Abstract
Neuronal dysfunctions in the cortical GABAergic system have been widely documented in neuropsychiatric disorders with prenatal infectious etiologies, including schizophrenia. At least some of these abnormalities may stem from transcriptional impairments in the GABAergic transcriptome. However, the extent to which prenatal exposure to immune challenge can induce long-term alterations in GABAergic gene transcription remains largely elusive. Here, we use an established mouse model of prenatal immune activation induced by maternal gestational administration of the viral mimetic poly(I:C) (= polyriboinosinic-polyribocytidilic acid) to demonstrate that prenatal immune activation causes maturation-dependent alterations in prefrontal GABAergic gene expression. The spectrum of abnormalities included altered mRNA expression levels of enzymes regulating γ-aminobutyric acid (GABA) biosynthesis (glutamic acid decarboxylase 65-kDa [GAD65] and GAD67), vesicular GABA transporter (VGAT), alpha-subunits of the GABA(A) receptor (α2, α3, α4, and α5), and the chloride transporters sodium-potassium-chloride cotransporter 1 and potassium-chloride cotransporter 2. Additional western blot analyses confirmed the deficits in prefrontal GAD65/GAD67 and VGAT expression at the protein level. Intriguingly, the prefrontal GABAergic transcriptome was found to be more strongly affected in adult compared with peripubertal offspring born to immune-challenged mothers, and these age-dependent changes in GABAergic gene expression were paralleled by an adult onset of working memory deficiency. Collectively, our data emphasize a critical impact of prenatal immune-related insults on long-term GABAergic changes relevant to neuropsychiatric disorders with prenatal infectious etiologies, especially for those with delayed onset in early adulthood.
Key words: GABA, immune activation, infection, prefrontal cortex, schizophrenia
Introduction
Prenatal exposure to maternal infection has been associated with increased risk of developmental neuropsychiatric disease, most notably schizophrenia.1,2 This epidemiological link has also been supported by a plethora of experimental work in animals demonstrating schizophrenia-relevant behavioral, cognitive, and neuroanatomical alterations following prenatal exposure to infectious pathogens or immune-activating agents.3–5 Accumulating evidence suggests that cytokine-associated inflammatory events,6,7 together with downstream pathophysiological processes such as oxidative stress,8 hypoferremia,9 and zinc deficiency,10 are critical in mediating the negative influences of prenatal infection on brain and behavioral development.
The central γ-aminobutyric acid (GABA) system is one of the neuronal networks that is highly sensitive to the disrupting effects of prenatal immune challenge. Indeed, prenatal exposure to viral infection or maternal immune activation has been repeatedly shown to cause GABAergic abnormalities at the cellular and neurochemical levels,11–16 some of which are directly implicated in the pathophysiology of schizophrenia.17 It is important to note that prenatal immune activation is capable of changing cellular GABAergic markers in the offspring’s brain without concomitantly inducing marked neurodegeneration and reactive gliosis.14 One implication is that such GABAergic abnormalities are unlikely to be the result of persistent necrotic and/or apoptotic processes, but they may rather be caused by changes in the expression of the corresponding genes. However, the extent to which prenatal immune activation can cause long-lasting changes in GABAergic gene expression remains essentially unexplored.
This study sought evidence for this hypothesis by measuring the transcription levels of various pre- and postsynaptic GABAergic genes in offspring subjected to prenatal immune activation relative to controls. We used a well-characterized mouse model of prenatal immune challenge induced by maternal gestational treatment with the viral mimetic poly(I:C) (= polyriboinosinic-poly ribocytidilic acid), which is known to capture a variety of cellular GABAergic abnormalities and parallel behavioral dysfunctions relevant to schizophrenia and autism.3,4 Poly(I:C) is a synthetic analog of double-stranded RNA that induces a cytokine-associated viral-like acute phase response in maternal and fetal compartments, including the fetal brain.3,4
Various GABAergic markers were assessed in the medial prefrontal cortex (mPFC), a brain region known to be sensitive to the disrupting effects of prenatal immune challenge.3,4,11–13 The examination of presynaptic GABAergic markers included the 2 isoforms of the rate-limiting enzyme for GABA biosynthesis, glutamic acid decarboxylase 65-kDa (GAD65) and GAD67, as well as the vesicular GABA transporter (VGAT) that is responsible for uptake and storage of GABA by synaptic vesicles.18 In addition to these presynaptic markers, we ascertained the expression of the 5 distinct alpha-subunits (α1–α5) of the GABA(A) receptor because of their suggested pathophysiological relevance to schizophrenia.19 We further measured the expression levels of the sodium-potassium-chloride cotransporter 1 (NKCC1) and the potassium-chloride cotransporter 2 (KCC2), which mediate chloride uptake and extrusion, respectively, thereby regulating the extent to which GABA signaling is predominately excitatory or inhibitory.18 All gene expression analyses were conducted in peripuberty and adulthood so as to assess possible maturation- dependent effects of prenatal immune activation.
Materials and Methods
Animals
C57BL/6 mice were used throughout the study. Female and male breeders were obtained from our in-house specific pathogen-free colony at the age of 10–14 weeks. Breeding began after 2 weeks of acclimatization to the new animal-holding room, which was a temperature and humidity controlled (21±11C, 55±5%) holding facility under a reversed light-dark cycle (lights off: 08:00–20:00h). All animals had ad libitum access to food (Kliba 3430, Klibamühlen, Kaiseraugst, Switzerland) and water unless specified otherwise. All procedures described in this study had been previously approved by the Cantonal Veterinarian’s Office of Zurich and are in agreement with the principles of laboratory animal care in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 86-23, revised 1985).
Maternal Immune Activation During Pregnancy
For the purpose of the maternal immunological manipulation during pregnancy, female mice were subjected to a timed mating procedure as described previously.12–14 Pregnant dams on gestation day 17 (GD17) received either a single injection of poly(I:C) (potassium salt; Sigma-Aldrich) at a dose of 5mg/kg or vehicle (sterile pyrogen-free 0.9% NaCl) according to protocols established before.12–14 The late gestational stage (ie, GD17) was selected because of our previous findings showing that GD17 poly(I:C) treatment is capable of inducing cellular abnormalities in the adult central GABAergic system and of inducing multiple cognitive deficits in adulthood.12,14,16 Poly(I:C) was dissolved in sterile pyrogen-free 0.9% NaCl (= vehicle) solution to yield a final concentration of 1.0mg/ml and was administered via the intravenous (iv) route at the tail vein under mild physical constraint. All solutions were freshly prepared at the day of administration and injected with a volume of 5ml/kg.
Allocation and Testing of Offspring
All offspring were weaned and sexed at postnatal day (PND) 21. Littermates of the same sex were caged separately and maintained in groups of 2–4 animals/cage as described above. Only male subjects were included in all molecular and cognitive tests to circumvent bias arising from sexual dimorphism. They stemmed from multiple independent litters (N = 14 for poly(I:C) and N = 15 for vehicle control litters) to avoid possible confounds arising from litter effects.3 Gene and protein expression analyses (see below) were conducted when the offspring reached either the peripubertal (PND 35) or the adult (PND 100) stage of development. Cognitive testing (see below) was similarly performed both in peripuberty (PND 28–40) and adulthood (PND 95–106). Peripubertal and adult stages were defined based on the gradual attainment of sexual maturity and age-specific behavioral discontinuities from younger to older animals.20 These 2 developmental stages roughly correspond to a period between 11–14 years and 20 years onward, respectively, in humans. Separate cohorts of behaviorally naïve male animals were used for all gene and protein expression analyses and cognitive testing in order to avoid possible confounds and carry-over effects arising from prior testing.
Collection of Brain Samples
Following decapitation of the animals, the brains were immediately extracted from the skull and placed dorsal side up on an ice-chilled plate. This was directly followed by preparing 1-mm coronal brain sections using razorblade cuts and subsequent microdissection of the mPFC region as established before and fully described elsewhere.16 All gene and protein expression analyses were performed on mPFC tissue (Bregma: +2.3 to +1.3) that included anterior cingulate, prelimbic, and infralimbic subregions.16 Both left and right brain hemispheres were collected and were used for either gene or protein expression analyses (see below), with right and left hemispheres being counterbalanced across the different molecular analyses (gene vs protein expression analyses) and prenatal treatment groups (control vs poly(I:C) offspring). Brain specimen were collected in 96-well microtiter plates kept on dry ice and allowed to freeze before storage at −80°C until further use.
RNA Preparation and Gene Expression Analysis by Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated by a single step of guanidinium isothiocyanate/phenol extraction, using PureZol RNA isolation reagent (Bio-Rad Laboratories s.r.l. Italia) according to the manufacturer’s instructions, and quantified by spectrophotometric analysis. Following total RNA extraction, the samples were processed for real-time polymerase chain reaction (RT-PCR) to assess various pre- and postsynaptic GABAergic markers using protocols established before.21 An aliquot of each sample was treated with DNase to avoid DNA contamination. RNA was then analyzed by TaqMan qRT-PCR instrument (CFX384 real-time system, Bio-Rad Laboratories) using the iScript one-step RT-PCR kit for probes (Bio-Rad Laboratories). The samples were run in 384-well formats in triplicates as multiplexed reactions with a normalizing internal control (36B4), and some of the results were additionally validated using 2 other internal standards, namely β-actin and 18S rRNA. Probe and primer sequences were purchased from Eurofins MWG-Operon. Thermal cycling was initiated with incubation at 50°C for 10min (RNA retrotranscription) and then at 95°C for 5min (TaqMan polymerase activation). After this initial step, 39 cycles of PCR were performed. Each PCR cycle consisted of heating the samples at 95°C for 10s to enable the melting process and then for 30s at 60°C for the annealing and extension reactions. The primer sequences of interest demonstrated high amplification efficiency across a wide range of serial dilutions of our samples (see online supplementary table 1) and are summarized in table 1. Relative target gene expression was calculated according to the 2(-Delta Delta C(T)) method.22 In addition, we divided the normalized NKCC1 mRNA levels (normalized to percent expression in saline control animals) by the normalized KCC2 levels with the aim to analyze the NKCC1:KCC2 ratio.
Table 1.
Sequences of Forward and Reverse Primers Used in Real-Time Polymerase Chain Reaction Analysis
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| gad65 | 5’-ACCGAGAAGGCTATGAAATGG-3’ | 5’-TCTGGCTTTAATCACTGGCG-3’ | 5’-AGGCGGCTCATTCTCTCTTCATTGTC-3’ |
| gad67 | 5’-GCAGATCCTGGTTGACTGTAG-3’ | 5’-GAACATATTGGTATTGGCAGTCG-3’ | 5’-TGGTTTAGCTGGTGAATGGCTGACA-3’ |
| vgat | 5’-ACGACAAACCCAAGATCACG-3’ | 5’-AAGATGATGAGGAACAACCCC-3’ | 5’-CAGCACGAACATGCCCTGAATGG-3’ |
| alpha1 | 5’-TGAGAGCTGAATGCCCAATG-3’ | 5’-TCTGCTACAACCACTGAACG-3’ | 5’-CCTGCCCACTAAAATTCGGAAGCTATGC-3’ |
| alpha 2 | 5’-CCATGAGGCTTACAGTCCAAG-3’ | 5’-ACGGAGTCAGAAGCATTGTAAG-3’ | 5’-CGTAGCTTCCAAATTTCAGTGGGCA-3’ |
| alpha 3 | 5’-ACAATATGACCACACCCAACA-3’ | 5’-AGCTTCCAAACTTCAGTGGG-3’ | 5’-CAATACACGCTGAATGCCCCATGC-3’ |
| alpha 4 | 5’-GCCTGCCCTTTGAAATTTGG-3’ | 5’-GATACAGTCTGCCCAATGAGG-3’ | 5’-ATCTACACCTGGACCAAAGGCCC-3’ |
| alpha 5 | 5’-GGGAATGGACAATGGAATGC-3’ | 5’-TGTCATTGGTCTCGTCTTGTAC-3’ | 5’-CATTTGCGAAAAGCCAAAGTGACTGGA-3’ |
| nkcc1 | 5’-GCATTCAATCCGTCTTTCTGG-3’ | 5’-GGCCACAGATCATTAAACCAAC-3’ | 5’-AGTAAAGCAGGCCGTGAGTTTGGA-3’ |
| kcc2 | 5’-TCAAACAGATGCACCTCACC-3’ | 5’-CCTCTGGCTTCTCCTCATTG-3’ | 5’-CTCCCGTTCCCGCTCGTTCTT-3’ |
| 36B4 | 5’-AGATGCAGCAGATCCGCAT-3’ | 5’-GTTCTTGCCCATCAGCACC-3’ | 5’-CGCTCCGAGGGAAGGCCG-3’ |
| β-actin | 5’-ACCTTCTACAATGAGCTGCG-3’ | 5’-CTGGATGGCTACGTACATGG-3’ | 5’-TCTGGGTCATCTTTTCACGGTTGGC-3’ |
| 18S rRNA | 5’-GTAACCCGTTGAACCCCATT-3’ | 5’-CCATCCAATCGGTAGTAGCG-3’ | 5’-TGCAATTATTCCCCATGAACGAGG-3’ |
Protein Extraction and Western Blot Analysis
Western blot analysis was used to investigate GAD65/67 and VGAT protein levels in the total homogenate of mPFC tissue. Brain samples were manually homogenized using a glass-glass potter in a pH 7.4 cold buffer (containing 0.32M sucrose, 0.1mM EGTA, 1mM HEPES solution in presence of a complete set of protease [Roche], and phosphatase [Sigma-Aldrich] inhibitors) and then sonicated for 10 s at a maximum power of 10%–15% (Bandelin Sonoplus). Total protein content was measured according to the Bradford Protein Assay procedure (Bio-Rad Laboratories), using bovine serum albumin as calibration standard.
Equal amounts of protein were run under reducing conditions on Any Kd Criterion TGX precast gels (Bio-rad Laboratories) and then electrophoretically transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The blots were blocked with 10% nonfat dry milk and then incubated with the primary antibodies. Because of the limited availability of mPFC tissue in small animals such as mice, we were constrained to focus our western blot analyses on 2 analyses only. Therefore, we used an anti-GAD65/67 polyclonal antibody (1:1000, 4°C overnight; Millipore) that is able to simultaneously recognize both GAD isoforms (65 and 67kDa) and a primary anti-VGAT polyclonal antibody (1:1000, 4°C overnight, Millipore). Membranes were then incubated for 1h at room temperature with a peroxidase-conjugated antirabbit immunoglobin G (IgG; 1:1000 for GAD65/67, 1:2000 for VGAT) and immunocomplexes were visualized by chemiluminescence using the Chemidoc MP imaging system (Bio-Rad Laboratories). Results were standardized using β-actin as the control protein, which was detected by evaluating the band density at 43kDa after probing the membranes with a polyclonal antibody (1:10 000, Sigma-Aldrich) followed by a 1:10 000 dilution of peroxidase-conjugated antimouse IgG (Sigma-Aldrich). Protein levels of GAD65/67 (measured together without discriminating between bands) and VGAT were calculated using an up-to-date Image Lab software (Bio-Rad Laboratories).
Working Memory in a Matching-to-Position Dry Maze Paradigm
Working memory was assessed using a matching-to-position paradigm in the dry maze using an apparatus and established procedures fully described elsewhere.23,24 Before testing, all animals were progressively food deprived during the initial habituation phase (days 1–5) until a minimal 90% free-feeding weight was reached. Following habituation, the animals were then pretrained in the dry maze using the visual cue for 2 consecutive trials on 2 consecutive days (days 6 and 7).23,24 Working-memory testing was then started, and it lasted for 5 testing days (days 8–12).
The working-memory task was based on the matching-to-position paradigm, in which the animals were required to learn the novel position of a rewarded hole (75 μl freshly prepared condensed milk; Alicommerce SAS, Liebefeld-Bern, Switzerland) revealed to them on trial 1 of each day in order to navigate effectively to the same location (ie, matching) on the subsequent trial on the same day. Each test day included 2 trials. The reward remained in the same position across trials on a given test day but took a new position on each test day.23,24 The allocation of the rewarded holes to a specific spatial location was counterbalanced across the 2 prenatal treatment conditions. Working memory was indexed by the reduction in distance moved and time needed to find the location of the reward in trial 2 relative to trial 1.
Statistical Analyses
All gene expression (RT-PCR) and protein expression (Western blot) data were analyzed using independent Student’s t tests (two-tailed). In the dry maze matching-to-position paradigm of working memory, the distance moved and latency to locate the reward served as the critical test read-out and was analyzed using a 2×2 × 5 (prenatal treatment × trial × day) repeated-measures analysis of variance (ANOVA), followed by Fisher’s least significant difference post hoc comparisons or restricted ANOVAs whenever appropriate. In addition, a 2×5 (prenatal treatment × day) repeated-measures ANOVA of the improvement scores (ie, [time or distance in trial 1] – [time or distance in trial 2]) was conducted in the dry maze matching-to-position paradigm of working memory. All data were separately analyzed for peripubertal and adult subjects. Statistical significance was set at P < .05. All statistical analyses were performed using the statistical software StatView (version 5.0) implemented on a personal computer running the Windows XP operating system.
Results
Prenatal Immune Activation Induces Age-Dependent Changes in Prefrontal Expression of Presynaptic GABAergic Genes
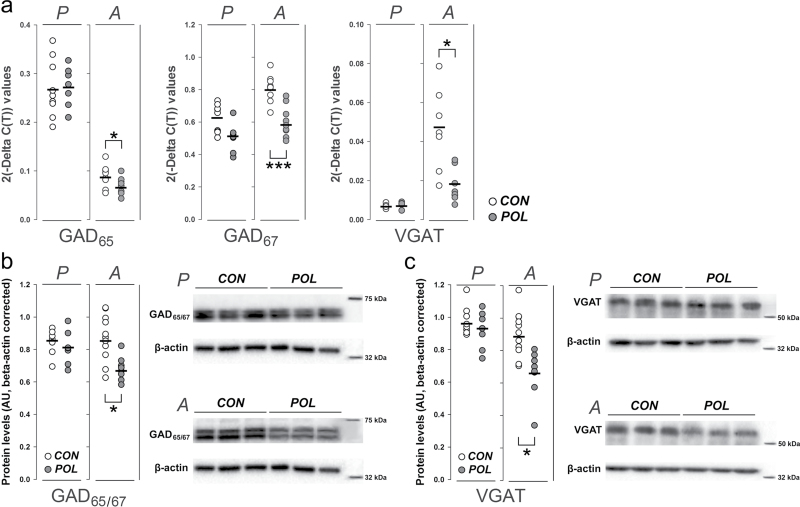
First, we studied the effects of prenatal immune challenge on the transcription levels of presynaptic GABAergic genes in the mPFC of peripubertal and adult offspring. Using 36B4 as internal normalizing gene, we found that adult offspring born to immune-challenged mothers displayed a marked deficit in mRNA expression levels of the enzymes regulating GABA biosynthesis (GAD65 and GAD67) and vesicular GABA storage (VGAT; figure 1a). Notably, these effects were not manifest when the offspring reached the peripubertal stage of development (figure 1a), suggesting that prenatal immune activation induces age-dependent changes in prefrontal expression of these presynaptic GABAergic genes. Importantly, the direction and magnitude of the observed changes between poly(I:C)-exposed and control animals remained stable when we analyzed mRNA expression of GAD65, GAD67, and VGAT using 2 other internal house-keeping genes, namely β-actin and 18S rRNA (see online supplementary figures 1 and 2). These additional investigations highlight that the effects of prenatal immune activation on GABAergic gene expression are readily attributable to significant alterations in the expression of the target genes and are not biased by possible effects on internal standard genes. Furthermore, we also confirmed the age-dependent effects of prenatal immune activation on presynaptic GABAergic markers at the protein level using western blot analyses; adult but not peripubertal offspring born to immune-challenged mothers displayed a significant reduction in GAD65/67 and VGAT protein in the mPFC (figure 1b,c).
Fig. 1.
Gene and protein expression profiling of presynaptic GABAergic markers in prefrontal cortex following prenatal immune activation. (a) The graph depicts the 2(-Delta C(T)) values for each individual control (CON; white symbols) or poly(I:C)-exposed (POL, gray symbols) offspring at peripubertal (P) or adult (A) age. Individual mRNA levels are given for the 2 isoforms of the rate limiting enzyme for GABA biosynthesis (GAD65 and GAD67) and the vesicular GABA transporter (VGAT). The bars represent the mean of individual 2(-Delta C(T)) values for each group/age. *P < .05, **P < .01, and ***P < .001, based on independent Student’s t tests (two-tailed). (b) GAD65/67 protein levels for individual POL and CON offspring at peripubertal (P) or adult (A) age assessed using Western blot analysis. The photomicrographs show Western blot samples for GAD65/67 protein in the prefrontal cortex of 3 representative CON and POL offspring. β-actin is shown as control for comparison. *P < .05, based on independent Student’s t tests (two-tailed). (c) VGAT protein levels for individual POL and CON offspring at peripubertal (P) or adult (A) age assessed using Western blot analysis. The photomicrographs depict Western blot samples for VGAT protein in the prefrontal cortex of 3 representative CON and POL offspring. β-actin is shown as control for comparison. *P < .05, based on independent Student’s t tests (two-tailed). N(CON/peripuberty) = 9, N(POL/ peripuberty) = 7, N(CON/adulthood) = 11, N(POL/ adulthood) = 9.
Prenatal Immune Activation Induces Age-Dependent Changes in Prefrontal Transcription of GABA(A) Alpha-Receptor Subunits
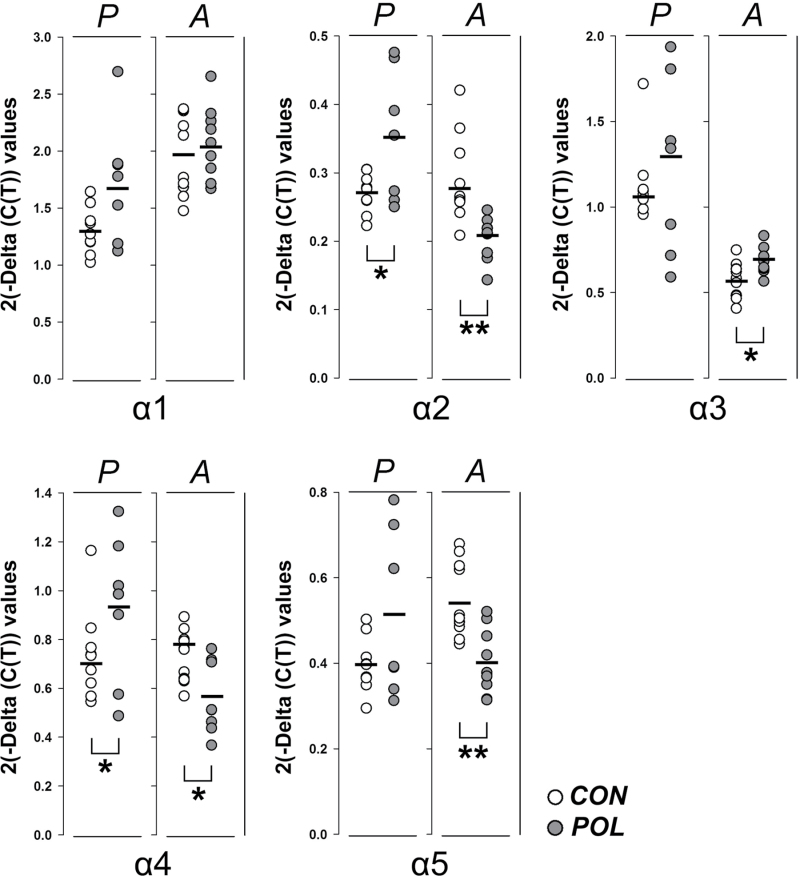
We also sought evidence for the possibility that prenatal immune activation may affect the transcription of GABA(A) receptor genes. To this end, we focused on the expression of the 5 alpha subunits (α1–α5) of the GABA(A) receptor because previous investigations have detected cellular changes in the expression of these subunits following prenatal immune activation in mice.13,14,25 Here, we show that prenatal immune activation leads to age-dependent alterations in the expression of the alpha-subunits of GABA(A) receptor genes (figure 2): whereas prefrontal mRNA levels of the α2 and α4 subunits were significantly increased in immune-challenged offspring at peripubertal age, expression of the same genes was down-regulated in immune-exposed offspring when they reached adulthood. Furthermore, adult but not peripubertal offspring showed a significant increase and decrease in the expression of α3 and α5 mRNA levels, respectively (figure 2).
Fig. 2.
Analysis of GABA(A) receptor alpha-subunits mRNA levels in prefrontal cortex following prenatal immune activation. The graph depicts the 2(-Delta C(T)) values of the α1–5 subunits measured in each individual control (CON, white symbols) or poly(I:C)-exposed (POL, gray symbols) offspring at peripubertal (P) or adult (A) age. The bars represent the mean of individual 2(-Delta C(T)) values for each group/age. *P < .05 and **P < .01, based on independent Student’s t tests (two-tailed). N(CON/peripuberty) = 9, N(POL/ peripuberty) = 7, N(CON/adulthood) = 11, N(POL/ adulthood) = 9.
Prenatal Immune Activation Induces Age-Dependent Changes in Genes Regulating the GABA Excitatory/Inhibitory Shift
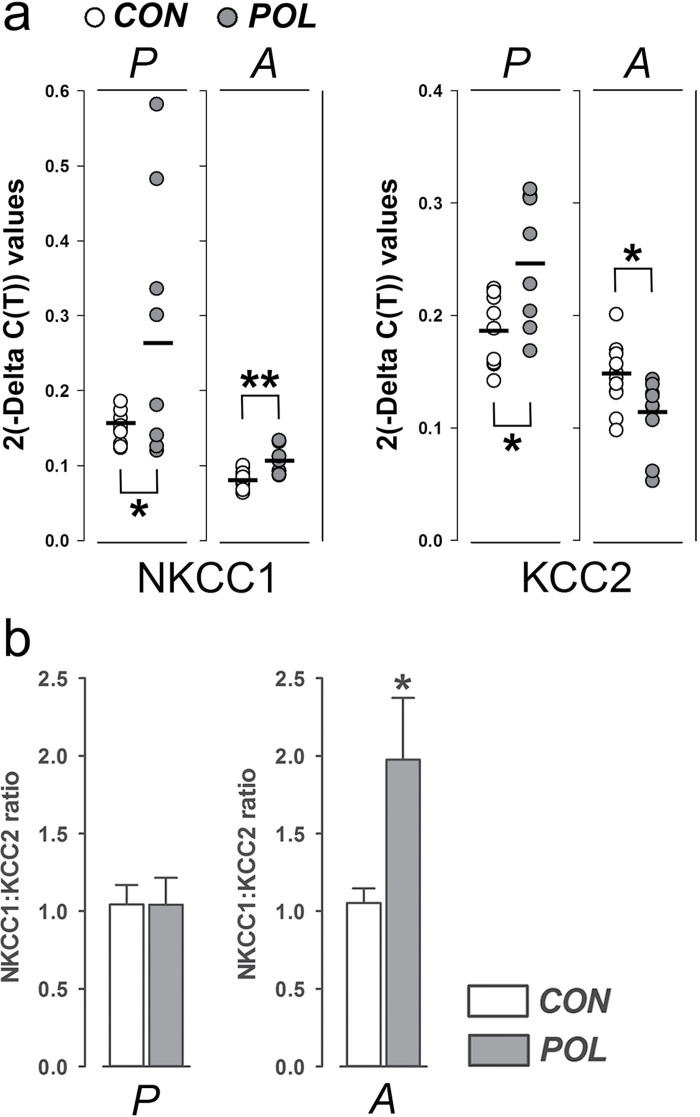
The age-dependent alterations of presynaptic and receptors-associated GABAergic gene expression manifest in immune-challenged offspring may be indicative of altered maturation of the prefrontal GABA system. To further seek evidence for this possibility, we measured the gene transcription levels of NKCC1 and KCC2, which regulate the extent to which GABA signaling is either excitatory or inhibitory.18 KCC2 is a potassium chloride cotransporter that mediates chloride extrusion, thereby facilitating the inhibitory actions of GABA.18 It is expressed primarily in mature neurons; therefore, its expression peaks only after full brain maturation in adulthood.27 In contrast, NKCC1 shows peak levels during early postnatal brain development, a time when GABA signaling is primarily excitatory.26 Therefore, the relative expression of NKCC1 vs KCC2 is typically taken as a molecular index of the functional maturation of the central GABA system.
We found that prenatal immune activation increased the transcription levels of both NKCC1 and KCC2 in the mPFC of peripubertal offspring (figure 3a). Because of this concomitant enhancement, the NKCC1:KCC2 ratio was highly similar between immune-challenged offspring and controls at peripubertal age (figure 3b). In marked contrast, adult offspring born to immune-challenged mothers displayed a significant increase and decrease in the transcription of NKCC1 and KCC2, respectively (figure 3a). This imbalanced expression was further evident in the NKCC1:KCC2 ratio, which was significantly increased in immune-challenged offspring relative to controls at adult age (figure 3b). Taken together, these findings support the hypothesis that prenatal immune activation disrupts the normal maturation of the prefrontal GABA system, leading to molecular signs of an immature GABAergic system in adulthood.
Fig. 3.
Analysis of NKCC1 and KCC2 mRNA levels in prefrontal cortex following prenatal immune activation. (a) The graph depicts the 2(-Delta C(T)) values of the sodium-potassium-chloride cotransporter 1 (NKCC1) and the potassium-chloride cotransporter 2 (KCC2) measured in each individual control (CON, white symbols) or poly(I:C)-exposed (POL, gray symbols) offspring at peripubertal (P) or adult (A) age. The bars represent the mean of individual 2(-Delta C(T)) values for each group/age. *P < .05 and **P < .01, based on independent Student’s t tests (two-tailed). (b) The bar plots show the means ± standard error mean (SEM) of normalized NKCC1:KCC2 ratios for peripubertal (P) or adult (A) offspring. N(CON/peripuberty) = 9, N(POL/ peripuberty) = 7, N(CON/adulthood) = 11, N(POL/ adulthood) = 9.
Prenatal Immune Activation Induces an Adult Onset of Working Memory Impairments
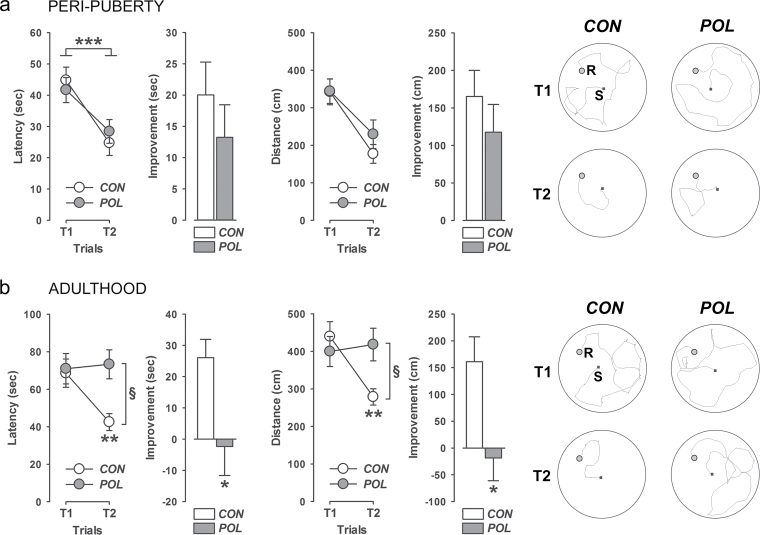
Because of its critical role in regulating neuronal synchronization, the prefrontal GABAergic system critically contributes to a variety of cognitive functions, including working memory.17 Impairments in prefrontal GABAergic signaling are thus believed to significantly contribute to working memory deficits in psychiatric disorders, especially in schizophrenia.17 In view of our findings demonstrating maturation-dependent alterations in prefrontal GABAergic gene transcription following prenatal immune activation (figure 1–3), we further ascertained whether the presence of these molecular changes might be accompanied by cognitive dysfunctions in the form of working-memory impairments. Because the prefrontal GABAergic transcriptome is arguably more affected in adult compared with peripubertal offspring born to immune-challenged mothers (figure 1–3), one clear expectation would be that the negative influences of prenatal immune challenge on cognitive functions may be readily noticeable in adulthood compared with earlier maturational stages.
The assessment of working-memory performance in immune-challenged and control offspring confirmed this expectation. Using a matching-to-position paradigm, we found that adult but not peripubertal offspring born to immune-challenged mothers displayed a severe working-memory deficit (figure 4). Indeed, at the peripubertal stage, both immune-challenged and control subjects showed a clear reduction in the latency and distance moved to find the rewarded hole in trial 2 relative to trial 1 (when the position of the rewarded hole was unknown to the animals; figure 4a). In marked contrast, such improvement in performance from trial 1 to trial 2 was no longer evident in adult offspring subjected to prenatal immune activation, while at the same time, adult control offspring still displayed highly significant reduction in the latency and distance moved to find the rewarded hole in trial 2 relative to trial 1 (figure 4b).
Fig. 4.
Working-memory performance following prenatal immune activation. The line plots show the latency (sec) and distance moved (cm) to find the rewarded hole in trial 1 (T1) and T2, and the bar plots depict the improvement in these measures from T1 to T2. The drawings illustrate computer-generated search path of representative control (CON) and poly(I:C)-exposed (POL) offspring in T1 and T2 of the working memory test. S, Starting position; R, location of the rewarded hole. (a) Working-memory performance in POL and CON offspring at peripubertal age. ***P < .001, reflecting the significant main effect of trials [latency: F (1,25) = 14.63, P < 0.001; distance: F (1,25) = 11.75, P < 0.001] obtained by a 2×2 × 5 (prenatal treatment × trial × day) repeated-measures ANOVA. N(CON) = 13, N(POL) = 14. All values are means ± SEM. (b) Working-memory performance in POL and CON offspring at adult age. **P < .01, reflecting the significant main effect of trials [latency: F (1,16) = 7.92, P < .01; distance: F (1,16) = 8.95, P < .01] in adult CON animals following the presence of a significant prenatal treatment × trial interaction [latency: F (1,29) = 4.58, P < .05; distance: F (1,29) = 6.48, P < .05]; § P < .01, reflecting the significant difference between CON and POL animals in T2 based on post hoc comparisons; *P < .05, reflecting the significant difference between CON and POL animals associated with the significant main effect of prenatal treatment [latency: F (1,29) = 5.01, P < .05; distance: F (1,29) = 5.82, P < .05] obtained by 2×5 (prenatal treatment × day) repeated-measures ANOVA of the improvement scores. N(CON) = 17, N(POL) = 14. All values are means ± SEM.
Discussion
Our data provide the first line of experimental evidence showing that prenatal exposure to immune activation has long-lasting consequences on GABAergic mRNA expression in the mPFC. Some of the gene expression changes identified in adult immune-challenged offspring, including reduced prefrontal mRNA levels of GAD67, α4, and α5, have been repeatedly noted in schizophrenia and other neurodevelopmental disorders with prenatal infectious etiologies, including autism.17,19,27,28 On the other hand, our findings do not match the reports of decreased and increased α1 and α2 mRNA levels, respectively, in cortical areas of schizophrenic patients.19,27 Our experimental data can thus be taken to support the hypothesis that prenatal immune challenge is a significant environmental risk factor for some but not all long-term GABAergic abnormalities commonly found in neuropsychiatric disorders with developmental components.
However, we would like to emphasize that our experimental data may be more readily reminiscent of and relevant for neuropsychiatric disorders with a delayed onset in early adulthood, primarily schizophrenia. This is because the deleterious effects of prenatal immune activation on GABAergic pathology (and associated cognitive impairments) were found to be more pronounced when the offspring reached adulthood. Indeed, one of the major findings here was that the magnitude and/or direction of the prenatal infection-induced changes in mRNA expression were highly dependent on the postnatal age of the offspring. For example, deficits in the expression levels of enzymes regulating GABA biosynthesis (GAD65 and GAD67) and VGAT were only clearly manifest in immune-challenged offspring once they had reached adulthood. Furthermore, adult offspring subjected to prenatal immune activation also showed qualitatively (and quantitatively) more severe changes in mRNA expression of the GABA(A) receptor alpha-subunits compared with immune-challenged offspring at peripubertal age. Consistent with its maturational impact on dopaminergic development,29 these findings emphasize that prenatal immune activation does not induce static effects on GABAergic gene expression. Rather, the prenatal immunological insult may change early neurodevelopmental trajectories that may then interact with maturational processes to precipitate the full spectrum of GABAergic abnormalities in adulthood. This proposition would also be congruent with the complex temporal dynamics underlying the normal maturation of the central GABA system, in which even subtle changes during development can exert a profound impact on long-term GABAergic signaling.30
Our findings of altered NKCC1 and KCC2 mRNA expressions in prenatally immune-challenged offspring further support the hypothesis of altered GABAergic maturation following exposure to the prenatal immunological insult. The expression of these 2 chloride transporters is developmentally regulated, which in turn is pivotal in shifting the functional properties of GABA from initially excitatory in the developing immature brain to its predominantly inhibitory actions in the adult nervous system.26 Whereas the mRNA expression levels of NKCC1 and KCC2 were concomitantly increased in peripubertal offspring born to immune-challenged mothers, the relative expression of NKCC1 vs KCC2 was strongly shifted toward increased and decreased NKCC1 and KCC2 levels, respectively, when these offspring reached adult age. Owing to the normal developmental regulation of these chloride transporters, the presence of such imbalanced NKCC1 and KCC2 expressions in the adult brain is typically taken as an index of an immature GABAergic phenotype.26 Interestingly, a similar pattern of imbalanced NKCC1 and KCC2 expressions has recently been documented in the hippocampus of schizophrenic patients.31 In addition, some of the kinases strongly regulating KCC2 and NKCC1 activities were found to be altered in the dorsolateral prefrontal cortex of schizophrenic patients.32 Hence, alterations in molecular mechanisms regulating the nature of GABA neurotransmission (inhibitory vs excitatory) are noticeable both in human subjects with schizophrenia and in the present experimental model system relevant to schizophrenia and related disorders.
This study also confirms the deleterious impact of prenatal immune activation on working memory.13,23,24,33 Working memory is a special short-term memory buffer used to hold relevant information temporarily active in order to guide on-going behavior, and its disruption is a cardinal cognitive symptom in schizophrenia.17 The profound disruption of working memory in adult offspring born to immune-challenged mothers is comparable with our own previous investigations using the same working-memory paradigm,24 and with other studies performed by other independent laboratories documenting significant working-memory deficits following prenatal poly(I:C) exposure on GD17 in mice.33 Intriguingly, Brown and colleagues have recently provided a first line of evidence showing that deficits in executive functions and working memory are more pronounced in schizophrenic cases with a positive history of prenatal infection compared with schizophrenic cases without such a history.34 Hence, prenatal immune challenge seems to be a contributing factor for schizophrenia-associated cognitive functions both in the human clinical conditions and in in vivo animal model systems.
Our study further revealed a maturational correlation between multiple pre- and postsynaptic GABAergic deficits in the mPFC and working-memory disruption, both of which appeared to be more pronounced in adult compared with peripubertal offspring born to immune-challenged mothers. This association may not be unprecedented because the integrity of prefrontal GABAergic signaling is pivotal for normal working-memory functions.17 The age-dependent changes at the molecular and cognitive levels further highlight that this prenatal immune activation model captures developmental aspects of abnormal brain structure and functions. This feature is particularly relevant to the neurodevelopmental perspective of schizophrenia because the disorder’s pathophysiological and neuropathological mechanisms are assumed to be progressive in nature.35 However, we acknowledge that our study falls short in dissecting the relative contribution of specific GABAergic abnormalities to the emergence of working-memory impairments. Indeed, even though the temporal association between the prenatal infection-induced working impairments and multiple GABAergic changes is intriguing, we did not further attempt to delineate the functional contribution of GABAergic abnormalities to the induction of cognitive impairments. Additional work will be needed to further address this issue using pharmacological and/or genetic approaches, which could serve to mitigate the working-memory deficiency by targeting distinct GABAergic abnormalities.
It is also of note that the mRNA expression levels of some of the selected GABAergic genes were variable especially in peripubertal subjects, which in turn may be related to individual maturational differences of prefrontal cortical development in pubescence.30 Therefore, it seems important to replicate some of our findings using larger cohorts of offspring. Another limitation of our study is that we did not confirm all of the identified gene expression changes to the protein level because of the limited availability of mPFC tissue. However, the successful verification of the effects of prenatal immune activation on GAD65/67 and VGAT using Western blot protein analyses suggests that at least some of the identified GABAergic gene transcription changes are readily translatable to changes at the protein level. Furthermore, our study does not provide information about up-stream molecular mechanisms modifying GABAergic gene transcription in offspring subjected to prenatal immune activation. For example, it remains uncharted whether the identified reductions in GAD65 and GAD67 protein and mRNA levels could be accounted for by reduced numbers of functionally intact interneurons and/or by altered transcriptional activity of the corresponding enzymes. On speculative grounds, a feasible and testable possibility would be that prenatal immune activation could induce epigenetic changes in the central GABA system, which is known to be highly accessible to epigenetic modifications.36
In conclusion, our experimental data suggest that prenatal immune activation induces maturation-dependent alterations in the prefrontal GABAergic transcriptome. The importance of GABAergic changes present in schizophrenia and related disorders, together with our experimental data here, highlights the critical impact of prenatal immune-related insults on long-term GABAergic changes relevant to neuropsychiatric disorders with prenatal infectious etiologies. It is also highly interesting to note that a variety of pre- or perinatal manipulations that negatively affect early brain development are capable of inducing similar long-term GABAergic pathology, including lesions of the neonatal ventral hippocampus37 and prenatal exposure to the antimitotic agent methylazoxymethanol acetate.38 Hence, alterations in the adult central GABA system may represent a critical pathological convergence point for various early-life adversities implicated in the etiology of neurodevelopmental brain abnormalities.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
The European Union’s Seventh Framework Programme (FP7/2007–2011;259679 to UM).
Supplementary Material
Acknowledgments
We remain indebted to the laboratory animal technician team, Schwerzenbach, for animal husbandry and care. All authors declare that they have no conflicts of interest to disclose.
References
- 1. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010; 167: 261–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, Sørensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophr Bull. 2007; 33: 741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009; 33: 1061–1079 [DOI] [PubMed] [Google Scholar]
- 4. Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010; 90: 285–326 [DOI] [PubMed] [Google Scholar]
- 5. Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010; 24: 881–897 [DOI] [PubMed] [Google Scholar]
- 6. Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007; 27: 10695–10702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008; 13: 208–221 [DOI] [PubMed] [Google Scholar]
- 8. Lanté F, Meunier J, Guiramand J, et al. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus. 2008; 18: 602–609 [DOI] [PubMed] [Google Scholar]
- 9. Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS ONE. 2010; 5: e10967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coyle P, Tran N, Fung JN, Summers BL, Rofe AM. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res. 2009; 197: 210–218 [DOI] [PubMed] [Google Scholar]
- 11. Fatemi SH, Emamian ES, Kist D, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999; 4: 145–154 [DOI] [PubMed] [Google Scholar]
- 12. Meyer U, Nyffeler M, Engler A, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006; 26: 4752–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008; 22: 469–486 [DOI] [PubMed] [Google Scholar]
- 14. Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006; 143: 51–62 [DOI] [PubMed] [Google Scholar]
- 15. Harvey L, Boksa P. A stereological comparison of GAD67 and reelin expression in the hippocampal stratum oriens of offspring from two mouse models of maternal inflammation during pregnancy. Neuropharmacology. 2012; 62: 1767–1776 [DOI] [PubMed] [Google Scholar]
- 16. Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuro psychopharmacology. 2010; 35: 2462–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005; 6: 312–324 [DOI] [PubMed] [Google Scholar]
- 18. Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007; 160: 59–87 [DOI] [PubMed] [Google Scholar]
- 19. Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011; 21: 999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000; 24: 417–463 [DOI] [PubMed] [Google Scholar]
- 21. Guidotti G, Calabrese F, Auletta F, et al. Developmental influence of the serotonin transporter on the expression of npas4 and GABAergic markers: modulation by antidepressant treatment. Neuropsychopharmacology. 2012; 37: 746–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 23. Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010; 13: 981–996 [DOI] [PubMed] [Google Scholar]
- 24. Vuillermot S, Joodmardi E, Perlmann T, Ögren SO, Feldon J, Meyer U. Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments. J Neurosci. 2012; 32: 436–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samuelsson AM, Jennische E, Hansson HA, Holmäng A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006; 290: R1345–R1356 [DOI] [PubMed] [Google Scholar]
- 26. Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012; 18: 467–486 [DOI] [PubMed] [Google Scholar]
- 27. Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008; 165: 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blatt GJ, Fatemi SH. Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat Rec. 2011; 294: 1646–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010; 30: 1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004; 27: 400–406 [DOI] [PubMed] [Google Scholar]
- 31. Hyde TM, Lipska BK, Ali T, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011; 31: 11088–11095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arion D, Lewis DA. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry. 2011; 68: 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Connor CM, Dincer A, Straubhaar J, Galler JR, Houston IB, Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr Res. 2012; 140: 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown AS, Vinogradov S, Kremen WS, et al. Association of maternal genital and reproductive infections with verbal memory and motor deficits in adult schizophrenia. Psychiatry Res. 2011; 188: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Insel TR. Rethinking schizophrenia. Nature. 2010; 468: 187–193 [DOI] [PubMed] [Google Scholar]
- 36. Guidotti A, Auta J, Chen Y, et al. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011; 60: 1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tseng KY, Lewis BL, Hashimoto T, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008; 28: 12691–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006; 23: 279–284 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.