Abstract
Background:
Schizophrenia is a chronic syndrome of unknown etiology, predominantly defined by signs of psychosis. The onset of the disorder occurs typically in late adolescence or early adulthood. Efforts to study pathophysiological mechanisms in early stages of the disease are crucial in order to prompt intervention.
Methods:
Case-control study of first-episode psychotic (FEP) patients and matched controls. We recruited 117 patients during the first year after their FEP according to the DSM-IV criteria and recruited 106 gender-, race-, and age-matched controls between September 2010 and June 2011.
Results:
Biochemical studies carried out in peripheral mononuclear blood cells (PMBC) and plasma evidence a significant increase in intracellular components of a main proinflammatory pathway, along with a significant decrease in the anti-inflammatory ones. Multivariate logistic regression analyses identified the expression of inducible isoforms of nitric oxide synthase and cyclooxygenase in PMBC and homocysteine plasma levels as the most reliable potential risk factors and the inhibitor of the inflammatory transcription factor NFκB, IκBα, and the anti-inflammatory prostaglandin 15d-PGJ2 as potential protection factors.
Discussion:
Taken as a whole, the results of this study indicate robust phenotypical differences at the cellular machinery level in PMBC of patients with FEP. Although more scientific evidence is needed, the determination of multiple components of pro- and anti-inflammatory cellular pathways including the activity of nuclear receptors has interesting potential as biological markers and potential risk/protective factors for FEP. Due to its soluble nature, a notable finding in this study is that the anti-inflammatory mediator 15d-PGJ2 might be used as plasmatic biomarker for first episodes of psychosis.
Key words: first-episode psychosis, inflammatory balance, schizophrenia, biomarker
Introduction
Schizophrenia is a chronic syndrome of unknown etiology, predominantly defined by signs of psychosis. The onset of the disorder occurs typically in late adolescence or early adulthood and includes positive, negative, affective and cognitive symptoms.1 While around 3% of the general population suffers a first episode of psychosis (FEP) along their life, schizophrenia affects approximately 1% of the population worldwide.2
After a century of scientific effort to better understand the nature of the disease, we are still far from a clear control of the symptoms, and there is a need to change the strategies followed to understand the pathophysiology of the disorder and the possible therapeutic targets for new drugs.3,4
Early intervention seems to mitigate progression and improve therapeutic outcomes of the disease.5 Establishment of biomarkers as soon as possible after the onset of the disease will enable early disease prevention and thus improve the prognosis.6 Notably, research in the onset of illness is especially significant because it avoids the effect of confounding variables, such as chronicity. Therefore, in this vein, patients with a FEP are an excellent target to study the risk factors linked to the development of schizophrenia and other psychotic disorders.
Among the different pathophysiological mechanisms involved in schizophrenia and other psychosis, several hypotheses involving inflammatory processes, caused both by external and endogenous factors, have been proposed.7 An appreciable body of evidence indicates a spectrum of immunological dysfunctions in schizophrenia.8 This includes genome-wide association study results implicating immune- or inflammatory-related genes as risk factors of this disorder.9 In addition, recent meta-analyses and reviews have reported favorable effects of add-on classic, nonsteroidal anti-inflammatory drugs to antipsychotics on total, positive and negative symptoms in schizophrenia.10,11
A lot of additional data support the inflammatory hypothesis in the pathophysiology of schizophrenia: (a) an elevation of proinflammatory cytokines8; (b) a decrease of anti-inflammatory cytokines and of the interleukin-1 antagonist receptor (IL-1ra)12,13; (c) anticytokine effect shown by some antipsychotics14–16; (d) increased plasma levels of the inflammatory mediator prostaglandin E2 (PGE2), the major product of inducible cyclooxygenase-2 (COX-2),17 and increased COX activity18; (e) microglial activation suggested by postmortem and positron emission tomography studies, at least in subpopulations of individuals with schizophrenia,19,20 and the identification of inflammation-related genes upregulated in schizophrenic brains.21,22
The inflammatory response is an adaptive mechanism that allows the organism to cope with diverse threatening challenges, but under pathological and long-lasting conditions, the maintenance of this response could become deleterious. The precise regulation of the whole process involves complex endogenous counterbalancing mechanisms that control the effects of potentially deleterious proinflammatory mediators. Thus, apart from all data showing inflammatory mechanisms in schizophrenia or psychosis, several studies have focused on the role of anti-inflammatory signaling pathways in both experimental and clinical settings,23 with data showing a clear misbalance in some proinflammatory/anti-inflammatory mediators in blood of patients with long-lasting schizophrenia at protein expression level.24 However, there are no data regarding the state of inflammatory mediators and their balance in early phases of the disease, such as after a FEP.
A major proinflammatory pathway is the one triggered by the activation of the nuclear factor κB (NFκB). Stimuli of diverse nature trigger a series of multienzymatic routes that cause the degradation of its inhibitory complex IκB.25–27 NFκB then translocates to the nucleus where it recognizes specific DNA sequences in the promoter of target genes, among which are those that codify for the proinflammatory enzymes, inducible nitric oxide synthase (iNOS) and the isoform 2 of the enzyme cyclooxygenase-2 (COX-2). The overactivation of these enzymes can produce an accumulation of oxidative and nitrosative mediators (ie, nitric oxide, peroxinitrite anion, and PGE2), which can cause the depletion of endogenous antioxidant defenses and attack membrane phospholipids causing cell damage in a process known as lipid peroxidation.23
However, in the last few years, some endogenous counterbalancing mechanisms, activated in response to an inflammatory/immune stimulus, have been also described.28 One of these mechanisms is the activation of peroxisome proliferator activated receptors (PPARs).29 These nuclear receptors act as ligand-dependent transcription factors, binding to DNA in specific regions and regulating the expression of proinflammatory genes.29,30 They are expressed in the great majority of brain and peripheral immune cells,31 and recent studies demonstrated that PPARs (mainly their gamma isoform, PPARγ) are master regulators of cerebral physiology and potential therapeutic targets for the treatment of several neuropathological conditions, including stress-related conditions.23,32 Interestingly, several COX-derived products, such as the prostaglandin 15-deoxy-PGJ2 (15d-PGJ2), act as endogenous anti-inflammatory agents by targeting PPARγ.33 Thus, 15d-PGJ2/PPARγ pathway is involved in the endogenous compensatory mechanism regulating the inflammatory process. This pathway can also be stimulated pharmacologically, representing not only a potential biomarker but also an important new candidate therapeutic target in neurologic/neuropsychiatric diseases, with inflammation taking part in their physiopathology.
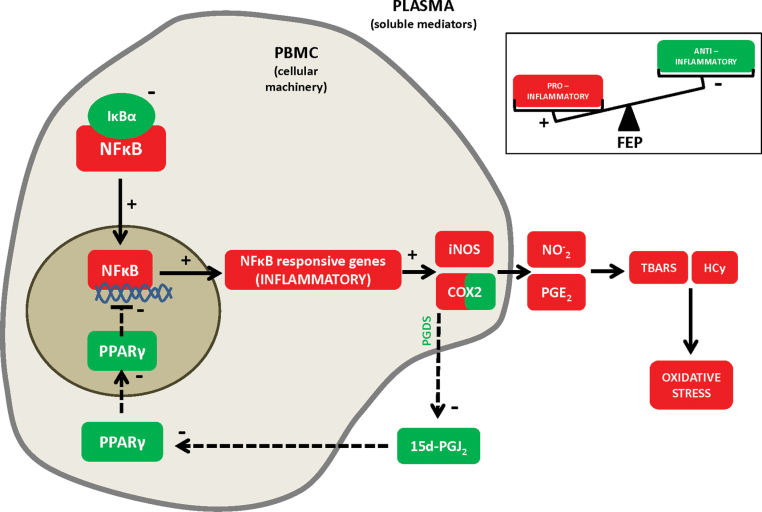
Based on this background, we hypothesized that the physiological balance between these interrelated proinflammatory/anti-inflammatory pathways may be disrupted in FEP (see figure 3). Our purpose is to study in detail all the elements of these pathways from the main single nucleotide polymorphisms (SNPs) to their protein expression level and activity in plasma and Peripheral Blood Mononuclear Cells (PBMC) samples from control and FEP patients, taking advantage of a Spanish multicenter, longitudinal, naturalistic, follow-up study, designed to evaluate clinical, neuropsychological, neuroimaging, biochemical, and genetic variables of a strictly recruited FEP patient sample between 2010–2011 (PEPs study). Finally, multivariate logistic regression analyses were conducted to identify potential risk/protective factors for FEP.
Fig. 3.
Inflammatory dysregulation in peripheral mononuclear blood cells and plasma from patients with FEP. Increase in some intracellular components of the main proinflammatory pathway (in red, straight lines) has been also demonstrated: increase in NFκB transcriptional activity and increase in the expression of 2 of the main inflammatory and oxido/nitrosative inducible enzymes, iNOS and COX-2. On the other hand, a decrease in various components of the anti-inflammatory pathway (in green, dotted lines) has been also demonstrated: decrease in the production of 15d-PGJ2, decrease in PPARγ transcriptional activity and decrease in the expression of the NFκB inhibitory subunit, IκBα. +: activation; −: inhibition.
Methods
See online supplementary material for complete details of each subheading.
Subjects
We recruited 117 patients during the first year after their first episode of psychosis according to the DSM-IV criteria34 and 106 gender-, race-, and age-matched controls. In adults, diagnosis was established according to DSM-IV criteria (SCID-I and II).34 For participants under 18 years of age, diagnosis was made using the Kiddie-Schedule for Affective Disorders & Schizophrenia, Present & Lifetime Version (K-SADS-PL).35 The duration of untreated psychosis (DUP) was defined as the number of days elapsed between the first onset of positive psychotic symptoms (the first week with the Positive and Negative Syndrome Scale [PANSS] items P1, P3, P5, P6, or G9 scoring four or more) and the beginning of the first adequate treatment of psychosis. Full recruitment details are shown in supplementary information (see Methods and online supplementary data 1).
Baseline demographic details of patients involved in the study are detailed in table 1. To ensure diagnosis stability, clinical evaluations were repeated after 6 months of the inclusion of the patients. In order to not exclude early-onset psychotic patients, there was a broad age of inclusion allowed. Inclusion criteria for patients were (a) age: 9–35 years at the first evaluation; (b) presence of psychotic symptoms of less than 12 months of duration; (c) speaking Spanish correctly; and (d) having signed the informed consent. Exclusion criteria for patients were (a) mental retardation per the DSM-IV criteria, including not only an intelligence quotient below 70 but also impaired functioning; (b) history of head trauma with loss of consciousness; and (c) organic disease with mental repercussions.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | Patients (N = 117) | Controls (N = 106) |
|---|---|---|
| Demographic characteristics | ||
| Age(y) | 23.91±5.83 | 25.43±6.43 |
| Sex, n (%) | ||
| Male | 81 (69.2) | 70 (66.0) |
| Female | 36 (30.8) | 36 (34.0) |
| Socioeconomic status | ||
| High | 22 (18.8) | 14 (13.2) |
| Medium-high | 12 (10.3) | 17 (16) |
| Medium | 48 (41) | 54 (50.9) |
| Medium-low | 27 (23.1) | 19 (17.9) |
| Low | 8 (6.8) | 2 (1.9) |
| Ethnic group | ||
| Caucasian | 110 (94) | 96 (90.6) |
| Gipsy | 1 (0.9) | 0 (0) |
| Maghrebian | 1 (0.9) | 2 (1.9) |
| Asian | 1 (0.9) | 0 (0) |
| Caribbean | 1 (0.9) | 0 (0) |
| Hispanic | 3 (2.6) | 6 (5.7) |
| Others | 0 | 2 (1.9) |
| Psychiatric history | ||
| Duration of untreated psychosis (DUP) in d | 98.02±114.38 | — |
| Diagnosis, n (%) | ||
| Affective psychosis | 21 (17.9) | — |
| Non-affective psychosis | 96 (82.1) | — |
| Psychopathology score | ||
| PANSS | ||
| Total | 53.10±19.50 | — |
| Positive | 11.17±6.05 | — |
| Negative | 14.32±6.03 | — |
| General | 27.62±10.07 | — |
| Young | 1.90±4.76 | — |
| Montgomery-Asberg | 6.51±6.37 | — |
| Overall functioning score (GAF) | 67.20±13.70 | — |
| Baseline antipsychotic medication, n (%) | ||
| Risperidone | 43 (36.8) | — |
| Olanzapine | 15 (12.8) | — |
| Aripiprazole | 11 (9.4) | — |
| Paliperidone | 9 (7.7) | — |
| Clozapine | 8 (6.8) | — |
| Quetiapine | 7 (6.0) | — |
| Ziprasidone | 2 (1.7) | — |
| None | 22 (18.8) | — |
| Lithium use, n (%) | 10 (8.5) | — |
| Baseline body mass index | 24.92±4.07* | 23.14±3.16 |
| Baseline Cannabis use, n (%) | 31 (26.5) | 14 (16.0) |
| Baseline Cannabis per month use | 11.38±34.02* | 1.15±6.36 |
| Baseline tobacco use, n (%) | 56 (57.7)* | 20 (23.0) |
| Baseline tobacco per month use | 212.43±248.63* | 45.38±119.32 |
Note: Mann-Whitney U test, *P-value < .05. The bold values in the table represent the values reaching statistical significance (P-value < .05).
Healthy controls were selected from the same geographic areas. Inclusion criteria were (a) same gender as patients; (b) similar age (± 10%) as patients; (c) similar parental socioeconomic status as patients, measured by the Hollingshead-Redlich scale (±1 level); (d) no past or present psychiatric disorder per DSM-IV criteria34; (e) speaking Spanish correctly; and (f) having signed the informed consent. The exclusion criteria for controls were (a) mental retardation according to DSM-IV criteria34 including not only an intelligence quotient below 70, but also impaired functioning; (b) history of head trauma with loss of consciousness; (c) organic disease with mental repercussions; and (d) history of psychotic disorder among first-degree relatives.
Clinical assessment of patients and controls included a complete medical history and physical examination, laboratory tests, electrocardiogram, weight, height, and body mass index. The exclusion criteria were ongoing infections, fever, allergies, or the presence of other serious medical conditions (autoimmune, cardiac, pulmonary, endocrine, and chronic infectious diseases and neoplasms). Having designed a real-life patient, naturalistic study, substance use was not an exclusion criterion. Neither the FEP patients nor the healthy control subjects were receiving immunosuppressive drugs or vaccinations for at least 6 months prior to inclusion in the study or anti-inflammatory analgesics the 2 days prior to the extraction of the blood sample.
The study was approved by the Ethics Committee of the 6 participant hospitals. The subjects participated after receiving a full explanation of the study and providing written informed consent in accordance with the Declaration of Helsinki II.
Specimen Collection and Preparation
Venous blood samples (10ml) were collected between 8:00 and 10:00h after fasting overnight. All the sample collection and preparation protocols in Flamm-PEPs study are available at www.cibersam.es. Samples were maintained at 4ºC until preparation after approximately 1h.
Blood tubes were centrifuged (641g × 10min, 4ºC). The resultant plasma samples were collected and stored at −80ºC. The rest of the sample was 1:2 diluted in culture medium (Roswell Park Memorial Institute [RPMI] 1640, Invitrogen) and a gradient with Ficoll-Paque (GE Healthcare) was used to isolate mononuclear cells by centrifugation (800g × 40min, room temperature [RT]). PBMC layer was aspired and resuspended in RPMI and centrifuged (1116g × 10min, RT). The supernatant was removed and the mononuclear cell-enriched pellet was manually resuspended in RPMI and stored at −80ºC.
For genetic studies, genomic DNA was isolated from 25 µl of the resuspended mononuclear cell-enriched pellet using Puregene (Gentra Systems) in accordance with the manufacturer’s protocol. The DNA concentration was determined by means of absorbance (ND1000, NanoDrop).
Biochemical Determinations in Plasma
Prostaglandin Levels.
Plasma levels of COX by-products PGE2 and 15d-PGJ2 were measured by enzyme immunoassay (EIA) using reagents in PGE2 EIA Kit-Monoclonal; Cayman Chemical Europe and 15-deoxy-Δ12,14- PGJ2 Enzyme-linked immunosorbent assay (ELISA) Kit DRG Diagnostics, respectively.
Nitrites.
NO− 2, the final and stable product of nitric oxide, were measured using the Griess method.
Lipid Peroxidation.
This was determined by Thiobarbi turic Acid Reactive Substances (TBARS) assay (Cayman Chemical Europe), based on the reaction of malondialdehyde and thiobarbituric acid under high temperature (95ºC) and acidic conditions.
Plasma Levels of Homocysteine.
Plasma levels of homocysteine (Hcy) were determined using an enzymatic assay (Axis-Shield Diagnostics) according to manufacturer’s instructions.
Biochemical Determinations in PBMC
To carry out all biochemical determinations, PBMC samples were first fractionated in cytosolic and nuclear extracts. For preparation of cytosolic and nuclear extracts, a modified procedure based on the Schreiber et al.36 method was used. Determination of proinflammatory p65 NFκB subunit and anti-inflammatory PPARγ respective transcriptional activities were carried out in nuclear extracts from peripheral mononuclear blood cells (PMBC):
Nuclear Factor Kappa B activity.
Activation of Nuclear factor kappa B (NFκB) occurs by enzymatic degradation of the bound inhibitory protein (IκBα), allowing movement of the p50/65 subunits from the cytoplasm to the nucleus where they bind to consensus κB sequences in DNA. The presence of p65 subunit in cell nuclei is considered an index of activity. The activity of NFκB was measured in nuclear extracts (obtained as described above) through a commercially available NFκB (p65) Transcription Factor Assay (Cayman Chemicals) following the manufacturer’s instructions.
PPARγ Transcription Factor Assay.
PPARγ activity was determined in nuclear extracts from PBMC using ELISA-based kits, which allow the detection and quantification of PPARγ specific transcriptional activity (Cayman Chemical Europe).
Western Blot Analysis.
The protein levels of the inhibitory subunit of NFκB, IκBα, and the proinflammatory enzymes COX-2 and iNOS in cytosolic extracts from PBMC were quantified by Western blot (WB) analysis. In addition, PPARγ protein expression was quantified in nuclear extracts from PBMC also by WB analysis. In the WB carried out in cytosolic extracts, the housekeeping genes β-actin and GAPDH were used as loading control (blots shown in the respective figures). In the case of PPARγ, the loading control was the nuclear factor SP1. For clarity, in the figures two WB results are presented, representative of all the samples studied (in each different gel, n = 3 per group—control or FEP sample). The insets were the most representative of statistical AU data after densitometric analysis as stated above. All densitometry results are expressed in percent from control.
Gene Studies.
A total of 40 SNPs were selected in 5 candidate gene regions (NFKB, NOS2–iNOS-, COX-2, PPARG, and PGDS: prostaglandin-D synthase, the synthesizing enzyme of PGD2, from which PGJ2 is derived non enzymatically, covering target loci and upstream and downstream regions) by tagging analysis (as implemented in Haploview 4.1) at an r 2 threshold of 0.8 to capture 98% of the most common HapMap phase II variants based on the CEU panel (minor allele frequency > 0.05) (range 91%–100% for individual genes). Three SNPs were rejected prior to genotyping for assay rules. The remaining 37 tag SNPs were genotyped by the MassARRAY genotyping system (Sequenom Inc.).
Statistical Analysis
Differences between baseline characteristics for patients and controls were assessed using Chi-square, t test, or nonparametric Mann-Whitney U tests, according to the distribution and scales of the variables.
To assess the effect of psychotropic medication, linear regression models were performed for each biomarker, and we followed the consensus method described by Gardner et al., 201037 to calculate the potency equivalents compared with Chlorpromazine.
To calculate the association between FEP and the level of biological markers, we used hierarchical logistic regression models. In order to explore mechanisms explaining the association, we used 5 models for each biological marker, in which we gradually controlled for potential confounders (age, gender, body mass index [BMI], cannabis use per month, and tobacco use per month). Model 1 included the level of biological marker. Model 2 additionally included terms for age and gender. Model 3 additionally included BMI. Model 4 additionally included cannabis use per month and finally, Model 5 additionally included tobacco use per month. Only biological markers significantly associated (P < .05) with FEP in model 4 in the previous analyses were selected for the following steps. Logistic regression analyses were again calculated with the same system, and all the biological markers chosen were kept and analyzed together in a new model 1. Model 2 additionally included terms for age and gender. Model 3 additionally included BMI. Model 4 additionally included cannabis use per month (final model). Model 5 additionally included tobacco use per month (final model).
To estimate the independent contribution of each SNP, genotype frequencies were assessed by means of multivariate methods based on logistic regression analysis and analyzed under codominant, dominant, overdominant, recessive, and additive models.
Results
Demographic and Clinical Features
The demographic and clinical characteristics of the FEP patients and healthy control group are presented in table 1 and online supplementary data 1. Patient and control groups did not differ in gender, age, and race because the case-control match had been designed. The patients and control subjects differed in body mass index (BMI; 24.92±4.07 vs 23.14±3.16, P < .05) and in baseline number of cannabis cigarettes smoked per month (11.38±34.02 vs 1.15±6.36, P < .05) although no differences were found in the percentage of active cannabis users at the study’s baseline.
In addition, there are also differences in baseline tobacco use (56 patients [57.7%] vs 20 controls [23.0%]) and in baseline tobacco cigarettes per month use (212.43±248.63 vs 45.38±119.32).
The clinical characteristics of the sample were similar to other Spanish and European studies with FEP,38,39 taking into account that subjects younger than 18 years were allowed to participate.
Patients had been diagnosed and treated for 6 months. The PANSS mean total score was 53.10±19.50, and the mean Global Assessment of Functioning (GAF) score was 67.20±13.70, although these patients had been diagnosed and treated for 6 months. The mean age of inclusion was 23.91±5.83 years, while the mean DUP was 98.02±114.38 days. The 82.1% of the patients had been diagnosed of nonaffective psychotic disorders. Only 21 subjects (17.9%) were oriented as affective disorders with psychotic features. Risperidone and olanzapine were the most frequent antipsychotics used. Twenty-two patients had discontinued the antipsychotic treatment at the inclusion time, 13 of which were oriented as affective psychosis and 6 (27.27%) were taking lithium. The clinical variables PANSS, Young, Montgomery-Asberg, GAF, age of inclusion, and DUP were not associated with any of the inflammatory markers studied separately.
However, the lineal regression analysis made to elucidate whether the antipsychotic treatment modify the levels of any of the inflammatory and oxido/nitrosative markers studied showed that only the levels of TBARS are significantly modified (P = .048) for the effect of antipsychotic medication. Thus, for each increased unit of chlorpromazine equivalents per day, TBARS levels decrease 0.003 units.
Inflammatory and Oxido/Nitrosative Markers in Control and First-Episode Psychotic Patients
The transcription factor NFκB is a master regulator of the inflammatory and oxido/nitrosative (I&ON) status of a cell. In nuclear extracts from PMBC, the activity of its p65 subunit is increased in samples from FEP patients compared with controls (table 2 and figure 1a). Similarly, the expression of the 2 main enzymatic sources of I&ON soluble mediators, iNOS and COX-2, were significantly higher in FEP group than those in the control subjects (table 2 and figure 1b and c). However, at this particular stage of the disease, the mean levels of the I&ON markers in plasma NO− 2, PGE2, TBARS, and Hcy were increased in FEP patients, but they did not reach statistical significance (P > .05; table 2 and figure 1d–g, respectively).
Table 2.
Biological Markers
| Marker | Patients, Total N = 117 | Controls, Total N = 106 | Statistics | df | P-Value |
|---|---|---|---|---|---|
| NFκB -act- | 12.79±22.24* (n = 53) | 4.36±3.28 (n = 35) | U = 612.0 | — | .007 |
| iNOS -WBc- | 125.36±49.71* (n = 91) | 96.60±40.50 (n = 88) | U = 2585.5 | — | <.001 |
| COX2 -WBc- | 145.28±145.50* (n = 90) | 94.58±61.92 (n = 88) | U = 2517.0 | — | <.001 |
| NO− 2 -sol- | 14.65±5.90 (n = 50) | 12.75±4.67 (n = 61) | U = 1270.0 | — | .131 |
| PGE2 -sol- | 524.89±739.41 (n = 111) | 373.35±264.95 (n = 104) | U = 5522.5 | — | .584 |
| TBARS -sol- | 3.49±3.52 (n = 105) | 2.68±2.69 (n = 104) | U = 4748.5 | — | .109 |
| Hcy -sol- | 16.30±17.41 (n = 71) | 9.87±6.86 (n = 41) | U = 1194.0 | — | .114 |
| IκBα -WBc- | 84.81±47.63* (n = 91) | 103.66±47.13 (n = 88) | U = 2922.0 | — | .002 |
| 15dPGJ2 -sol- | 571.32±154.63* (n = 108) | 618.87±158.42 (n = 104) | U = 4737.0 | — | .049 |
| PPAR -WBn- | 77.64±32.91* (n = 14) | 103.12±27.92 (n = 16) | t = 2.30 | 28 | .029 |
| PPAR -act- | 1.39±0.96* (n = 71) | 1.88±1.45 (n = 41) | U = 2532.5 | — | .005 |
Note: Mean differences (SD) on biomarkers levels between FEP and controls. Two-tailed t test was assessed for PPARγ expression because its distribution meets the assumption of normality in the Kolmogorov-Smirnov (with Lillierfors correction) test. For the rest of variables, two-tailed nonparametric Mann-Whitney U test was used. *P-value < .05. The bold values in the table represent the values reaching statistical significance (P-value < .05). Analyses carried out in WB: protein expression, determined by western blot in PBMC (WBc: in cytoplasmatic fraction; WBn: in nuclear fraction); sol: plasma levels of soluble compounds; act: activity assay in nuclear extracts. See Methods for details.
Fig. 1.
Mean differences (SD) on biomarkers between FEP and controls (univariate analysis). (a) NFκB activity in PBMC nuclear extracts from FEP patients (n = 53) and controls (n = 35); (b) Western blot analysis of proinflammatory proteins iNOS (patients n = 91, controls, n = 88); (c) COX-2 in PBMC cytosolic extracts from FEP patients (n = 90) and controls (n = 88); and (d) plasma levels of nitrites (NO2−; patients n = 50, controls, n = 61), (e) proinflammatory prostaglandin E2 (patients n = 111, controls, n = 104), (f) thiobarbituric acid reactive substances (patients n = 105, controls, n = 104), and (g) homocysteine from FEP patients (n = 71) and controls (n = 41). AU, arbitrary units. Two-tailed nonparametric Mann-Whitney U test was used. ° represents an atypical value and * an extreme value.
Anti-inflammatory Markers in Control and First-Episode Psychotic Patients
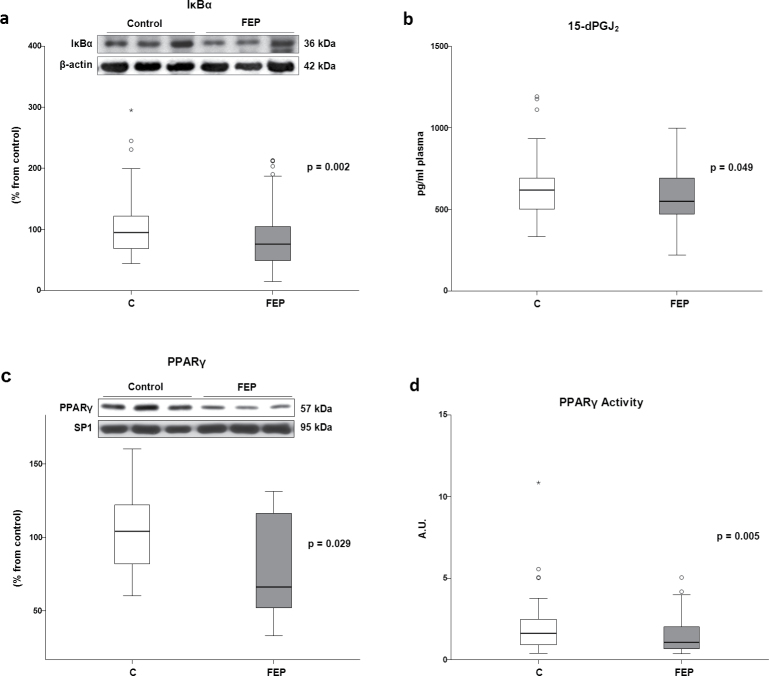
Levels of the NFκB inhibitory subunit IκBα (which maintains NFκB dimers in the cytoplasm of unstimulated cells, blocking its translocation to the nucleus) in cytosolic extracts were decreased in patients compared with those in healthy subjects (table 2 and figure 2a), suggesting the presence of a chronic proinflammatory status in PBMC from patients.
Fig. 2.
Mean differences (SD) on (a) Western blot analysis of IκBα in PBMC cytosolic extracts (patients n = 91, controls, n = 88); (b) plasma levels of anti-inflammatory prostaglandin 15d-PGJ2 (patients n = 108, controls, n = 104); (c) Western blot analysis of peroxisome proliferator activated receptor γ (PPARγ; patients n = 14, controls, n = 16); and (d) transcriptional activity of PPARγ (patients n = 78, controls, n = 87) in PBMC nuclear extracts. Two-tailed t test was assessed for PPARγ, and for the rest of variables, two-tailed nonparametric Mann-Whitney U test was used. ° represents an atypical value and * an extreme value.
On the contrary, the plasma levels of the anti-inflammatory prostaglandin 15d-PGJ2 were significantly lower in FEP patients than those in the control subjects (table 2 and figure 2b).
Given that 15d-PGJ2 acts as an endogenous ligand for PPARγ, which is considered a potent anti-inflammatory transcription factor, we explored the expression (by WB analysis) and transcriptional activity (by studying its ability to bind to its specific DNA response elements) of this receptor in nuclear extracts from PBMC. The WB analysis revealed lower PPARγ expression in patients compared with controls (table 2 and figure 2c). Moreover, the transcriptional activity of PPARγ, analyzed by its ability to bind to its specific DNA response elements, was also reduced in patients (table 2 and figure 2d).
Gene Studies
The results of the association analysis for the 35 SNPs studied in 5 candidate genes (NFκB, iNOS, COX-2, PPARG, and PGDS) are shown in online supplementary data 2. Two polymorphisms presented nominal pointwise, P < .027, the nominal P-value expected by chance (rs13348258, NFκB; rs2779248, iNOS). However, none of these showed significant empirical P-values after permutation correction for multiple testing.
Multivariate Analysis
In the final model, only 6 of the 11 biological markers studied were significantly associated with FEP after controlling for all possible confounders (table 3 and online supplementary data 3). The resulting equation was [0.428 × NFκB] + [0.055 × iNOS] + [0.072 × COX-2] + [0.432 × Hcy] + [−0.097 × IκBα] + [−0.069×15d-PGJ2] + [−0.152 × gender (female)] + [0.482 × age] + [1.185 × BMI] + [0.182 × cannabis use per month] + [0.011 × tobacco use per month].
Table 3.
Multivariate Logistic Regression Analysis
| B | SE | Wald | OR (95 % CI) | P-Value | |
|---|---|---|---|---|---|
| NFκB | 0.428 | 0.234 | 3.242 | 1.534 (0.963–2.443) | .072 |
| iNOS | 0.055 | 0.028 | 4.041 | 1.057 (1.001–1.115) | .044 |
| COX2 | 0.072 | 0.037 | 3.889 | 1.075 (1.000–1.154) | .049 |
| Hcys | 0.432 | 0.202 | 4.560 | 1.541 (1.036–2.291) | .033 |
| IκBα | −0.097 | 0.042 | 5.403 | 0.908 (0.837–0.985) | .020 |
| 15d-PGJ2 | −0.069 | 0.032 | 4.697 | 0.933 (0.876–0.993) | .030 |
Note: Association between FEP and level of biomarker. All the biomarkers were analyzed together and adjusted for age, gender, body mass index, cannabis use per month, and tobacco use per month. The bold values in the table represent the values reaching statistical significance (P-value < .05).
Among the proinflammatory markers, the highest odds ratio (OR) observed was the Hcy (OR = 1.541), meaning that for each unit increased of this biomarker, the risk40 of FEP increased by 54.1% [(e 0.432×1 − 1) × 100] after controlling for remaining biological markers and all possible confounders. Similarly, the results were 53.4% for NFκB (not significant), 5.6% for iNOS, 7.5% for COX-2.
Among the anti-inflammatory markers, the IκBα variable had the lowest OR observed (OR = 0.908), meaning that the association went in the inverse direction of proinflammatory markers, because the risk decreased by 10.2% [(e 0.097×1 − 1) × 100] for each unit of increased biomarker after controlling for remaining biological markers and all possible confounders. Similarly, the result for 15d-PGJ2 was 7.1%.
PPARγ protein expression data were excluded of the multivariate analysis due to its small sample size although it was significant (P < .05) in its individual regression model controlled for all confounders.
Discussion
In this study, we have found evidence of systemic inflammatory conditions in FEP patients. Specifically we have identified a significant increase in some intracellular components of a main proinflammatory pathway, along with a significant decrease in the anti-inflammatory ones. This is, to our knowledge, the first description of such imbalance in this particular clinical sample. All together, these results describe an imbalanced, proinflammatory phenotype in FEP patients. The multivariate logistic regression analyses conducted allows us to identify Hcy plasma levels as the most reliable potential risk factor, along with iNOS and COX-2, and IκBα and 15d-PGJ2 as potential protection factors. Due to its soluble nature, a notable finding in this study is that the anti-inflammatory 15d-PGJ2 might be used as plasmatic biomarker for FEP.
The results of the multivariate analysis applied is of special translational importance because it tries to simulate what actually happens in biological pathways, including both pro- and anti-inflammatory markers in the same statistical model, allowing them to interact even with possible sociodemographic confounders. So in the clinical practice, we could calculate the association between FEP and one of the markers (eg, iNOS) once the influence of other markers (PGE2, COX-2, Hcy, and 15d-PGJ2) and confounders (age, sex, BMI, cannabis, and tobacco) have been controlled. The association calculated is quiet stable because this did not change after adjusting for possible confounders. The strength of the association was supported by the stability of OR in the different models calculated.
Taken as a whole, the results of this study indicate phenotypical differences at the cellular machinery level in PBMC of FEP patients, 80% of whom are at the beginning of a multi-episode chronic severe mental illness such as schizophrenia or bipolar disorder.41 It is worth noting that, contrarily to what occurs in schizophrenia patients, the majority of the proinflammatory soluble elements are not significantly altered although all the parameters follow the same tendency to increase. In addition, the levels of TBARS, a final consequence of cellular damage produced by oxidative stress, follows the same profile. This lack of statistical significance could be explained in base of the heterogeneity of the FEP samples; in fact, only a part of these subjects will develop a full-blown schizophrenia. Longitudinal studies with the same patients will clarify the relevance of the potential oxido/nitrosative cellular damage in FEP subjects, taking into account the increase in lipid hydroperoxides reported in a similar sample.42
There are few studies focused on diagnostic tools in FEP; some image studies indicate subtle brain abnormalities43 and others clinical slight symptoms.44 In terms of the inflammation process, our results show that while the soluble final products are not significantly modified, their enzymatic sources iNOS and COX-2, both inducible isoforms regulated by the IκBα/NFκB pathway,45,46 are overexpressed in PMBC. This suggests that FEP patients are at the onset of the inflammatory process.
The great majority of studies reporting inflammatory/immune alterations are described in full-blown schizophrenic patients,15,17,24,47–50 a situation in which tissue of plasma antioxidant mechanisms are exhausted.51 Similarly, hyperhomocysteinemia can cause oxidative stress via a number of mechanisms such as auto-oxidation of Hcy to form reactive oxygen species.52 Previous studies53 showed a correlation between the increased amount of Hcy and nitrotyrosine in plasma proteins or plasma TBARS,54 thus being considered as a risk factor for the disease. In this vein, our multivariate statistical approach has identified Hcy levels as a very reliable risk factor for FEP.
There is also a decrease in the counterbalancing pathway mainly controlled by 15d-PGJ2. Indeed, this mechanism is considered as a possible endogenous regulator of the inflammatory response in neurodegenerative conditions and stress-related diseases.23 Our group recently described a decrease in this pathway in male, chronic schizophrenic inpatients in acute relapse phase.24 Now, the data presented here indicate that the changes in 15d-PGJ2/PPARγ pathway are also present at the very early stages of the disease.
This study suggests an active role for the anti-inflammatory signaling pathway in the pathophysiology of the disorder, which adds support to pharmacological strategies involving the stimulation of the PPARγ activity. Of special interest is the possible use of some thiazolidinediones, potent agonists of PPARγ, used as insulin-sensitizing drugs for the treatment of type 2 diabetes.55 Pharmacological activation of PPARγ is a multifaceted therapeutic target due to its anti-inflammatory/antioxidant/antiexcitotoxic/proenergetic profile, reported in some inflammatory-related scenarios (neurological and stress-related diseases).23,56 Recently, PPARγ activation has been presented as a putative treatment for neurocognitive deficits associated with mood and psychotic syndromes.57
In addition to the putative neuroprotective effects of PGD2/15d-PGJ2/PPARγ for the negative and cognitive symptomatology treatment of schizophrenia, classical studies already suggested a relevant mechanism to elucidate a specific role for PGD2 in the management of the positive symptoms. In these studies, PGD2 stimulated the production of cyclic adenosine monophosphate and thereby exerted functional antagonism of dopamine-D2 receptors.58 Therefore, PGD2 and its metabolites could be counteracting the biochemical and behavioral effects of dopamine, and deficient PGD2/PGJ2 signaling in the brain could influence dopamine transmission.59
After the analysis of the most common SNP variables of pro/anti-inflammatory mediators, the lack of correlation with their studied gene variants could suggest a possible role for epigenetic factors, other less-studied SNPs, or other candidate genes, as well as the need for new methods to detect genetic effects. For example, a recent study used a SNP-based analysis of neuroactive pathways implicates PGE2 as a novel mediator of antipsychotic treatment response using data from the multiphase, randomized controlled trial Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE).60
On the other hand, the absence of findings related to genetic makers and the lack of any clinical correlates of the biomarkers does not yield support to these biomarkers being of etiological relevance. The effects reported could well be reflecting an epiphenomenon related to stress or metabolic complications, which anyway does not diminish their value as putative therapeutic targets. Fortunately, Flamm-PEPs is still an open study, and future longitudinal studies are now conducted with 2 years of follow-up and that will help elucidate these controversial issues and evaluate the utility of this pro-/anti-inflammatory signaling pathway as biological marker for possible second episodes.
Some limitations in this study should be noted:
First, we used a single control group of healthy subjects instead of using 2 control groups, 1 from healthy subjects and another including other psychiatric conditions with the aim of controlling and thereby increasing the specificity of our results. In fact, changes in both inflammatory and anti-inflammatory COX-derived pathways occur after acute and chronic stress exposure.23,30 Second, 81.2% of the FEP patients included in our study were receiving atypical antipsychotic treatment, and there is some evidence on the potential anti-inflammatory effects of antipsychotics,13–15 most of them at anti-cytokine level. Nevertheless, we have tried to control the possible confounding effect of antipsychotic treatment through a multiple linear regression analysis, and we found only marginal effects on the plasma levels of TBARS. As commented above, increased lipid peroxidation has been found in early-onset first-episode psychosis, but the specific effects of antipsychotic medication were not addressed.42 Third, a small group (8%) of patients required lithium. Although there are no clear references about lithium and inflammation, the possibility of being a confounding factor was also assessed. However, the result did not modify the association showed in this study. Fourth, the total number of subjects taking part of this study is 117 patients and 106 matched controls, but we could not measure all of the parameters in all the subjects. In general, for the parameters measured in plasma (eg, the 2 prostaglandins) almost all subjects were used, but for the determinations made in the cytosolic/nuclear extracts of PBMC, some methodological limitations existed and the quantity of sample obtained is relatively low. With this limitation in mind, we have tried to get a reasonable number of subjects for each parameter studied to carry out a reliable statistical analysis. No major changes were found between the differences in the whole sample and those in the subsets that were finally analyzed. It is worth noting that PPARγ protein expression data were not chosen and kept together with the other markers selected because of the sample size was small although PPARγ protein was significant in its individual regression model controlled for all confounders. Keeping these data in mind, we cannot discard a role for PPARγ as a potential protective factor in FEP patients. In fact, PPARγ expression and activity are significant in the two-tailed Chi-square tests on categorical data used to identify differences between baseline characteristics for patients and control subjects, both in our study and in schizophrenic inpatients in acute relapse phase.24
Despite these limitations, key strengths of the study deserve mention: The sample was very homogeneous in the moment in the course of illness and originated from specific areas of 2 major and 3 middle European cities. The diagnostic evaluation was performed with a very comprehensive protocol, and inclusion-exclusion criteria were applied in a strict manner. Finally, another unique feature of the study is that it includes a wide spectrum of biochemical inflammatory markers in both PBMC and plasma samples, allowing in-depth insights and relationships between multiple components of the pro- and anti-inflammatory signaling pathways.
Efforts in describing biological markers for schizophrenia in pathway approaches are claimed by psychiatrists as tools to help early diagnosis and monitor evolution of the disease; this would greatly assist preventive strategies by identifying at-risk individuals who could then be monitored and treated in a way to minimize subsequent morbidity.
In conclusion, and regarding to implications for clinical practice, the importance of early detection and intervention in psychosis has renewed interest in subtle psychopathology beyond positive and negative symptoms and also in the search for biological markers of the disease. Although more scientific evidence is needed, the determination of multiple components of pro- and anti-inflammatory cellular pathways has interesting potential as biological risk/protective markers for FEP. Their pharmacological modulation can be a promising (see figure 3) therapeutic target to take into account in the future.
Funding
CIBERSAM Intramural Projects 2010 (P02): Flamm-PEPs , Inflammatory alterations in schizophrenia: search of biological markers in first-psychotic episodes. Spanish Ministry of Economy and Competiveness, Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias (PI 1100325).
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The results presented here conforms a supplementary hypothesis added to the initial hypothesis in the wide PEP study (ISCIII 2009–2011). The authors declare no conflict of interest.
B.G.B. wrote the first version of the article and performed some of the biochemical determinations; M.B. managed and analyzed the clinical data; K.S.M.D. performed biochemical determinations in plasma and in cells and prepared subcellular samples; M.F.B. and J.S. performed the statistical and the first version of the figures; M.M.C., L.P., R.R.J., and P.S. collected the biological samples and the clinical data; C.C. performed the Hcy determination; A.L. performed the gene study and analyzed the data; A.G.P., M.P., G.R., and M.P.G.P. analyzed the clinical data; J.A.M. analyzed the oxidative data; M.B. coordinated PEP study and analyzed the clinical data; J.C.L. coordinated Flamm-PEP study, designed the study, and wrote the article. All of the authors contributed to the final version of the article.
References
- 1. van Os J, Kapur S. Schizophrenia. Lancet. 2009; 374: 635–645 [DOI] [PubMed] [Google Scholar]
- 2. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007; 64: 19–28 [DOI] [PubMed] [Google Scholar]
- 3. Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006; 12: 1016–1022 [DOI] [PubMed] [Google Scholar]
- 4. Insel TR. Rethinking schizophrenia. Nature. 2010; 468: 187–193 [DOI] [PubMed] [Google Scholar]
- 5. Kuehn BM. FDA: Gene tests need premarket approval. JAMA. 2010; 304: 145 [DOI] [PubMed] [Google Scholar]
- 6. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009; 110: 1–23 [DOI] [PubMed] [Google Scholar]
- 7. Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006; 147 Suppl 1: S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Müller N, Schwarz MJ. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci. 2008; 258 Suppl 2: 97–106 [DOI] [PubMed] [Google Scholar]
- 9. Shi J, Levinson DF, Duan J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009; 460: 753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012; 73: 414–419 [DOI] [PubMed] [Google Scholar]
- 11. Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011; 132: 96–110 [DOI] [PubMed] [Google Scholar]
- 12. Arolt V, Rothermundt M, Wandinger KP, Kirchner H. Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Mol Psychiatry. 2000; 5: 150–158 [DOI] [PubMed] [Google Scholar]
- 13. Maes M, Bosmans E, Ranjan R, et al. Lower plasma CC16, a natural anti-inflammatory protein, and increased plasma interleukin-1 receptor antagonist in schizophrenia: effects of antipsychotic drugs. Schizophr Res. 1996; 21: 39–50 [DOI] [PubMed] [Google Scholar]
- 14. Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009; 33: 303–307 [DOI] [PubMed] [Google Scholar]
- 15. Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995; 29: 141–152 [DOI] [PubMed] [Google Scholar]
- 16. Pollmächer T, Haack M, Schuld A, Kraus T, Hinze-Selch D. Effects of antipsychotic drugs on cytokine networks. J Psychiatr Res. 2000; 34: 369–382 [DOI] [PubMed] [Google Scholar]
- 17. Kaiya H, Uematsu M, Ofuji M, et al. Elevated plasma prostaglandin E2 levels in schizophrenia. J Neural Transm. 1989; 77: 39–46 [DOI] [PubMed] [Google Scholar]
- 18. Das I, Khan NS. Increased arachidonic acid induced platelet chemiluminescence indicates cyclooxygenase overactivity in schizophrenic subjects. Prostaglandins Leukot Essent Fatty Acids. 1998; 58: 165–168 [DOI] [PubMed] [Google Scholar]
- 19. Steiner J, Bielau H, Brisch R, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008; 42: 151–157 [DOI] [PubMed] [Google Scholar]
- 20. van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008; 64: 820–822 [DOI] [PubMed] [Google Scholar]
- 21. Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007; 7: 46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, et al. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int J Neuropsychopharmacol. 2010; 13: 1369–1381 [DOI] [PubMed] [Google Scholar]
- 23. García-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 2008; 32: 1136–1151 [DOI] [PubMed] [Google Scholar]
- 24. Martínez-Gras I, Pérez-Nievas BG, García-Bueno B, et al. The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophr Res. 2011; 128: 15–22 [DOI] [PubMed] [Google Scholar]
- 25. Bierhaus A, Wolf J, Andrassy M, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003; 100: 1920–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madrigal JL, Hurtado O, Moro MA, et al. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002; 26: 155–163 [DOI] [PubMed] [Google Scholar]
- 27. Middleton G, Hamanoue M, Enokido Y, et al. Cytokine-induced nuclear factor kappa B activation promotes the survival of developing neurons. J Cell Biol. 2000; 148: 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galea E, Heneka MT, Dello Russo C, Feinstein DL. Intrinsic regulation of brain inflammatory responses. Cell Mol Neurobiol. 2003; 23: 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008; 13: 1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. García-Bueno B, Madrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC. Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry. 2005; 57: 885–894 [DOI] [PubMed] [Google Scholar]
- 31. Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007; 1771: 1031–1045 [DOI] [PubMed] [Google Scholar]
- 32. Feinstein DL. Therapeutic potential of peroxisome proliferator-activated receptor agonists for neurological disease. Diabetes Technol Ther. 2003; 5: 67–73 [DOI] [PubMed] [Google Scholar]
- 33. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA. 1997; 94: 4312–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. First M, Gibbon M, Spitzer RL, Williams JBW, Smith J. Entrevista clínica estructurada para los trastornos del eje-I del DSM-IV. Barcelona, Spain: Masson; 1999. [Google Scholar]
- 35. Ulloa RE, Ortiz S, Higuera F, et al. [Interrater reliability of the Spanish version of Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime version (K-SADS-PL)]. Actas Esp Psiquiatr. 2006; 34: 36–40 [PubMed] [Google Scholar]
- 36. Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989; 17: 6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010; 167: 686–693 [DOI] [PubMed] [Google Scholar]
- 38. Castro-Fornieles J, Parellada M, Gonzalez-Pinto A, et al. The child and adolescent first-episode psychosis study (CAFEPS): design and baseline results. Schizophr Res. 2007; 91: 226–237 [DOI] [PubMed] [Google Scholar]
- 39. Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008; 371: 1085–1097 [DOI] [PubMed] [Google Scholar]
- 40. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 41. Alvarez-Jiménez M, Parker AG, Hetrick SE, McGorry PD, Gleeson JF. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011; 37: 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Micó JA, Rojas-Corrales MO, Gibert-Rahola J, et al. Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry. 2011; 11: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arango C, Rapado-Castro M, Reig S, et al. Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry. 2012; 69: 16–26 [DOI] [PubMed] [Google Scholar]
- 44. Schultze-Lutter F, Ruhrmann S, Fusar-Poli P, Bechdolf A, Schimmelmann BG, Klosterkötter J. Basic symptoms and the prediction of first-episode psychosis. Curr Pharm Des. 2012; 18: 351–357 [DOI] [PubMed] [Google Scholar]
- 45. Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994; 269: 4705–4708 [PubMed] [Google Scholar]
- 46. Newton R, Kuitert LM, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem Biophys Res Commun. 1997; 237: 28–32 [DOI] [PubMed] [Google Scholar]
- 47. Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry. 2009; 65: 481–488 [DOI] [PubMed] [Google Scholar]
- 48. Das I, Khan NS, Puri BK, Sooranna SR, de Belleroche J, Hirsch SR. Elevated platelet calcium mobilization and nitric oxide synthase activity may reflect abnormalities in schizophrenic brain. Biochem Biophys Res Commun. 1995; 212: 375–380 [DOI] [PubMed] [Google Scholar]
- 49. Yokota O, Terada S, Ishihara T, et al. Neuronal expression of cyclooxygenase-2, a pro-inflammatory protein, in the hippocampus of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004; 28: 715–721 [DOI] [PubMed] [Google Scholar]
- 50. Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull. 2004; 30: 923–934 [DOI] [PubMed] [Google Scholar]
- 51. Ghosh N, Ghosh R, Mandal SC. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic Res. 2011; 45: 888–905 [DOI] [PubMed] [Google Scholar]
- 52. Jones BG, Rose FA, Tudball N. Lipid peroxidation and homocysteine induced toxicity. Atherosclerosis. 1994; 105: 165–170 [DOI] [PubMed] [Google Scholar]
- 53. Brown AS, Bottiglieri T, Schaefer CA, et al. Elevated prenatal homocysteine levels as a risk factor for schizophrenia. Arch Gen Psychiatry. 2007; 64: 31–39 [DOI] [PubMed] [Google Scholar]
- 54. Dietrich-Muszalska A, Malinowska J, Olas B, et al. The oxidative stress may be induced by the elevated homocysteine in schizophrenic patients. Neurochem Res. 2012; 37: 1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995; 270: 12953–12956 [DOI] [PubMed] [Google Scholar]
- 56. García-Bueno B, Caso JR, Pérez-Nievas BG, Lorenzo P, Leza JC. Effects of peroxisome proliferator-activated receptor gamma agonists on brain glucose and glutamate transporters after stress in rats. Neuropsychopharmacology. 2007; 32: 1251–1260 [DOI] [PubMed] [Google Scholar]
- 57. McIntyre RS, Soczynska JK, Lewis GF, MacQueen GM, Konarski JZ, Kennedy SH. Managing psychiatric disorders with antidiabetic agents: translational research and treatment opportunities. Expert Opin Pharmacother. 2006; 7: 1305–1321 [DOI] [PubMed] [Google Scholar]
- 58. Ono N, Abiru T, Sugiyama K, Kamiya H. Influences of cyclooxygenase inhibitors on the cataleptic behavior induced by haloperidol in mice. Prostaglandins Leukot Essent Fatty Acids. 1992; 46: 59–63 [DOI] [PubMed] [Google Scholar]
- 59. Condray R, Yao JK. Cognition, dopamine and bioactive lipids in schizophrenia. Front Biosci (Schol Ed). 2011; 3: 298–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Adkins DE, Khachane AN, McClay JL, et al. SNP-based analysis of neuroactive ligand-receptor interaction pathways implicates PGE2 as a novel mediator of antipsychotic treatment response: data from the CATIE study. Schizophr Res. 2012; 135: 200–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.