregnancy-induced hypertension, pre-eclampsia (PE) or HELLP syndrome [19-24]. The definition of hypertensive disorders may be challenging since PE features (proteinuria and hypertension after the 20th gestational week) overlap with those of diabetic nephropathy. Moreover, definitions of "superimposed PE" are non-univocal [25-26].
The improvements in maternal-fetal care and the increased recognition of the effects of kidney disease in pregnancy have contributed to significant changes in the risk-benefit balance of "high risk" pregnancies and changed the prognosis for preterm babies [31-33]. Furthermore, epigenetic and developmental studies have underlined the importance of early exposure to pathologic noxae in the development of adult diseases, thus raising long-term concerns about preterm, growth-restricted babies and children born of mothers affected by different disorders [34-39].
Despite the fact that there are several recent reviews on the changing clinical spectrum of diabetic nephropathy, no review has analyzed the outcomes of pregnancy in patients with diabetic nephropathy in type 1 diabetes [27-30]. The main reason for performing the present systematic review is the need for constantly updated, evidence-based information for counseling, a pivotal task in the era of patient empowerment [40-41].
Methods
Search strategy
Simultaneous searches were performed in Pubmed, Embase and the Cochrane Library (in the last week of November 2012). The search was deliberately broad to increase sensitivity, according to the guidelines of the Cochrane Collaboration. Search terms included pregnancy as MESH, Emtree and free term and diabetic nephropathy as MESH and free term. The reference lists of reviews and selected papers were also searched for papers that had not been retrieved by the previous search strategy.
Selection criteria
Case series with less than 5 patients and patients with kidney or pancreas-kidney transplant were excluded. Likewise, papers dealing with long-term effects on mother and offspring (over 6 months) were not considered for the present analysis which is limited to pregnancy-related outcomes.
The limitations imposed on the selection of papers related both to patients and to time of publication (as provided by Medline). The period from 1980 to 2012 was selected because of the profound changes in diabetes care that have occurred since the early nineteen-eighties. Although the search was not limited to English, language barriers impaired the evaluation of four papers published in Japanese [42-45].
The search was performed in duplicate by GBP and RC. They worked independently and subsequently matched results. Abstracts and titles were screened by GBP, RC, and GC; controversies were resolved by discussion. The final paper selection was approved by the whole group and data were extracted in duplicate. The study group "Rene e Gravidanza" monitored the retrieved data and the final results.
Data collection and analysis
The following data were collected: title, author, objective, year, journal, period of study, study centers, country, type of study, number of cases, control group (when present), maternal age, parity, hypertension, PE, proteinuria, glycemic control, drugs, additional care, gestational age, birth weight, indication for delivery, preterm delivery, stillbirth/neonatal death, small for gestational age (SGA), intrauterine growth restriction (IUGR), admission to intensive care unit (NICU), malformations, other neonatal complications (whenever reported), maternal and fetal follow-up, definition of diabetic nephropathy, and inclusion criteria.
Papers were divided into three major periods: 1980-1989; 1990-1999 and 2000 to present. As the definition of PE has changed over time, the older term "gestosis" was considered equivalent to PE. Relevant definitions (for example as for HELLP or pregnancy-induced hypertension) were also collected.
The decision to perform a narrative or meta-analytical systematic review was dependent on the type and quality of the evidence retrieved. Since we were expecting to deal with a high degree of heterogeneity, a descriptive narrative review was planned for the main results and outcomes, while data pooling was envisaged for each period according to the degree of diabetic nephropathy. Data analysis was performed using SPSS version 18.0.
Results
Retrieving the evidence and summary data
Our search on diabetic nephropathy (type 1 diabetes) and pregnancy identified 209 articles from 1058 references. Thirty-four satisfied the selection criteria, one article was added from the reference lists (Figure 1) [12, 46-79]. Sixteen studies (719 pregnancies) published in 2000-2012 (Table 1), 15 studies (719 pregnancies) published in 1990-1999 (Table 2), and 4 studies (73 pregnancies) published in 1980-1989 were selected (Table 3).
Figure 1. Study design.
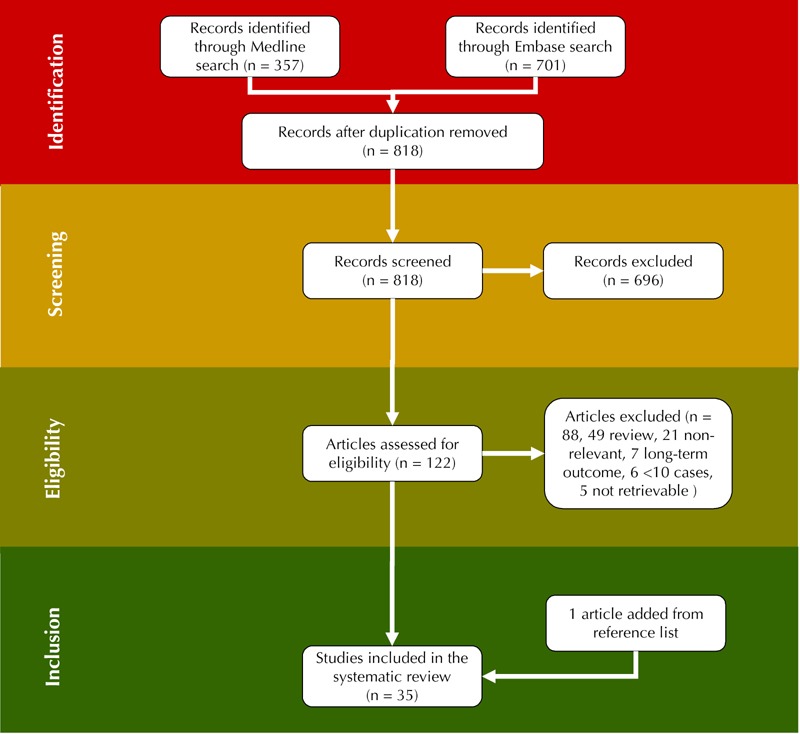
In total, 818 articles have been retrieved from database screening. After removal of duplicate and non-relevant articles, 122 have been assessed for eligibility. Of these 122 articles, reviews and further non-relevant articles have been excluded such that finally 35 studies could be included in the present study.
Table 1. General data on selected studies from 2000-2012.
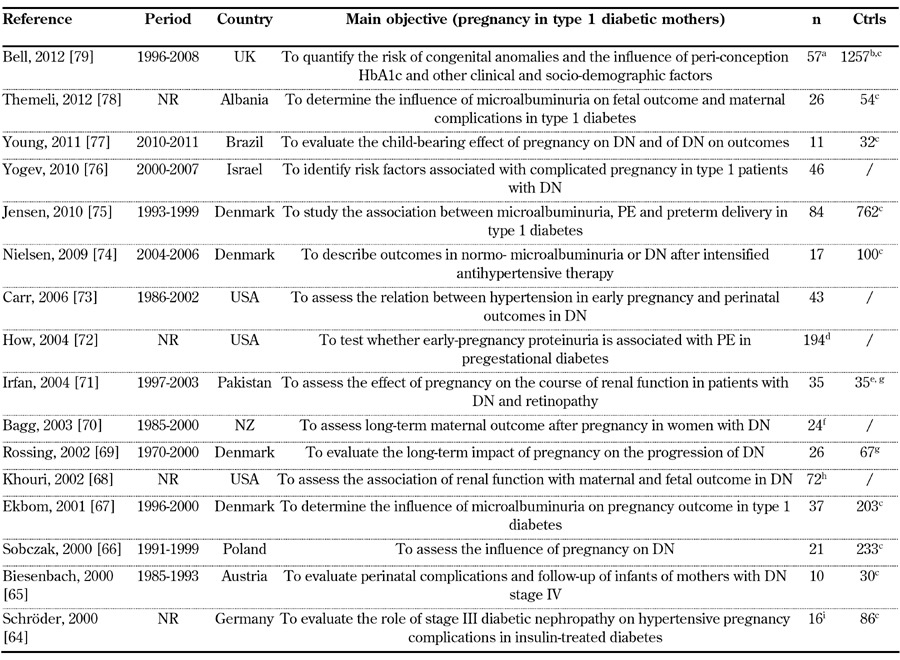
Legend: a 3 cases with type 2 diabetes were not considered. b The study reports on 1314 pregnancies in type 1 diabetes, 363 in type 2 diabetes. c Pregnant type 1 diabetic patients without nephropathy. d Cases: 94 with proteinuria <190 mg/24 h, 35 proteinuria 190-499 mg/24 h, 65 proteinuria >499 mg/24 h. e Controls are mentioned, data are not supplied. f 14 women. g Non-pregnant women with diabetic nephropathy. h 58 women. i Cases: 8 type 1 diabetes with UAE 30-300 mg/day, 5 gestational diabetes with UAE 30-300 mg/day, 3 with UAE > 300 mg/day. Abbreviations: Ctrls – controls, DN – diabetic nephropathy, IDDM – insulin-dependent diabetes mellitus, n – number of pregnancies, NR – not reported, PE – pre-eclampsia, Pro – prospective, Pts – patients, Ret – retrospective. References [64-79].
Table 2. General data on selected studies from 1990-1999.
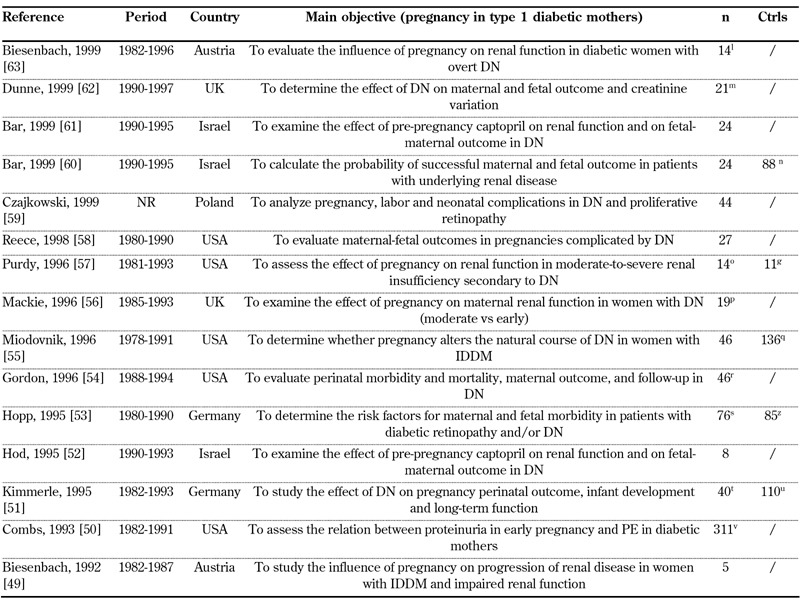
Legend: g Non-pregnant women with diabetic nephropathy.l 12 women. m 18 women. n 65 women: 38 with primary renal disease, 27 with functioning renal allograft. o 11 women. p 17 women. q 13 women developed diabetic nephropathy. r 45 women. s Retinopathy or nephropathy. t 33 women. u 91 women. v 190 proteinuria < 190 mg/day, 45 proteinuria 190-499 mg/day, 62 proteinuria >= 500 mg/day. z Patients without severe microangiopathy (White C D). Abbreviations: Ctrls – controls, DN – diabetic nephropathy, IDDM – insulin-dependent diabetes mellitus, n – number of pregnancies, NR – not reported, PE – pre-eclampsia, Pro – prospective, Pts – patients, Ret - retrospective. References [49-63].
Table 3. General data on selected studies from 1980-1989.
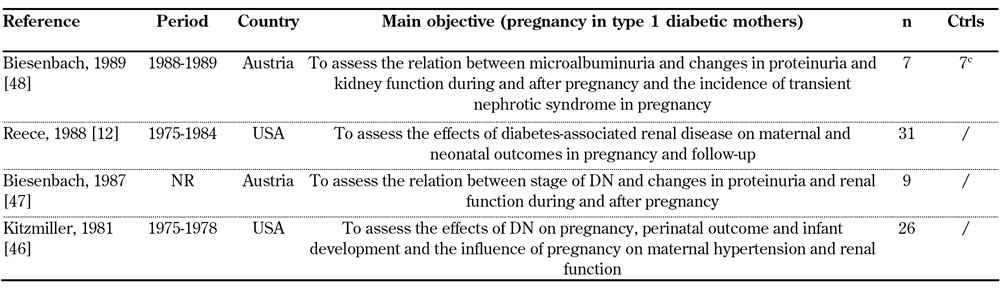
Most studies were monocentric. Because case reports were not included, the number of observed patients ranged from 5 to 311. Two studies only included more than 100 pregnancies and stratified the patients according to albuminuria levels.
The studies originated from all over the world: ten were from North America, eighteen from Europe, one from Asia, one from New Zealand, one from South America and four from Israel. Overall, Europe and USA are the main sources of the data. The studies were heterogeneous with regard to duration (from 1 to over 20 years) and period of study (1966-1981 through 2010-2011) (Tables 1-3). Seventeen studies included "controls". Of these studies, nine included type 1 pregnant diabetic patients without nephropathy as controls.
Definitions and staging of diabetic nephropathy
Most of the studies gave the definition of diabetic nephropathy or the selection criteria employed when they dealt with retrospective analyses (Tables 4-6, in the Appendix). However, the definitions differed over time. Diabetic nephropathy was more often defined by albumin excretion rates. The cut-off point most frequently applied since 2000 was 300 mg/day, while 500 mg/day was generally used before that date. Some studies included serum creatinine in the definition (e.g. Yogev, 2010 [76]), while others stressed the fact that urinary albumin excretion (UAE) should be considered when bacteriuria and other signs of kidney disease (e.g. Biesenbach, 1999 [63]) are absent or diabetic retinopathy (e.g. Bagg, 2003 [70]) or hypertension (i.e. Schröder, 2000 [64]) are present.
Table 4. Main definitions of diabetic nephropathy in type 1 diabetes, diabetes control, and subgroups considered from 2000-2012.
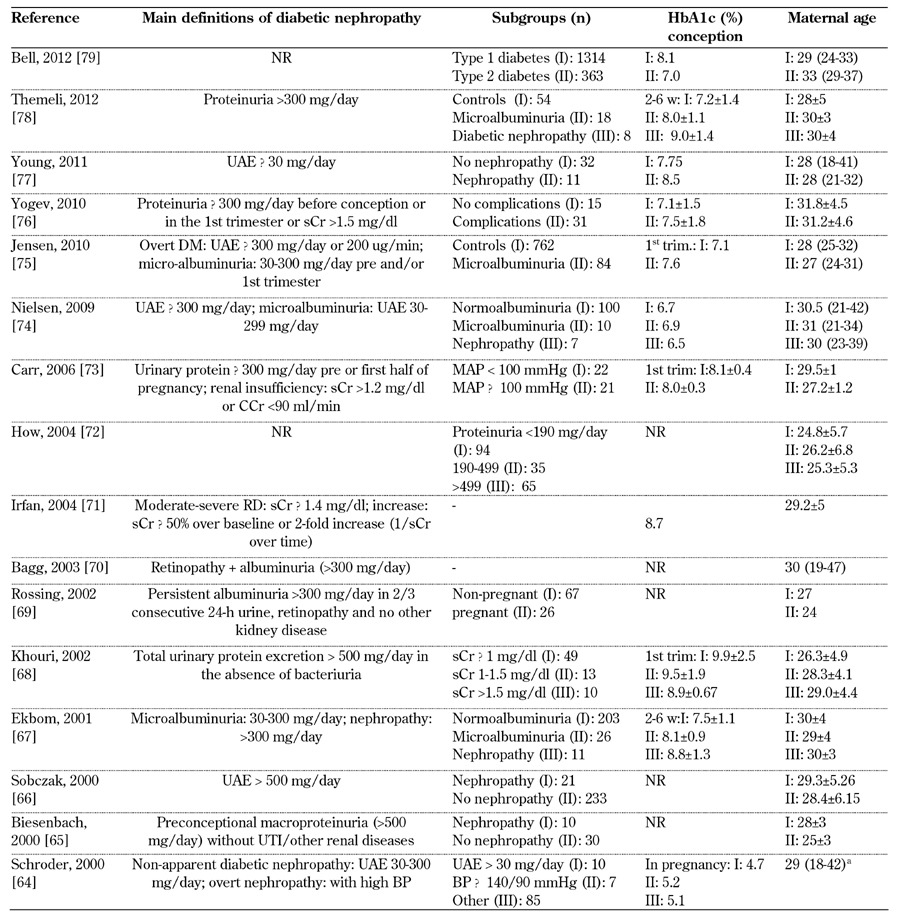
Legend: a without any subgroup specification. b value as SD above mean. c White class B: onset at age 20 or older or duration < 10 years; class C: onset at age 10-19 or duration 10-19 years; class D: onset < 10 or duration > 20 years; class E: overt diabetes mellitus with calcified pelvic vessels; class F: diabetic nephropathy; class R: proliferative retinopathy; class RF: retinopathy and nephropathy; class H: ischemic heart disease; class T: prior kidney transplant. Abbreviations: PE – preeclampsia, SBP – systolic blood pressure, DBP – diastolic blood pressure, μg – microgram, sCR – serum creatinine, CCr – creatinine clearance rate, MAP – mean arterial pressure, UAE – urinary albumin excretion, ESRD – end-stage renal disease. ACOG – American College of Obstetricians and Gynecologists, AKF – acute kidney failure, PtU – proteinuria, wk – week, trim – trimester, DN - diabetic nephropathy, NR – not reported, HbA1c – glycated hemoglobin. References [64-79].
Table 6. Main definitions of diabetic nephropathy in type 1 diabetes, diabetes control, and subgroups considered from 1980-1989.
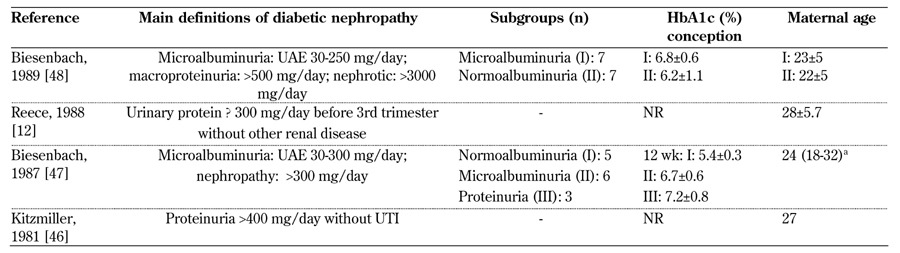
Microalbuminuria represents a "huge area", occasionally defined as "non-apparent diabetic nephropathy" (Schröder, 2000 [64]) or included in "diabetic nephropathy" (Young, 2011 [77]) (Tables 4-6, in the Appendix). In line with the changes in age at conception observed in the last decades, mean/median age has progressively increased in Western countries from the 20s to the 30s, in particular over the periods of 1980-1990 and 2010-2013. This may reflect a delay in the onset of diabetic nephropathy (Tables 4-6, in the Appendix).
Diabetes control, reported at different intervals during pregnancy, is also variable as a reflection of study settings and patient selection (Tables 4-6, in the Appendix).
Main maternal outcomes
Main maternal outcomes are reported in Tables 7-9 (in the Appendix). According to the search strategy, papers containing information on short-term pregnancy-related outcomes were included in the analysis. Five papers were mainly dealing with long-term outcomes, but also included short-term data, and were thus selected as well.
Table 7. Main maternal outcomes from 2000-2012.
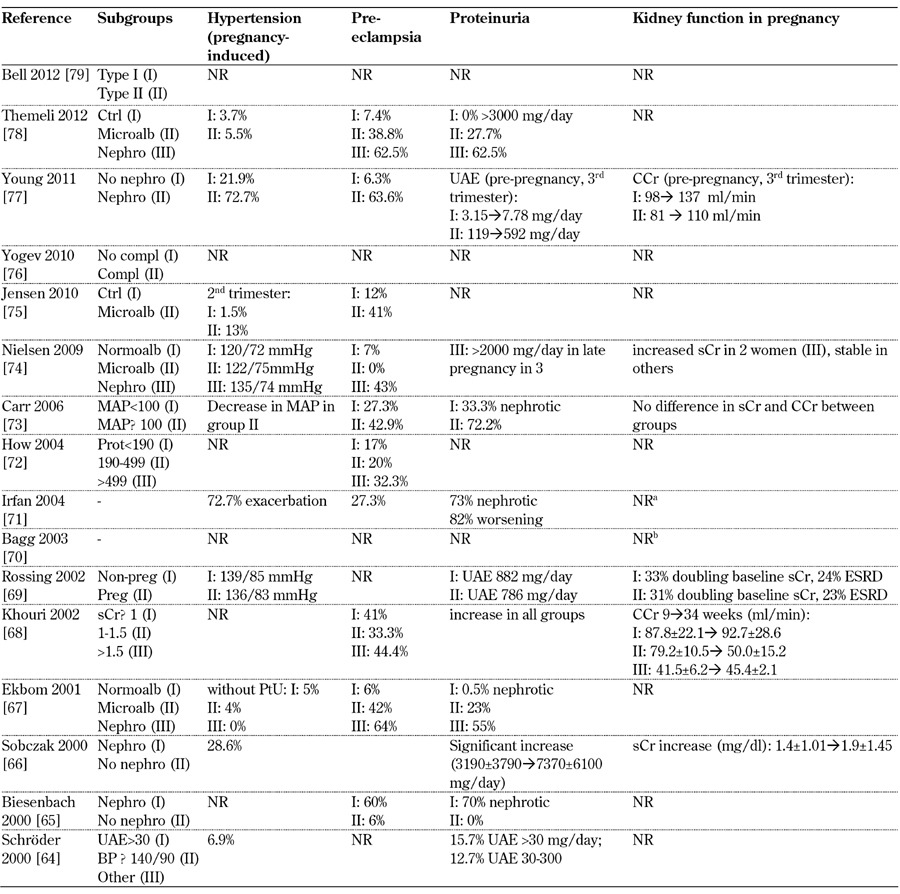
Legend: Medium-long term outcomes: a Irfan 2004: 63.6% need dialysis/transplantation (26 months from parturition). b Bagg 2003: 36% begun dialysis (follow-up 6 years). Abbreviations: ATG – above target group, BP – blood pressure, BTG – below target group, CCr – creatinine clearance, ctrl – control, DBP – diastolic blood pressure, ESRD – end-stage renal disease, GFR – glomerular filtration rate, DN – diabetic nephropathy, LN – lupus nephritis, MAP – mitogen-activated protein, microalb – microalbimuria, nephro – nephropathy, normoalb – normoalbimuria, NR – not reported, OR – odds ratio, PE – preeclampsia, PN – pyelonephritis, preg – pregnant, prot – proteinuria, PtU – proteinuria, sCr – serum creatinine, SBP – systolic blood pressure, trim – trimester, UAE – urinary albumin excretion, UTI – urinary tract infection, wk – week. *with severe microangiopathy. References [64-79].
Table 9. Main maternal outcomes from 1980-1989.
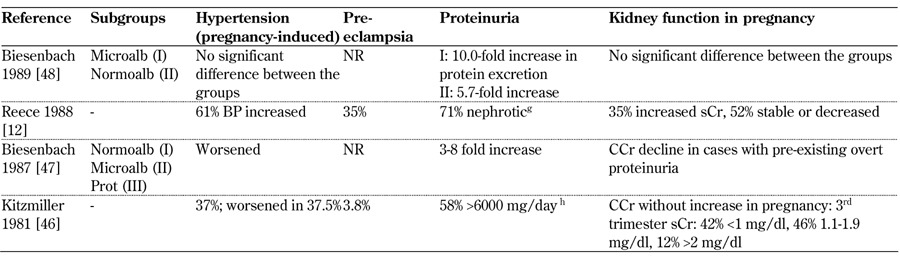
Legend: Medium-long term outcomes: g Reece 1988: proteinuria increases reverted in puerperium. h Kitzmiller 1981: proteinuria declined >50% in 65.2% 6-35 months after pregnancy White class B: onset at age 20 or older or duration < 10 years; class C: onset at age 10-19 or duration 10-19 years; class D: onset < 10 or duration > 20 years; class E: overt diabetes mellitus with calcified pelvic vessels; class F: diabetic nephropathy; class R: proliferative retinopathy; class RF: retinopathy and nephropathy; class H: ischemic heart disease; class T: prior kidney transplant. Abbreviations: ATG – above target group, BP – blood pressure, BTG – below target group, CCr – creatinine clearance, ctrl – control, DBP – diastolic blood pressure, ESRD – end-stage renal disease, GFR – glomerular filtration rate, DN – diabetic nephropathy, LN – lupus nephritis, MAP – mitogen-activated protein, microalb – microalbimuria, nephro – nephropathy, normoalb – normoalbimuria, NR – not reported, OR – odds ratio, PE – preeclampsia, PN – pyelonephritis, preg – pregnant, prot – proteinuria, PtU – proteinuria, sCr – serum creatinine, SBP – systolic blood pressure, trim – trimester, UAE – urinary albumin excretion, UTI – urinary tract infection, wk – week. References [12, 46-48].
Proteinuria and hypertension are the main hallmarks of diabetic nephropathy during pregnancy. Pre-eclampsia is usually defined as proteinuria >300 mg/day, hypertension in the absence of proteinuria and pre-conception hypertension. However, the role of baseline microalbuminuria is not clear and none of the studies give any information on uteroplacental blood flows or other obstetrical or biochemical markers of PE (Tables 7-9, in the Appendix). The different definitions of PE may account for some of the differences recorded. PE and/or nephrotic proteinuria, considered together, are described in over 60% of cases with baseline nephropathy (for example Themeli 2012 [78], Young 2011 [77], Ekbom 2001 [67], Biesenbach 1999 [63] and 2000 [65]). In the few papers reporting on this medium-term outcome, proteinuria slowly decreases towards baseline in 3-6 months (Biesenbach 1999 [63], Biesenbach 1992 [49], Kitzmiller 1981 [46]) (Tables 7-9, in the Appendix).
Regarding the parameters of renal function, the wide variety of definitions and patient selection criteria did not allow data pooling. All possible outcomes are reported:
1. Physiological increase in glomerular filtration rate (GFR) throughout pregnancy (Young 2011 [77])
2. Stable kidney function (Bar 1999 [61])
3. Individual patterns (Mackie 1996 [56], Reece 1988 [12])
4. Frequent worsening (Biesenbach 1999 [63], Biesenbach 2000 [65], Rossing 2002 [69])
In those papers describing different stages of diabetic nephropathy, worsening is reported as more frequent in cases where there is functional impairment or severe proteinuria at baseline (Tables 7-9, in the Appendix). However, the inhomogeneity of the definitions prevents the pooling of data. In the context of good overall glycemic control, no clear link between glycated hemoglobin and outcomes would appear to be evident in this descriptive analysis.
Notably, maternal age reported in the papers showed an increasing trend, presumably in parallel to the increase in the overall population. All the series published in the first decade include women younger than 30 years (mean or median age). In the second decade, women aged less than 30 years make up 11/15 of the patients (7 <28 years) and this figure is 10/16 in the last period (2000-2012). In the last period, 2 series only include cases with a mean/median age <28 years.
Main fetal outcomes
The main fetal outcomes are reported in Tables 10-12 (in the Appendix). No paper reported control data in "normal", non-diabetic pregnancies. Interestingly, when the three papers (Themeli 2012 [78]; Nielsen 2009 [74]; Ekbom 2001 [67]) which use non-microalbuminemic patients, homogeneously defined, as controls are considered, a higher prevalence of adverse pregnancy-related events in cases with micro- and macro-albuminuria is confirmed as is also the case for SGA and preterm delivery (both at gestational age <34 and <37 weeks), although the prevalence of perinatal death and stillbirth is not higher, possibly because of the small number of cases (Table 13).
Table 10. Main fetal outcomes from 2000-2012.
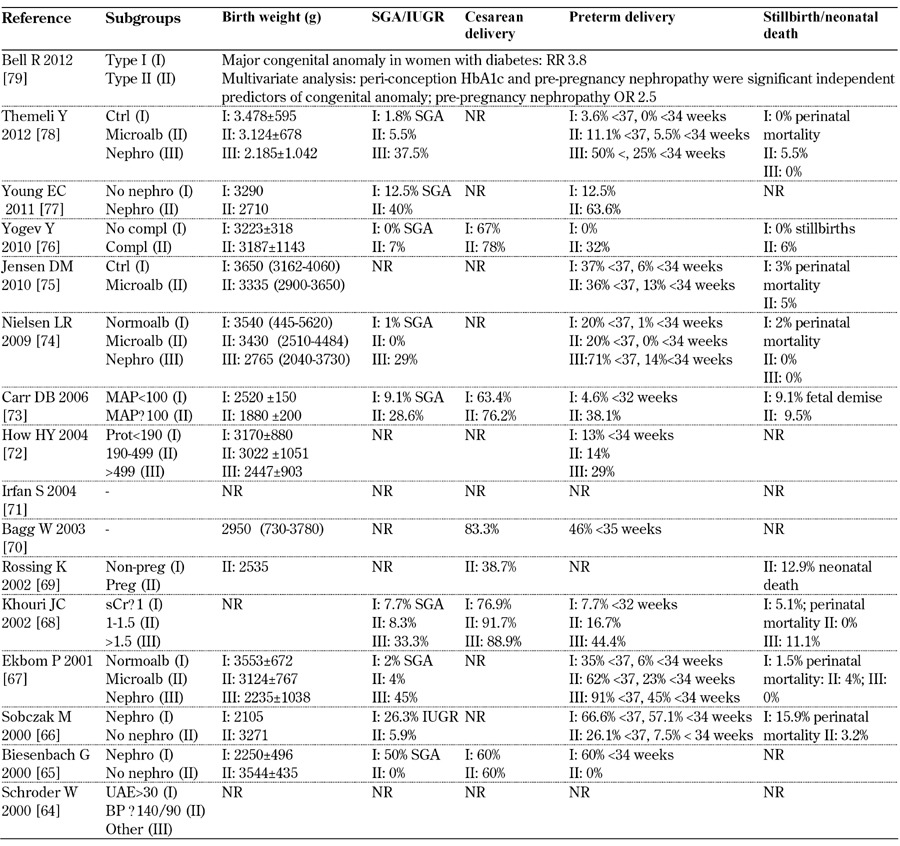
Legend: White class B: onset at age 20 or older or duration < 10 years; class C: onset at age 10-19 or duration 10-19 years; class D: onset < 10 or duration > 20 years; class E: overt diabetes mellitus with calcified pelvic vessels; class F: diabetic nephropathy; class R: proliferative reti-nopathy; class RF: retinopathy and nephropathy; class H: ischemic heart disease; class T: prior kidney transplant. Abbreviations: BP – blood pressure, Compl – complications, Ctrl – control, IUGR – intrauterine growth restriction, LN – lupus nephritis, Macroalb – macroalbuminuria, MAP – mitogen-activated protein, Microalb – microalbuminuria, MPGN – membranoproliferative glomerulonephritis, Nephro - nephropathy, Normoalb – normoalbuminuria, NR – not reported, OR – odds ratio, Preg – pregnant, Prot – proteinuria, RR – relative risk, sCr – serum creat-inine, SGA – small for gestational age, SLE – systemic lupus erythematosis, UAE - urinary albumin excretion. References [64-79].
Table 12. Main fetal outcomes from 1980-1989.
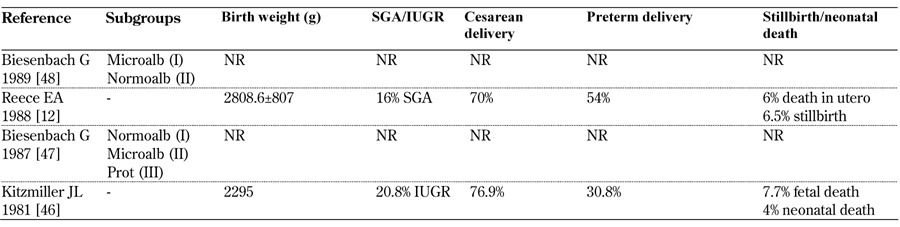
Legend: White class B: onset at age 20 or older or duration < 10 years; class C: onset at age 10-19 or duration 10-19 years; class D: onset < 10 or duration > 20 years; class E: overt diabetes mellitus with calcified pelvic vessels; class F: diabetic nephropathy; class R: proliferative reti-nopathy; class RF: retinopathy and nephropathy; class H: ischemic heart disease; class T: prior kidney transplant. Abbreviations: IUGR – in-trauterine growth restriction, Microalb – microalbuminuria, Normoalb – normoalbuminuria, NR – not reported, Prot – proteinuria, RR – rela-tive risk, SGA – small for gestational age. References [49-63].
Table 13. Main fetal outcomes in patients with type 1 diabetes, microalbuminuria, and nephropathy homogeneously defined in Themeli 2012, Nielsen 2009, and Ekbom 2001.
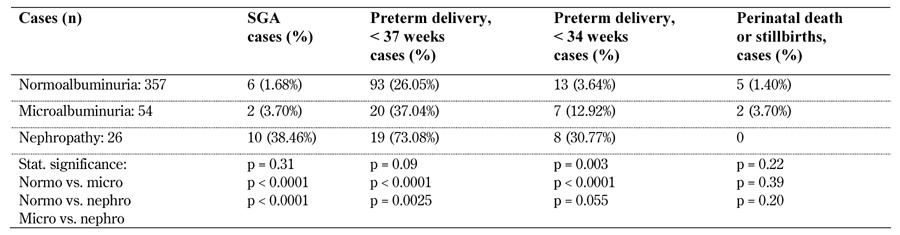
Legend: Normo – normoalbuminuria, Micro – microalbuminuria, Nephro – diabetic nephropathy, SGA – small for gestational age.
Caesarian section rates in microalbuminuric patients vary widely from none (Nielsen, 2009 [74]) to about 20%. Higher levels are recorded in patients with full-blown diabetic nephropathy (up to over 90% according to Khouri 2002 [68]). The incidence of preterm delivery is likewise high, within a wide range, and it is almost the rule in full-blown diabetic nephropathy. Again, there is an increasing trend in severe diseases (albeit differently defined) (Tables 10-12, in the Appendix).
Against a background of risk for macrosomal babies, the incidence of SGA and IUGR increased along with worsening of kidney disease. Once more, the definitions of SGA and IUGR were non-univocal and this may account for at least part of the reported variability (Tables 10-12, in the Appendix). As a reflection of the different definitions and populations studied, the prevalence of pre-term delivery remained high without a decreasing trend over the three decades analyzed.
It is worth noting that the incidence of stillbirth and neonatal death has remained high in recent years (5.5% according to Themeli 2012 [78], 6% according to Yogev 2010 [76]). However, there may be suggestions of an improving trend as compared with the cumulative incidence of neonatal and fetal death of over 10% in the eighties. The inhomogeneity of the definitions prevents further pooling of the data. In the context of overall good glycemic control, no clear link between glycated hemoglobin and outcomes would appear to be evident in this descriptive analysis. Only one paper specifically reported on malformations, described as increased in diabetic nephropathy as compared with diabetic patients (Bell, 2012 [78]).
Discussion
The most common complications of diabetes and pregnancy affect the kidney. It is not surprising therefore that several pregnancy-related adverse outcomes, such as hypertension, proteinuria and PE, were more common in diabetic patients, and even more common in patients with diabetes and kidney disease [10-17].
In an era when patients are increasingly required to participate in therapeutic choices, a systematic review of the literature may be helpful for counseling. Thus, the present review started from the early eighties, the time when the DCCT study set new standards and goals for diabetic care [80]. The review deals with short-term pregnancy-related outcomes, by definition within 6 months of delivery or after birth.
In our opinion, five major points emerge from our analysis which are potentially relevant to patient counseling. The first point is the relatively limited evidence available on this very important and challenging nephropathy (Tables 1-3). In fact, out of thirty-four papers retrieved, only two reported on more than 100 patients, while most studies (28/35) described less than fifty patients (Tables 1-2). The second point relates to the definitions of diabetic nephropathy. In fact, after the publication of the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, attention shifted to earlier stages of kidney disease, in part as a reflection of the change in the definition of chronic kidney disease, which was substantially modified in the new millennium [81] (Tables 4-6, in the Appendix). In diabetic nephropathy, as well as in clinical nephrology, a common language is urgently required [26]. The wide range of definitions of diabetic nephropathy is likely to affect the results. In recent reports, diabetic nephropathy has included albuminuria or required urinary proteins to reach 0.5 g/day. It may or may not take serum creatinine into account or require the presence of other signs of microvascular disease (Tables 4-6, in the Appendix).
These first two points should give rise to discussion of the limits and biases of the available evidence in the context of patient counseling. Given these limits, the third point is that, in each period of study, there was substantial agreement on a negative observation. Indeed, the risks for pregnancy-related adverse events were increased in patients with type 1 diabetes and diabetic nephropathy (Tables 1-12).
Furthermore, there would appear to be an overall increase in risk as diabetic nephropathy progresses, following a trend similar to that observed in other kidney disease [26, 82, 83]. Maternal outcomes are widely scattered and probably reflect several factors: the lack of univocal definitions of diabetic nephropathy, chronic kidney disease, and PE, increasing maternal age over time, different study aims, patient selection and the setting of several studies, small sample size, and single-center and care setting (Tables 1-12).
Within this framework, two further aspects may be emphasized. The risks do not decrease over time (Tables 7-9, in the Appendix). An increase in maternal age, observed over time, and presumably paralleling the ageing of the overall population, may be one of the clues which explains part of this phenomenon, as an increase in maternal age is associated with an independent risk for adverse pregnancy-related outcomes and proteinuria. Hypertension, proteinuria and PE are the most relevant challenges; however the relative role of each complication is difficult to determine, another reflection of the non-univocal definition of PE and "superimposed PE", in particular with respect to microalbuminuria (Tables 7-9, in the Appendix). In fact, the strict definition of PE (proteinuria and hypertension after the 20th gestational week in a previously normotensive, non proteinuric patient), was designed to identify cases without kidney damage at baseline, a situation that hardly applies to patients with microvascular disease [25].
Conversely, the risks of a worsening in kidney function are more difficult to summarize, as all possible occurrences are reported in the studies (increase, decrease, stable kidney function) and probably depend upon a complex interaction between baseline function and pregnancy-related outcomes. Furthermore, without a comparison of long-term outcomes in type 1 diabetic patients with a comparable degree of kidney disease but no pregnancies it is impossible to reach definitive conclusions (Tables 4-9, in the Appendix). Thus, counseling on the maternal risks should underline the increased risk of developing hypertension and proteinuria, either in the context of PE or as a specific response of baseline nephropathy to the challenge presented by hyperfiltration during pregnancy. Moreover, it is important to mention the highly unpredictable but observed risk of a worsening in kidney function during pregnancy and after delivery (Tables 7-9, in the Appendix). Remarkable differences in the reported outcomes suggested a relevant "center effect" and they are in favor of referral to experienced settings.
The last two points concern fetal outcomes. The first is related to the greatest risks of diabetic pregnancies: stillbirth or fetal death (Tables 10-12, in the Appendix). While the high risk of prematurity and SGA or intrauterine growth restriction is also shared by patients with other kidney disorders and increases as the kidney disease progresses, stillbirth or fetal death are shared only by patients with systemic lupus erythematous, another systemic syndrome with remarkable microvascular damage [26, 82, 83]. The risk has also been reported in diabetic patients without overt nephropathy, but appears to be increased in the setting of diabetic nephropathy (Tables 10-12, in the Appendix). Interestingly, even if the risks of stillbirth and fetal death have been reduced in the period from 2000 to date compared to the eighties (from over 10% to about 5%), the reduction is much less than that observed in the case of mothers on dialysis for example, which has decreased by 25% in each decade since the eighties, albeit starting from a much higher level [84-86]).
Appendix
Table 5. Main definitions of diabetic nephropathy in type 1 diabetes, diabetes control, and subgroups considered from 1990-1999.
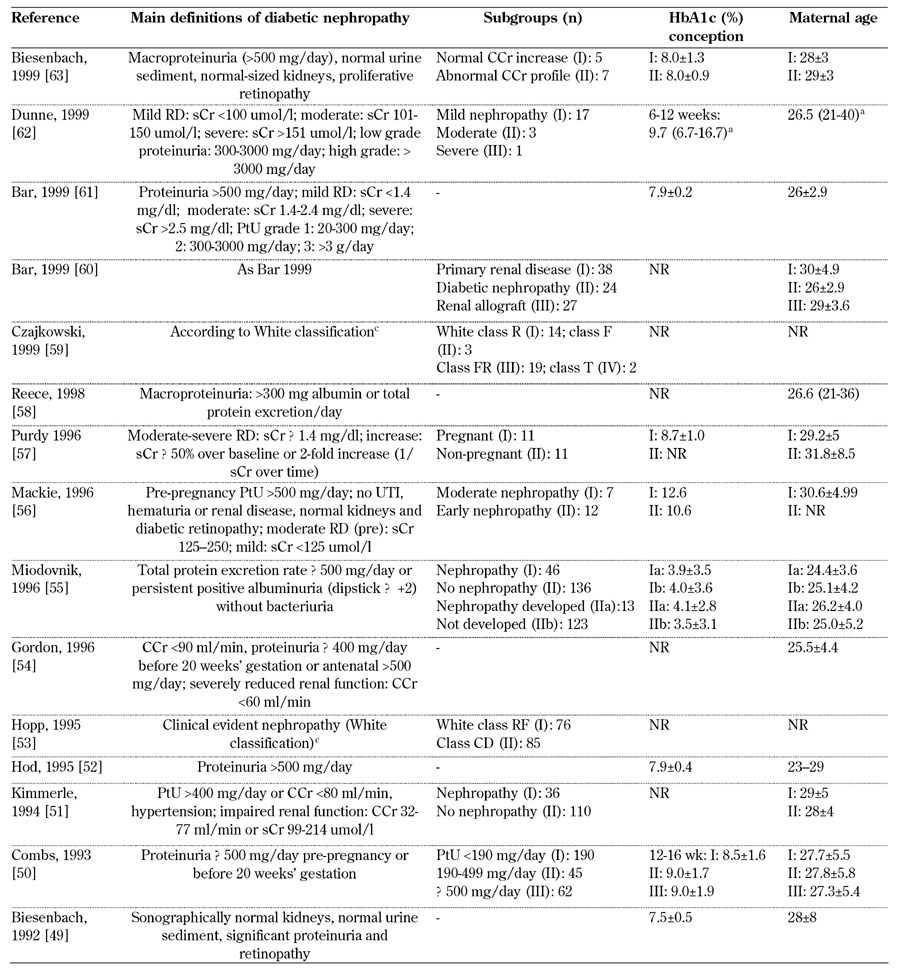
Legend: a without any subgroup specification. b value as SD above mean. c White class B: onset at age 20 or older or duration < 10 years; class C: onset at age 10-19 or duration 10-19 years; class D: onset < 10 or duration > 20 years; class E: overt diabetes mellitus with calcified pelvic vessels; class F: diabetic nephropathy; class R: proliferative retinopathy; class RF: retinopathy and nephropathy; class H: ischemic heart disease; class T: prior kidney transplant. Abbreviations: PE – preeclampsia, SBP – systolic blood pressure, DBP – diastolic blood pressure, μg – microgram, sCR – serum creatinine, CCr – creatinine clearance rate, MAP – mean arterial pressure, UAE – urinary albumin excretion, ESRD – end-stage renal disease. ACOG – American College of Obstetricians and Gynecologists, AKF – acute kidney failure, PtU – proteinuria, wk – week, trim – trimester, DN – diabetic nephropathy, NR – not reported, HbA1c – glycated hemoglobin. References [49-63].
Table 8. Main maternal outcomes from 1990-1999.
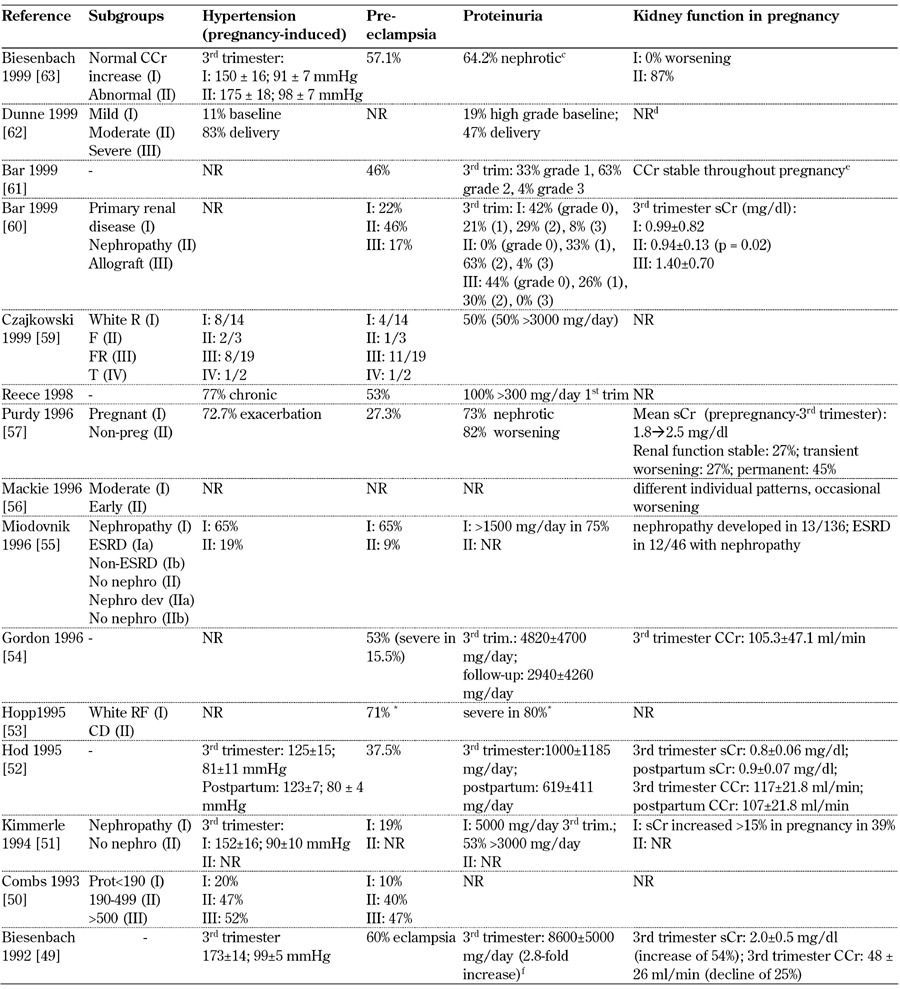
Legend: Medium-long term outcomes: c Biesenbach 1999: protein excretion to pre-conceptional values 3-6 months after delivery; d Dunne 1999: one patient reached ESRD and received hemodialysis; e Bar 1999: no deterioration in renal function in any patient after two years; f Biesenbach 1992: proteinuria returned to pre-pregnancy within 3-6 months. Abbreviations: ATG – above target group, BP – blood pressure, BTG – below target group, CCr – creatinine clearance, ctrl – control, DBP – diastolic blood pressure, ESRD – end-stage renal disease, GFR – glomerular filtration rate, DN – diabetic nephropathy, LN – lupus nephritis, MAP – mitogen-activated protein, microalb – microalbimuria, nephro – nephropathy, normoalb – normoalbimuria, NR – not reported, OR – odds ratio, PE – preeclampsia, PN – pyelonephritis, preg – pregnant, prot – proteinuria, PtU – proteinuria, sCr – serum creatinine, SBP – systolic blood pressure, trim – trimester, UAE – urinary albumin excretion, UTI – urinary tract infection, wk – week. * with severe microangiopathy. References [49-63].
Table 11. Main fetal outcomes from 1990-1999.
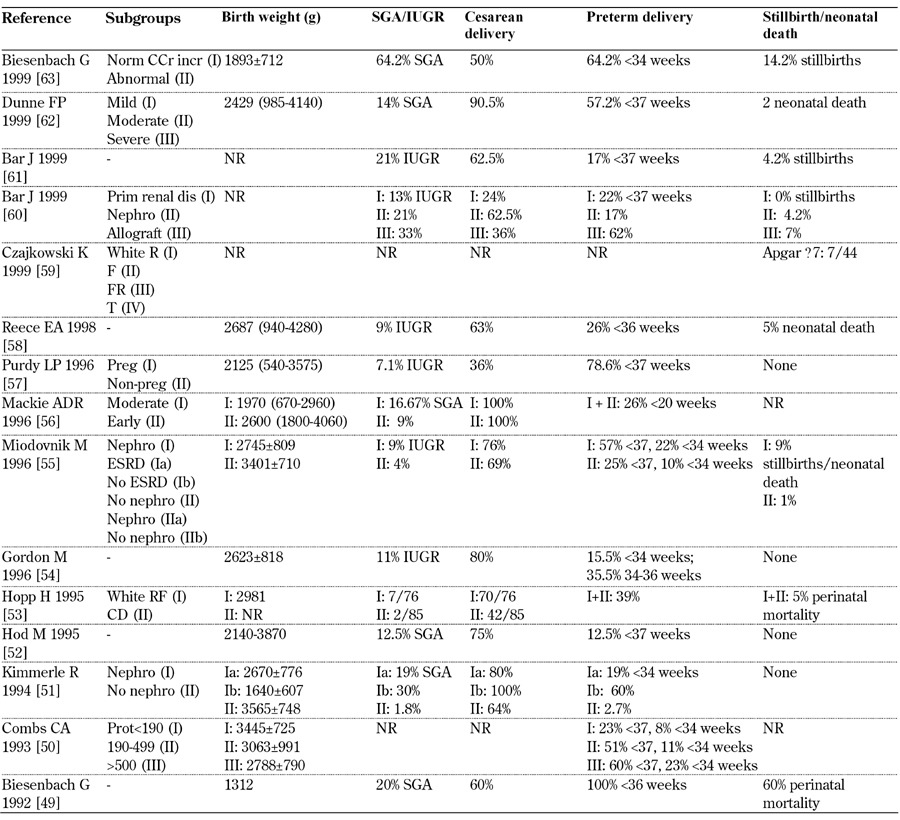
Legend: White class B: onset at age 20 or older or duration < 10 years; class C: onset at age 10-19 or duration 10-19 years; class D: onset < 10 or duration > 20 years; class E: overt diabetes mellitus with calcified pelvic vessels; class F: diabetic nephropathy; class R: proliferative reti-nopathy; class RF: retinopathy and nephropathy; class H: ischemic heart disease; class T: prior kidney transplant. Abbreviations: BP – blood pressure, Compl – complications, Ctrl – control, ESRD – end-stage renal disease, IUGR – intrauterine growth restriction, LN – lupus nephritis, Macroalb – macroalbuminuria, MAP – mitogen-activated protein, Microalb – microalbuminuria, MPGN – membranoproliferative glomeru-lonephritis, Nephro - nephropathy, Normoalb – normoalbuminuria, NR – not reported, OR – odds ratio, Preg – pregnant, Prot – proteinuria, RR – relative risk, sCr – serum creatinine, SGA – small for gestational age, SLE – systemic lupus erythematosis, UAE - urinary albumin excre-tion. References [49-63].
References
- 1.Reutens AT. Epidemiology of diabetic kidney disease. Med Clin N Am. 2013;97:1–18. doi: 10.1016/j.mcna.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Williams ME. Diabetic kidney disease in elderly individuals. Med Clin N Am. 2013;97:75–89. doi: 10.1016/j.mcna.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG DEMAND investigators. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 4.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q. et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 6.Unnikrishnan RI, Rema M, Pradeepa R, Deepa M, Shanthirani CS, Deepa R, Mohan V. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45) Diabetes Care. 2007;30:2019–2024. doi: 10.2337/dc06-2554. [DOI] [PubMed] [Google Scholar]
- 7.Rosolowsky ET, Skupien J, Smiles AM, Niewczas M, Roshan B, Stanton R, Eckfeldt JH, Warram JH, Krolewski AS. Risk for ESRD in type 1 Diabetes Remains High Despite Renoprotection. J Am Soc Nephrol. 2011;22:545–553. doi: 10.1681/ASN.2010040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab. 2012;38:291–297. doi: 10.1016/j.diabet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Hill CJ, Fogarty DG. Changing trends in end-stage renal disease due to diabetes in the United Kingdom. J Ren Care. 2012;38:12–22. doi: 10.1111/j.1755-6686.2012.00273.x. [DOI] [PubMed] [Google Scholar]
- 10.Landon MB. Diabetic nephropathy and pregnancy. Clin Obstet Gynecol. 2000;50:998–1006. doi: 10.1097/GRF.0b013e31815a6383. [DOI] [PubMed] [Google Scholar]
- 11.Kaaja R. Vascular complications in diabetic pregnancy. Thromb Res. 2011;127(Suppl 3):S53–S55. doi: 10.1016/S0049-3848(11)70015-9. [DOI] [PubMed] [Google Scholar]
- 12.Reece EA, Coustan DR, Hayslett JP, Holford T, Coulehan J, O'Connor TZ, Hobbins JC. Diabetic nephropathy: pregnancy performance and feto-maternal outcome. Am J Obstet Gynecol. 1988;159:56–66. doi: 10.1016/0002-9378(88)90494-2. [DOI] [PubMed] [Google Scholar]
- 13.Reece EA, Homko CJ. Diabetes-related complications of pregnancy. J Natl Med Assoc. 1993;85:537–545. [PMC free article] [PubMed] [Google Scholar]
- 14.Hawthorne G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:77–90. doi: 10.1016/j.bpobgyn.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Confidential Enquiry into Maternal and Child Health. Diabetes in pregnancy: are we providing the best care? Findings of a national enquiry: England, Wales and Northern Ireland. CEMACH; London: 2007. [Google Scholar]
- 16.Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, Gunderson EP, Herman WH, Hoffman LD, Inturrisi M. et al. Managing preexisting diabetes for pregnancy. Diabetes Care. 2008;31:1060–1079. doi: 10.2337/dc08-9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton P, Crowther CA, Simmonds L, Muller P. Different intensities of glycaemic control (tight versus very tight) for pregnant women with pre-existing diabetes. Cochrane Database Syst Rev. 2010;9:CD008540. doi: 10.1002/14651858.CD008540.pub2. [DOI] [PubMed] [Google Scholar]
- 18.McElvy SS, Miodovnik M, Rosenn B, Khoury JC, Siddiqi T, Dignan PS, Tsang RC. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000;9:14–20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Ali S, Dornhorst A. Diabetes in pregnancy: health risks and management. Postgrad Med J. 2011. 87:417–427. doi: 10.1136/pgmj.2010.109157. [DOI] [PubMed] [Google Scholar]
- 20.Gordin D, Kaaja R, Forsblom C, Hiilesmaa V, Teramo K, Groop PH. Pre-eclampsia and pregnancy-induced hypertension are associated with severe diabetic retinopathy in type 1 diabetes later in life. Acta Diabetol. 2012 doi: 10.1007/s00592-012-0415-0. In press. [DOI] [PubMed] [Google Scholar]
- 21.Mathiesen ER, Ringholm L, Feldt-Rasmussen B, Clausen P, Damm P. Obstetric nephrology: pregnancy in women with diabetic nephropathy - the role of antihypertensive treatment. Clin J Am Soc Nephrol. 2012;7:2081–2088. doi: 10.2215/CJN.00920112. [DOI] [PubMed] [Google Scholar]
- 22.Bramham K, Rajasingham D. Pregnancy in diabetes and kidney disease. J Ren Care. 2012;38:78–89. doi: 10.1111/j.1755-6686.2012.00270.x. [DOI] [PubMed] [Google Scholar]
- 23.Mathiesen ER, Ringholm L, Damm P. Pregnancy management of women with pregestational diabetes. Endocrinol Metab Clin North Am. 2011;40:727–738. doi: 10.1016/j.ecl.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Ringholm L, Mathiesen ER, Kelstrup L, Damm P. Managing type 1 diabetes mellitus in pregnancy - from planning to breastfeeding. Nat Rev Endocrinol. 2012;8:659–667. doi: 10.1038/nrendo.2012.154. [DOI] [PubMed] [Google Scholar]
- 25.Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S Guideline Development Group. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207. doi: 10.1136/bmj.c2207. [DOI] [PubMed] [Google Scholar]
- 26.Piccoli GB, Conijn A, Attini R, Biolcati M, Bossotti C, Consiglio V, Deagostini MC, Todros T. Pregnancy in chronic kidney disease: need for a common language. J Nephrol. 2011;24:282–299. doi: 10.5301/JN.2011.7978. [DOI] [PubMed] [Google Scholar]
- 27.Forbes JM, Cooper ME. Mechanism of diabetic compplications. Physiol Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 28.Melendez-Ramirez LY, Richards RJ, Cefalu WT. Complications of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:625–640. doi: 10.1016/j.ecl.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Dwyer JP, Lewis JB. Nonproteinuric diabetic nephropathy: when diabetics don't read the textbook. Med Clin North Am. 2013;97:53–58. doi: 10.1016/j.mcna.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Steinke JM, Mauer M. Lessons learned from studies of the natural history of diabetic nephropathy in young type 1 diabetic patients. Pediatr Endocrinol Rev. 2008;5:958–963. [PubMed] [Google Scholar]
- 31.Piccoli GB, Attini R, Vasario E, Conijn A, Biolcati M, D'Amico F, Consiglio V, Bontempo S, Todros T. Pregnancy and chronic kidney disease: a challenge in all CKD stages. Clin J Am Soc Nephrol. 2010;5:844–855. doi: 10.2215/CJN.07911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol. 2012;8:265–274. doi: 10.1038/nrneph.2012.38. [DOI] [PubMed] [Google Scholar]
- 33.Janvier A, Lorenz JM, Lantos JD. Antenatal counseling for parents facing an extremely preterm birth: limitations of the medical evidence. Acta Paediatr. 2012;101:800–804. doi: 10.1111/j.1651-2227.2012.02695.x. [DOI] [PubMed] [Google Scholar]
- 34.Stelloh C, Allen KP, Mattson DL, Lerch-Gaggl A, Reddy S, El-Meanawy A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl Res. 2012;159:80–89. doi: 10.1016/j.trsl.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roggero P, Gianni ML, Garbarino F, Mosca F. Consequences of prematurity on adult morbidities. Eur J Intern Med. 2013 doi: 10.1016/j.ejim.2013.01.011. In press. [DOI] [PubMed] [Google Scholar]
- 36.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;97:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 37.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 38.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013;131:E1240–E1263. doi: 10.1542/peds.2012-2177. [DOI] [PubMed] [Google Scholar]
- 39.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review ant meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aujoulat I, d'Hoore W, Deccache A. Patient empowerment in theory and practice: polysemy or cacophony? Patient Educ Couns. 2007;66:13–20. doi: 10.1016/j.pec.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Anderson RM, Funnell MM. Patient empowerment: reflections on the challenge of fostering the adoption of a new paradigm. Patient Educ Couns. 2005;57:153–157. doi: 10.1016/j.pec.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Furukawa T, Tokunaga S, Iwashita K, Sato K, Terashima M, Hora K, Oguchi H, Furuta S, Shigematsu H. Diabetic glomerulosclerosis with nephrotic syndrome in early pregnancy. Nippon Jinzo Gakkai Shi. 1987;29:541–545. [PubMed] [Google Scholar]
- 43.Omori Y. Medical management of pregnant women with diabetes. Nihon Rinsho. 2002;60:786–791. [PubMed] [Google Scholar]
- 44.Omori Y. Diabetes and pregnancy. Nihon Naika Gakkai Zasshi. 1996;85:379–383. [PubMed] [Google Scholar]
- 45.Omori Y. Diabetes and pregnancy. Horumon to Rinsho. 1981;29:789–793. [PubMed] [Google Scholar]
- 46.Kitzmiller JL, Brown ER, Phillippe M, Stark AR, Acker D, Kaldany A, Singh S, Hare JW. Diabetic nephropathy and perinatal outcome. Am J Obstet Gynecol. 1981;141:741–751. doi: 10.1016/0002-9378(81)90698-0. [DOI] [PubMed] [Google Scholar]
- 47.Biesenbach G, Stoger W, Zazgornik J. Changes in proteinuria and renal function in female type 1 diabetics during and after pregnancy dependent on the stage of preexisting diabetic nephropathy. Klin Wochenschr. 1987;65:1048–1053. doi: 10.1007/BF01726324. [DOI] [PubMed] [Google Scholar]
- 48.Biesenbach G, Zazgornik J. Incidence of transient nephrotic syndrome during pregnancy in diabetic women with and without pre-existing microalbuminuria. Br Med J. 1989;299:366–367. doi: 10.1136/bmj.299.6695.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biesenbach G, Stoger H, Zazgornik J. Influence of pregnancy on progression of diabetic nephropathy and subsequent requirement of renal replacement therapy in female type 1 diabetic patients with impaired renal function. Nephrol Dial Transplant. 1992;7:105–109. doi: 10.1093/oxfordjournals.ndt.a092077. [DOI] [PubMed] [Google Scholar]
- 50.Combs CA, Rosenn B, Kitzmiller JL, Khoury JC, Wheeler BC, Miodovnik M. Early-pregnancy proteinuria in diabetes related to preeclampsia. Obstet Gynecol. 1993;82:802–807. [PubMed] [Google Scholar]
- 51.Kimmerle R, Zass RP, Cupisti S, Somville T, Bender R, Pawlowski B, Berger M. Pregnancies in women with diabetic nephropathy: long-term outcome for mother and child. Diabetologia. 1995;38:227–235. [PubMed] [Google Scholar]
- 52.Hod M, van Dijk DJ, Karp M, Weintraub N, Rabinerson D, Bar J, Peled Y, Erman A, Boner G, Ovadia J. Diabetic nephropathy and pregnancy: the effect of ACE inhibitors prior to pregnancy on fetomaternal outcome. Nephrol Dial Transplant. 1995;10:2328–2333. doi: 10.1093/ndt/10.12.2328. [DOI] [PubMed] [Google Scholar]
- 53.Hopp H, Vollert W, Ebert A, Weitzel H, Glöckner E, Jährig D. Diabetic retinopathy and nephropathy - Complications during pregnancy and delivery. Geburtshilfe Frauenheilkd. 1995;55:275–279. doi: 10.1055/s-2007-1023317. [DOI] [PubMed] [Google Scholar]
- 54.Gordon M, Landon MB, Samuels P, Hissrich S, Gabbe SG. Perinatal outcome and long-term follow-up associated with modern management of diabetic nephropathy. Obstet Gynecol. 1996;87:401–409. doi: 10.1016/0029-7844(95)00420-3. [DOI] [PubMed] [Google Scholar]
- 55.Miodovnik M, Rosenn BM, Khoury JC, Grigsby JL, Siddiqi TA. Does pregnancy increase the risk for development and progression of diabetic nephropathy? Am J Obstet Gynecol. 1996;174:1180–1191. doi: 10.1016/s0002-9378(96)70660-9. [DOI] [PubMed] [Google Scholar]
- 56.Mackie AD, Doddridge MC, Gamsu HR, Brudenell JM, Nicolaides KH, Drury PL. Outcome of pregnancy in patients with insulin-dependent diabetes mellitus and nephropathy with moderate renal impairment. Diabet Med. 1996;13:90–96. doi: 10.1002/(SICI)1096-9136(199601)13:1<90::AID-DIA992>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 57.Purdy LP, Hantsch CE, Molitch ME, Metzger BE, Phelps RL, Dooley SL, Hou SH. Effect of pregnancy on renal function in patients with moderate-to-severe diabetic renal insufficiency. Diabetes Care. 1996;19:1067–1074. doi: 10.2337/diacare.19.10.1067. [DOI] [PubMed] [Google Scholar]
- 58.Reece EA, Leguizamon G, Homko C. Stringent controls in diabetic nephropathy associated with optimization of pregnancy outcomes. J Matern Fetal Med. 1998;7:213–216. doi: 10.1002/(SICI)1520-6661(199807/08)7:4<213::AID-MFM11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 59.Czajkowski K, Janczewska E, Jozwicka E, Demkow K, Bomba D. Pregnancy in women with diabetes complicated by nephropathy and proliferative retinopathy. Ginekol Pol. 1999;70:753–758. [PubMed] [Google Scholar]
- 60.Bar J, Ben-Rafael Z, Padoa A, Orvieto R, Boner G, Hod M. Prediction of pregnancy outcome in subgroups of women of renal disease. Clin Nephrol. 2000;53:437–444. [PubMed] [Google Scholar]
- 61.Bar J, Chen R, Schoenfeld A, Orvieto R, Yahav J, Ben-Rafael Z, Hod M. Pregnancy outcome in patients with insulin dependent diabetes mellitus and diabetic nephropathy treated with ACE inhibitors before pregnancy. J Pediatr Endocrinol Metab. 1999;12:659–665. doi: 10.1515/jpem.1999.12.5.659. [DOI] [PubMed] [Google Scholar]
- 62.Dunne FP, Chowdhury TA, Hartland A, Smith T, Brydon PA, McConkey C, Nicholson HO. Pregnancy outcome in women with insulin-dependent diabetes mellitus complicated by nephropathy. Q J Med. 1999;92:451–454. doi: 10.1093/qjmed/92.8.451. [DOI] [PubMed] [Google Scholar]
- 63.Biesenbach G, Grafinger P, Stöger H, Zazgornik J. How pregnancy influences renal function in nephropathic type 1 diabetic women depends on their pre-conceptional creatinine clearance. J Nephrol. 1999;12:41–46. [PubMed] [Google Scholar]
- 64.Schroder W, Heyl W, Hill-Grasshoff B, Rath W. Clinical value of detecting microalbuminuria as a risk factor for pregnancy-induced hypertension in insulin-treated diabetic pregnancies. Eur J Obstet Gynecol Reprod Biol. 2000;91:155–158. doi: 10.1016/s0301-2115(99)00266-3. [DOI] [PubMed] [Google Scholar]
- 65.Biesenbach G, Grafinger P, Zazgornik J, Stöger H. Perinatal complications and three-year follow up of infants of diabetic mothers with diabetic nephropathy stage IV. Ren Fail. 2000;22:573–580. doi: 10.1081/jdi-100100898. [DOI] [PubMed] [Google Scholar]
- 66.Sobczak M, Wilczyński J, Cypryk K. Pregnancy in women with diabetic nephropathy. Ginekol Pol. 2000;71:893–899. [PubMed] [Google Scholar]
- 67.Ekbom P, Damm P, Feldt-Rasmussen B, Feldt-Rasmussen U, Molvig J, Mathiesen ER. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care. 2001;24:1739–1744. doi: 10.2337/diacare.24.10.1739. [DOI] [PubMed] [Google Scholar]
- 68.Khoury JC, Miodovnik M, LeMasters G, Sibai B. Pregnancy outcome and progression of diabetic nephropathy. What's next? J Matern Fetal Neonatal Med. 2002;11:238–244. doi: 10.1080/jmf.11.4.238.244. [DOI] [PubMed] [Google Scholar]
- 69.Rossing K, Jacobsen P, Hommel E, Mathiesen E, Svenningsen A, Rossing P, Parving HH. Pregnancy and progression of diabetic nephropathy. Diabetologia. 2002;45:36–41. doi: 10.1007/s125-002-8242-4. [DOI] [PubMed] [Google Scholar]
- 70.Bagg W, Neale L, Henley P, MacPherson P, Cundy T. Long-term maternal outcome after pregnancy in women with diabetic nephropathy. N Z Med J. 2003;116:U566. [PubMed] [Google Scholar]
- 71.Irfan S, Arain TM, Shaukat A, Shahid A. Effect of pregnancy on diabetic nephropathy and retinopathy. JCPSP. 2004;14:75–78. [PubMed] [Google Scholar]
- 72.How HY, Sibai B, Lindheimer M, Caritis S, Hauth J, Klebanoff M, Macpherson C, Van Dorsten P, Miodovnik M, Landon M, Paul R, Meis P, Thurnau G, Dombrowski M, Roberts J. Is early pregnancy proteinuria associated with an increased rate of preeclampsia in women with pregestational diabetes mellitus? Am J Obstet Gynecol. 2004;190:775–778. doi: 10.1016/j.ajog.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 73.Carr DB, Koontz GL, Gardella C, Holing EV, Brateng DA, Brown ZA, Easterling TR. Diabetic nephropathy in pregnancy: suboptimal hypertensive control associated with preterm delivery. Am J Hypertens. 2006;19:513–519. doi: 10.1016/j.amjhyper.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Nielsen LR, Damm P, Mathiesen ER. Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy. Diabetes Care. 2009;32(1):38–44. doi: 10.2337/dc08-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen DM, Damm P, Ovesen P, Mølsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Moeller M, Mathiesen ER. Microalbuminuria, preeclampsia, and preterm delivery in pregnant women with type 1 diabetes. Diabetes Care. 2010;33:90–94. doi: 10.2337/dc09-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yogev Y, Chen R, Ben-Haroush A, Hod M, Bar J. Maternal overweight and pregnancy outcome in women with type-1 diabetes mellitus and different degrees of nephropathy. J Matern Fetal Neonatal Med. 2010;23:999–1003. doi: 10.3109/14767050903544744. [DOI] [PubMed] [Google Scholar]
- 77.Young EC, Pires ML, Marques LP, de Oliveira JE, Zajdenverg L. Effects of pregnancy on the onset and progression of diabetic nephropathy and of diabetic nephropathy on pregnancy outcome. Diabetes Metab Syndr. 2011;5:137–142. doi: 10.1016/j.dsx.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 78.Themeli Y, Bajrami V, Zaimi K, Mustafaraj K, Lulo J, Peci E, Gjoshe J, Shtylla A, Barbullushui M, Idrizi A, Ktona E, Capo J. Diabetic nephropathy in pregnant women with type 1 diabetes mellitus. G Ital Ostet Ginecol. 2012;34:323–327. [Google Scholar]
- 79.Bell R, Glinianaia SV, Tennant PW, Bilous RW, Rankin J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia. 2012;55:936–947. doi: 10.1007/s00125-012-2455-y. [DOI] [PubMed] [Google Scholar]
- 80.Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 81.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 82.Nevis IF, Reitsma A, Dominic A, McDonald S, Thabane L, Akl EA, Hladunewich M, Akbari A, Joseph G, Sia W, Iansavichus AV, Garg AX. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598. doi: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piccoli GB, Fassio F, Attini R, Parisi S, Biolcati M, Ferraresi M, Pagano A, Daidola G, Deagostini MC, Gaglioti P, Todros T. Pregnancy in CKD: whom should we follow and why? Nephrol Dial Transplant. 2012;27:iii111–iii118. doi: 10.1093/ndt/gfs302. [DOI] [PubMed] [Google Scholar]
- 84.Shahir AK, Briggs N, Katsoulis J, Levidiotis V. An observational outcomes study from 1966-2008, examining pregnancy and neonatal outcomes from dialysed women using data from the ANZDATA Registry. Nephrology (Carlton) 2013;18:276–284. doi: 10.1111/nep.12044. [DOI] [PubMed] [Google Scholar]
- 85.Abou-Jaoude P, Dubourg L, Bessenay L, Pincon A, Jolivot A, Guebre-Egziabher F, Cochat P, Bacchetta J. What about the renal function during childhood of children born from dialysed mothers? Nephrol Dial Transplant. 2012;27:2365–2369. doi: 10.1093/ndt/gfr617. [DOI] [PubMed] [Google Scholar]
- 86.Piccoli GB, Conijn A, Consiglio V, Vasario E, Attini R, Deagostini MC, Bontempo S, Todros T. Pregnancy in dialysis patients: is the evidence strong enough to lead us to change our counseling policy? Clin J Am Soc Nephrol. 2010;5:62–71. doi: 10.2215/CJN.05660809. [DOI] [PMC free article] [PubMed] [Google Scholar]


