Abstract
Previous neuroimaging studies have revealed that both gray matter (GM) and white matter (WM) are altered in several morphological aspects in schizophrenia patients. Although several studies reported associations between GM and WM alterations in restricted regions, the existence of a global association between GM and WM pathologies is unknown. Considering the wide distribution of GM morphological changes and the profound genetic background of WM abnormalities, it would be natural to postulate a global association between pathologies of GM and WM in schizophrenia. In this investigation, we studied 35 schizophrenia patients and 35 healthy control subjects using T1-weighted magnetic resonance imaging and diffusion tensor imaging (DTI) and investigated the association between GM thickness and WM fractional anisotropy (FA) as a proxy of pathology in each tissue. To investigate cortical thickness, surface-based analysis was used. The mean cortical thickness for the whole brain was computed for each hemisphere, and group comparisons were performed. For DTI data, mean FA for the whole brain was calculated, and group comparisons were performed. Subsequently, the correlation between mean cortical thickness and mean FA was investigated. Results showed that the mean cortical thickness was significantly thinner, and the mean FA was significantly lower in schizophrenia patients. Only in the patient group the mean cortical thickness and mean FA showed significant positive correlations in both hemispheres. This correlation remained significant even after controlling for demographic and clinical variables. Thus, our results indicate that the GM and WM pathologies of schizophrenia are intertwined at the global level.
Key words: schizophrenia, white matter integrity, diffusion tensor imaging, cortical thickness, surface-based approach
Introduction
Schizophrenia is a neurodevelopmental disorder with widespread brain alterations. Over the past decades, magnetic resonance imaging (MRI) studies have revealed volume reductions in multiple brain regions in schizophrenia. Voxel-based morphometry is one of the most frequently applied techniques used to explore the whole brain without specific presumptions about search areas, and volume reductions of widely distributed cortical/subcortical gray matter (GM) regions in schizophrenia, including the prefrontal and temporal cortices, have been reported.1–3 On the other hand, cortical volume is affected by cortical thickness and surface area, both of which can be altered independently in human brains.4 Cortical thickness is supposed to better reflect cytoarchitectural alterations than cortical volumes,5 such as the disorganization or low density of neuronal and glial cells, and alterations of synaptic spines and passing axons in schizophrenia.5–7 Recently, the surface-based approach8,9 has been increasingly utilized to analyze the cortical thickness in the whole brain, and it can be used to directly measure cortical thickness separately from cortical surface area. It has revealed reduced cortical thickness in various regions including prefrontal and temporal areas in schizophrenia.10,11
Although GM pathology was initially the focus of many brain imaging studies, white matter (WM) abnormality in schizophrenia is increasingly being investigated using neuroimaging techniques, especially diffusion tensor imaging (DTI),12 which provides information about WM tracts and their organization based on water diffusion. Fractional anisotropy (FA) is the most often used DTI index of WM integrity, and reduced FA in schizophrenia has been frequently reported in various regions, including the uncinate fasciculus (UF), cingulum bundle, superior longitudinal fasciculus (SLF), corpus callosum (CC), and cerebellar peduncles.13–18 As a result, the term “disconnection hypothesis,”19 which originally denoted the functional disconnection at the synaptic level, is often used to stress the relevance of WM pathology.
As mentioned above, the findings of altered GM and WM in schizophrenia patients are increasingly prevalent. However, whether and how GM and WM pathologies are interrelated in the neurodevelopmental process have yet to be investigated. Meanwhile, several candidate genetic susceptibility factors for schizophrenia have recently garnered attention, and some of the mutations in such susceptibility genes are thought to affect the formation and regression of synapses in the cortex, which can lead to pathological GM loss. Furthermore, these genes also affect the proliferation and differentiation of oligodendrocytes in the WM, as well as their capacity for myelination, which can lead to abnormal WM integrity.20,21 Based on these findings, it is tempting to hypothesize that structural abnormalities of GM and WM at the macroscopic level are intertwined in the pathological process of schizophrenia. In attempts to answer this question, some studies have reported associations between regional GM and WM pathologies.22–25 However, to date, no study has examined whether there is such an association between GM and WM pathologies at the global level. Considering the wide distribution of GM morphological abnormalities and WM integrity changes, and the putative genetic background of GM and WM abnormalities, it would be natural to postulate a global association between pathologies of GM and WM in schizophrenia.
In this study, we tried to elucidate the global association between GM cortical thickness and WM integrity alterations in schizophrenia, using a surface-based approach and DTI. We hypothesized that there would be a positive correlation between cortical thinning and WM integrity reduction in schizophrenia patients at the global level.
Methods
Participants
Thirty-five schizophrenia patients diagnosed with the patient edition of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) were recruited. None of the patients had comorbid psychiatric disorders. The Positive and Negative Syndrome Scale (PANSS)26 was used to assess the severity of clinical symptom with 5 subscales; negative, positive, activation, depressive, and cognitive factors.27 Thirty-five healthy controls, matched with the patient group in terms of age, gender, and education level were recruited. The controls had no history of psychiatric illness, as screened with the nonpatient edition of the SCID, and it was confirmed that their first-degree relatives had no history of psychotic disorders. Exclusion criteria for all individuals included a history of head trauma, neurological illness, serious medical or surgical illness, and substance abuse. All participants were physically healthy when they undertook the scanning. After receiving a complete description of the study, all participants gave written informed consent. The study design was approved by the Committee on Medical Ethics of Kyoto University.
MRI Data Acquisition
All participants underwent MRI scans on a 3-Tesla whole-body scanner equipped with a receiver-only 8-channel phased-array head coil with a 40-mT/m gradient (Trio, Siemens). The scanning parameters of the T1-weighted 3-dimensional magnetization-prepared rapid gradient-echo (3D-MPRAGE) sequences were as follows: echo time (TE) = 4.38ms; repetition time (TR) = 2000ms; inversion time = 990ms; field of view (FOV) = 225×240mm; 240×256 matrix; resolution = 0.9375×0.9375×1.0mm3; and 208 total axial sections without intersection gaps.
The scanning parameters for DTI data were as follows: TE = 96ms, TR = 10 500ms, 96×96 matrix, FOV = 192×192mm, 70 continuous axial slices of 2.0mm thickness, 81 noncollinear axis motion probing gradient, b = 1500 s/mm2. The b = 0 images were scanned preceding every 9 diffusion weighted images, thus consisting of 90 volumes in total.
Data Processing and Statistical Analysis
Cortical Thickness Analysis.
For cortical thickness analysis in the whole brain, a surface-based approach was applied using FreeSurfer tools28,29 (version 4.5.0, http://surfer.nmr.mgh.harvard.edu). The 3D-MPRAGE images were used to calculate the thickness of the cerebral cortex throughout the cortical mantle. Briefly, the processing stream included a Talairach transform of each of the subject’s native brain, removal of nonbrain tissue, and segmentation of GM/WM tissue. The GM/WM boundary was tessellated to generate multiple vertices across the whole brain. The cortical surface of each hemisphere was inflated to an average spherical surface to locate the pial surface and the GM/WM boundary. The entire cortex of each subject was visually inspected, and any topological defects were corrected manually, blind to subject identities. Cortical thickness was computed as the shortest distance between the pial surface and the GM/WM boundary at each vertex across the cortical mantle. Global mean cortical thickness for each subject was computed by averaging cortical thickness of each vertex, right and left hemispheres separately. The global mean cortical thicknesses were compared between groups, using two-tailed t tests on SPSS version 19.0 (SPSS, Chicago). The correlations of the global mean cortical thicknesses with demographic and clinical data were also assessed using Pearson’s correlation coefficient in SPSS. The statistical significance threshold was set at P < .05. For regional cortical thickness analysis, the thickness value at each vertex for each subject was mapped to the surface of an average brain template (http://surfer.nmr.mgh.harvard.edu/fswiki/FsAverage), and the cortical map of each subject was smoothed with a Gaussian kernel of 10mm full width at half maximum. Subsequently, the group comparison between healthy controls and schizophrenia patients was performed. The general linear model was implemented at each vertex in the whole brain to identify brain regions where schizophrenia patients showed significant differences in cortical thickness compared with healthy controls, using FreeSurfer’s mri_glmfit. The effects of age and gender were regressed out in these models. The analyses were performed for the right and left hemispheres separately. The cluster forming threshold and clusterwise significance threshold (for multiple comparison) were set at P < .05 by Monte Carlo simulation.
DTI Processing
All DTI data processing and statistical analysis were performed using the programs in FMRIB Software Library (FSL) ver. 4.1.4.30 All source data were corrected for eddy currents and head motion by registering all data to the first b = 0 image with affine transformation. The FA maps were calculated using the FMRIB’s Diffusion Toolbox (FDT) program. The tract-based spatial statistics ver. 1.2, which is part of the FSL program, was utilized to calculate average FA value for whole brain. First, all subjects’ FA data were normalized into a common space using the nonlinear registration tool FNIRT. Normalized FA images were averaged to create a mean FA image, which was then thinned to create a “skeleton,” taking only the centers of WM tracts common to all subjects. Voxel values of each subject’s FA map were projected onto the skeleton by searching the local maxima along the perpendicular direction from the skeleton. Skeleton mean FA for each subject was calculated using the fslstats program. Group comparison of this skeleton mean FA and its correlation with demographic and clinical data were performed using a two-tailed t test and Pearson’s correlation coefficients, respectively, in SPSS. The statistical significance level was set at P < .05.
Voxelwise permutation-based nonparametric inference31 was performed on skeletonized FA data, using FSL Randomize ver. 2.5. A group comparison was performed using an ANCOVA design, with age and gender as nuisance covariates. Both “healthy controls-schizophrenia” and “schizophrenia-healthy controls” contrasts were tested, with 10 000 permutations. Multiple comparisons were corrected using threshold-free cluster enhancement,32 and the significance level was set at P < .01.
Correlational Analyses of Cortical Thickness and Skeleton Mean FA
Subsequently, we investigated the correlations between global mean cortical thickness and skeleton mean FA, for both patients and controls on SPSS. Furthermore, in case the correlation between global mean cortical thickness and skeleton mean FA was significant, partial correlation coefficient was calculated. The demographic and clinical variables revealed to be correlated with global mean cortical thickness or skeleton mean FA were used as controlling factors.
Results
Demographic and Clinical Data
The demographic and clinical data are shown in table 1. The schizophrenia group consisted of mainly chronic patients with relatively mild symptoms. All of the schizophrenia patients were prescribed antipsychotics. Among our patients, 4 received typical antipsychotic medication, 22 received atypical antipsychotics, and 9 received both types of medication.
Table 1.
Demographic Data
| Hc (SD) | Scz (SD) | P-Value | |
|---|---|---|---|
| Number | 35 | 35 | — |
| Age | 37.51 (11.68) | 36.63 (10.31) | .74a |
| Gender (M/F) | 18/17 | 23/12 | .23b |
| Education (year) | 14.48 (2.20) | 13.66 (2.23) | .12a |
| PANSS negative factor | — | 16.09 (5.85) | — |
| Positive factor | — | 16.23 (5.54) | — |
| Activation factor | — | 7.43 (2.05) | — |
| Depressive factor | — | 8.31 (2.81) | — |
| Cognitive factor | — | 5.51 (1.62) | — |
| Medication (mg/day, haloperidol equivalent)c | — | 10.96 (8.45) | — |
| Duration of Illness (year) | — | 13.99 (10.52) | — |
Group Comparison of Cortical Thickness
The global mean cortical thicknesses of both hemispheres were significantly thinner in schizophrenia patients than in healthy controls (table 2).
Table 2.
Comparisons of Global Mean Cortical Thickness and Skeleton Mean FA Between Hc and Scz (Two-Tailed t Test)
| Hc | Scz | P-Value | |
|---|---|---|---|
| Global mean cortical thickness of GM in hemisphere (mm) | |||
| Left (SD) | 2.549 (0.105) | 2.478 (0.115) | .008* |
| Right (SD) | 2.553 (0.099) | 2.475 (0.123) | .005* |
| Skeleton mean FA in WM | |||
| 0.419 (0.149) | 0.408 (0.196) | .010* | |
Note: FA, fractional anisotropy; GM, gray matter; WM, white matter.
*Significant (P < .05).
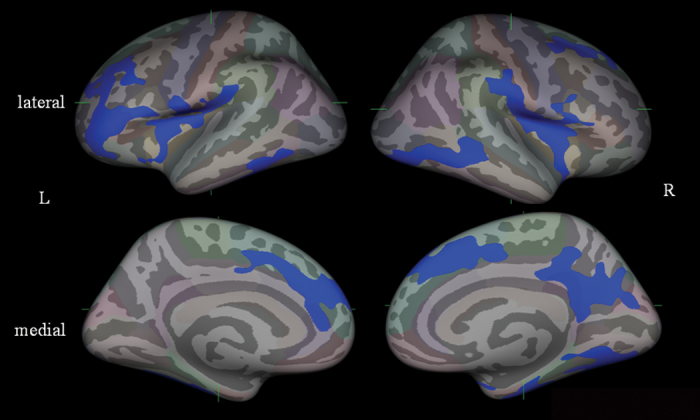
In the vertexwise analysis, the schizophrenia group exhibited reduced cortical thickness compared with the healthy group in several regions, including the bilateral superior to middle frontal gyri, bilateral insula, right precuneus region, and bilateral inferior temporal areas (figure 1). On the contrary, the healthy group had no cortical regions thinner than the schizophrenia group.
Fig. 1.
Statistical maps on FreeSurfer analysis of group comparison of gray matter cortical thickness between normal control and schizophrenia subjects (Monte Carlo simulation, P < .05). Maps are shown for right and left hemispheres in lateral and medical views, and significant reduction regions in schizophrenia are shown in blue color.
To check if the group difference found in global mean cortical thicknesses was caused by thinning in some specific region, we additionally calculated regional mean cortical thickness for each of the 5 cortical regions, namely, the frontal, parietal, temporal, and occipital lobes and cingulate cortex, according to Desikan et al.33 All regions, except for the left occipital lobe, were significantly thinner in schizophrenia patients than in healthy controls (P < .05; see online supplementary table 1).
Group Comparison of FA
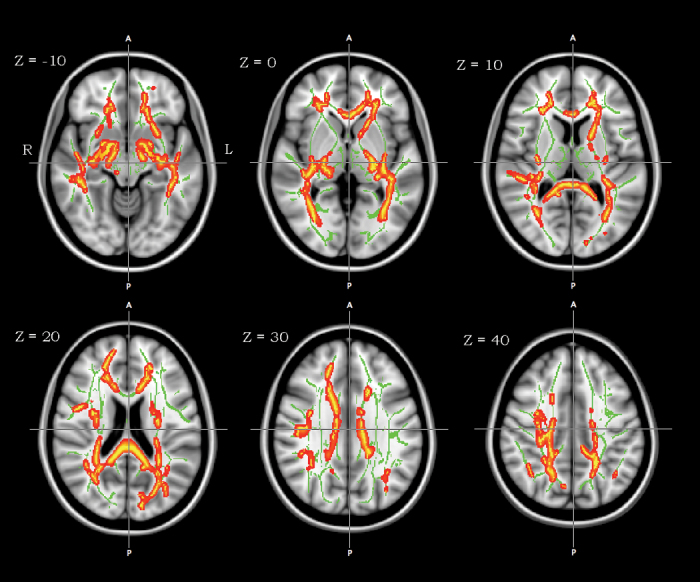
Skeleton mean FA was significantly lower in schizophrenia than in healthy controls (table 2). In the voxelwise analysis, there was a cluster of significant FA decreases in schizophrenia patients, which included widespread WM areas such as the CC, bilateral UF, corticospinal tracts, left SLF, and superior frontooccipital fasciculus (figure 2).
Fig. 2.
Tract-based spatial statistics group comparison of white matter fractional anisotropy (FA) between normal control and schizophrenia subjects (threshold-free cluster enhancement; P < .01). A cluster of significant FA reduction in schizophrenia subjects is shown using the tbss_fill implemented in FSL (red-yellow) on the mean FA map and FA skeleton (green color). Axial slices are from Z = −10 to 40 in Montreal Neurological Institute (MNI) coordinate. Left-right orientation is according to the radiological convention.
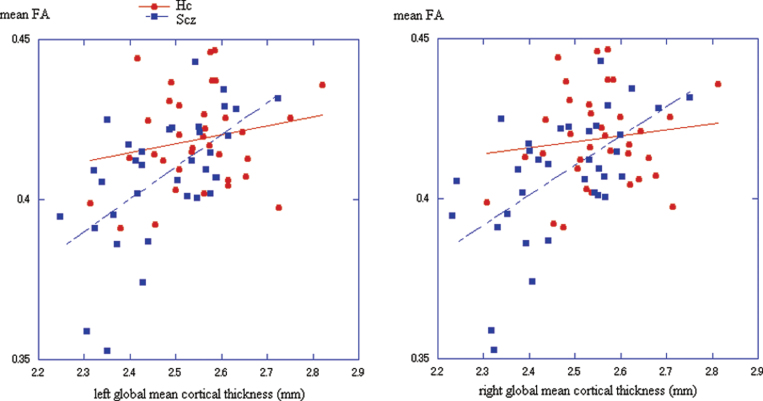
Correlation Between Global Mean Cortical Thickness and Skeleton Mean FA
In the schizophrenia group, the global mean cortical thickness of each hemisphere was significantly and positively correlated with the skeleton mean FA (table 3; figure 3). The global mean cortical thickness also showed significant correlation with age (left hemisphere r = −0.513, P = .002; right hemisphere r = −0.512, P = .002), and skeleton mean FA was significantly correlated with age (r = −0.566, P < .001), education (r = 0.532, P = .001), and duration of illness (r = −0.501, P = .002). None of the 5 subscales of PANSS were significantly correlated with the global mean cortical thickness or the skeleton mean FA. No other correlation with demographic or clinical variables was found for global mean cortical thickness or skeleton mean FA.
Table 3.
Correlational Analysis for Skeleton Mean FA With Global Mean Cortical Thickness in Hc and Scz
| Hc | Scz | |||
|---|---|---|---|---|
| Pearson’s Correlation Coefficient | P-Value | Pearson’s Correlation Coefficient | P-Value | |
| Global mean cortical thickness in left hemisphere | 0.199 | .25 | 0.587 | <.001* |
| Global mean cortical thickness in right hemisphere | 0.127 | .47 | 0.580 | <.001* |
*Significant (P < .05).
Fig. 3.
Scatter plot of the mean FA and global mean cortical thicknesses in healthy controls (Hc: red) and schizophrenia patients (Scz: blue) for each hemisphere.
Left panel: left hemisphere. Hc: r = 0.199, P = .25, Scz: r = 0.587, P < .001.
Right panel: right hemisphere. Hc: r = 0.127, P = .47, Scz: r = 0.580, P < .001.
The partial correlation between global mean cortical thickness and skeleton mean FA remained significant after controlling for age, education, and duration of illness (table 4).
Table 4.
Partial Correlational Analysis in the Correlation Between Global Mean Cortical Thickness and Skeleton Mean FA in Schizophrenia Group
| Correlation With Mean FA | No Items | + Age | + Age and Education | + Age, Education and Duration of Illness |
|---|---|---|---|---|
| Global mean cortical thickness in left hemisphere (P-value) | 0.587 (<.001)* | 0.420 (.014)* | 0.403 (.020)* | 0.400 (.023)* |
| Global mean cortical thickness in right hemisphere (P-value) | 0.580 (<.001)* | 0.410 (.016)* | 0.374 (.032)* | 0.368 (.038)* |
*Significant (P < .05).
By contrast, in the healthy group, there was no correlation between the global mean cortical thickness and skeleton mean FA. The global mean cortical thickness showed significant correlation with age (left hemisphere r = −0.631, P < .001; right hemisphere r = −0.620, P < .001). The skeleton mean FA was not significantly correlated with any demographic items.
Discussion
In this study we showed for the first time that global cortical thinning is positively correlated with global WM integrity reduction in schizophrenia. This finding supports the idea that GM and WM pathologies in schizophrenia are intertwined at the global level. It should be noted that a similar correlation was not found in normal populations, which suggests that this association is not a general occurrence in the human brain, such as in aging, but reflects a disease-specific pathological process.
As this is a macroscopic brain-imaging study, detailed discussion on the microstructural basis of these associations is difficult. However, recent neuropathological findings in schizophrenia, when considered in relation to our results, may bridge between 2 different levels. Global cortical thinning detected at a macroscopic level in our study may reflect the reduction of layer-specific neuropil density of pyramidal neurons,34 such as decreased density of dendritic spines on pyramidal neurons of cortical deep layer III revealed in the frontal regions of schizophrenia.35,36 On the other hand, reduced WM integrity at a macroscopic level may reflect reductions in the size and number of oligodendrocytes and disrupted myelination in schizophrenia.37
A principal mechanism controlling the formation and regression of synapses, which constitute the neuropil, is associated with the schizophrenia susceptibility gene products, such as disrupted-in-schizophrenia 1 (DISC1) and neuregulin (NRG)/ErbB, the latter of which modulates N-methyl-D-aspartate receptors (activation of which is involved in synapse formation and regression).20 On the other hand, loss of ErbB4 signaling leads to a decrease in the length of oligodendrocyte processes and thinner myelin sheaths.38 The intracellular ErbB family signaling cascade is triggered by NRG1, which is modulated by DISC1, and the alteration of these affects myelin synthesis, differentiation, and proliferation of oligodendrocytes. Importantly, disruption of normal myelination of axons has been shown to induce regression or failure of synapses in animal experiments.39,40 Thus, several schizophrenia susceptibility genes are involved in neurodevelopment, bridging between synapse formation/regression and oligodendrocyte function and myelination.41 Our results, at the macroscopic level, can be considered to support the existence of such coupled mechanisms between GM and WM. A schematic representation is shown in figure 4.
Fig. 4.
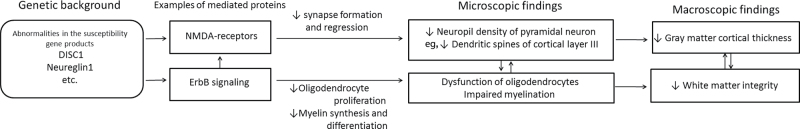
Schematic view of the hypothetical cascade inducing the pathology associated with schizophrenia, including the global association between cortical thinning and white matter integrity reduction. Note: DISC1, disrupted-in-schizophrenia 1; NMDA, N-methyl-D-aspartate.
Several previous studies have investigated the associations between GM and WM abnormalities in schizophrenia patients. Some of these15,22,25,42 focused on the regional GM and WM networks using structural MRI and DTI, while another43 combined functional MRI and DTI data. However, none of these studies have researched the pathological association between global GM and WM. Our study is the first to indicate the existence of the coupled mechanism between GM and WM mentioned above at the global level. In fact, both DISC1 and NRG1 are widely expressed in the animal44,45 and human brains,46–48 supporting our view.
We found an association between global cortical thickness and global WM integrity only for schizophrenia, suggesting that this association is a reflection of a disease-specific pathological process. However, if several candidate genes are commonly involved in the neurodevelopmental process of GM and WM, it is feasible that such a GM-WM association would also be found in a healthy population, albeit to a lesser extent. In fact, the low-pitched slopes for healthy controls in figure 3 give such an impression. On the other hand, the pathology of schizophrenia has traditionally been supposed to be some abnormal process that cannot be attributed to the variation in the normal sample. A study with a large sample size may be able to provide an answer to this question.
We did not find association between symptom severity and any imaging indices in this study. One possible interpretation of this is that the global association found in this study is not a “state” marker, but rather a “trait” marker of schizophrenia. On the other hand, schizophrenia patients show impairments in a broad range of cognitive functions such as general intelligence, working memory, and executive function. The global association found in this study may not underlie specific cognitive function impairments but may be the neural basis of the cognitive impairments in schizophrenia patients in general. We did not perform cognitive tasks in this study therefore we should be cautious about such speculation.
The global cortical thickness reduction in schizophrenia shown in this study is consistent with our previous study.10 The vertexwise analysis showed that such cortical thinning was most prominent in the prefrontal and temporal regions, again consistent with previous studies.10,11,49,50 The supplementary analysis of regional mean cortical thickness showed a diffuse pattern of reduction in cortical thickness in accordance with the results of a previous study,51 suggesting that the thinning of global cortical thickness was not due to an effect in some specific region but rather an effect that was global in nature. An extensive investigation of region-specific GM-WM association might be interesting in the future when the neurodevelopmental hypothesis proposed in this article turns out to be plausible.
Regarding WM analysis, FA was found to be reduced at the global level, consistent with an earlier study.52 Voxelwise analysis revealed that such an FA reduction was profound in widely distributed areas, including the CC, bilateral UF, bilateral corticospinal tracts, left SLF, and left superior frontooccipital fasciculus, largely compatible with previous studies.14–16,37
There are several limitations in this study. First, most of the schizophrenia participants had relatively mild symptoms and may not be representative of the general population of this disease. Second, all of the patients were taking antipsychotic medications. Although we confirmed that there was no significant correlation of medication level with either global cortical thickness or skeleton mean FA, we cannot exclude completely an effect of medication on brain morphology. Third, this study was a cross-sectional study, and not a longitudinal or cohort study. Therefore, it is unknown how these GM and WM changes occur during the development of schizophrenia pathology. Longitudinal studies, especially including high-risk populations, are needed in the future.
Despite of these limitations, our study is noteworthy because it reveals the global association of GM and WM pathologies in schizophrenia. Future studies will need to investigate the neurobiological mechanisms underlying such coupled abnormalities to better understand the pathophysiology of this disease.
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Funding
Ministry of Education, Culture, Sports, Science and Technology of Japan (Scientific Research B 23390290 and S 22220003 for T.M., Scientific Research on Innovative Areas 23118004 for T.M. and 23120009 for H.T.); Japan Society for the Promotion of Science (B 23791329 for J.M.); Mitsubishi Pharma Research Foundation (for T.M.); Uehara Memorial Foundation (for J.M.); NeuroCreative Lab (for T.M.); Takeda Science Foundation (for T.M.); Research Group for Schizophrenia, Japan (for T.M.).
Supplementary Material
Acknowledgments
The authors wish to extend their gratitude to Ms Miho Yoshizumi, Ms Shiho Ubukata, and Drs Mitsuaki Shimizu and Keita Uéda for their assistance in data acquisition and processing, to Dr Akiko Hayashi for assistance in statistical analyses, and most of all, to the patients and volunteers for participating in the study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005; 162: 2233–2245 [DOI] [PubMed] [Google Scholar]
- 2. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008; 64: 774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008; 165: 1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010; 53: 1135–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ehrlich S, Brauns S, Yendiki A, et al. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr Bull. 2012; 38: 1050–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J Anat. 2010; 217: 324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000; 57: 65–73 [DOI] [PubMed] [Google Scholar]
- 8. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999; 9: 179–194 [DOI] [PubMed] [Google Scholar]
- 9. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999; 9: 195–207 [DOI] [PubMed] [Google Scholar]
- 10. Kubota M, Miyata J, Yoshida H, et al. Age-related cortical thinning in schizophrenia. Schizophr Res. 2011; 125: 21–29 [DOI] [PubMed] [Google Scholar]
- 11. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003; 60: 878–888 [DOI] [PubMed] [Google Scholar]
- 12. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994; 66: 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009; 108: 3–10 [DOI] [PubMed] [Google Scholar]
- 14. Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007; 41: 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miyata J, Yamada M, Namiki C, et al. Reduced white matter integrity as a neural correlate of social cognition deficits in schizophrenia. Schizophr Res. 2010; 119: 232–239 [DOI] [PubMed] [Google Scholar]
- 16. Seal ML, Yücel M, Fornito A, et al. Abnormal white matter microstructure in schizophrenia: a voxelwise analysis of axial and radial diffusivity. Schizophr Res. 2008; 101: 106–110 [DOI] [PubMed] [Google Scholar]
- 17. Walterfang M, Wood SJ, Velakoulis D, Pantelis C. Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neurosci Biobehav Rev. 2006; 30: 918–948 [DOI] [PubMed] [Google Scholar]
- 18. Fujiwara H, Namiki C, Hirao K, et al. Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res. 2007; 95: 215–222 [DOI] [PubMed] [Google Scholar]
- 19. Friston KJ. The disconnection hypothesis. Schizophr Res. 1998; 30: 115–125 [DOI] [PubMed] [Google Scholar]
- 20. Benett J. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Prog Neurobiol. 2011; 95: 275–300 [DOI] [PubMed] [Google Scholar]
- 21. Karlsgodt KH, Sun D, Jimenez AM, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008; 20: 1297–1327 [DOI] [PubMed] [Google Scholar]
- 22. Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007; 130: 2375–2386 [DOI] [PubMed] [Google Scholar]
- 23. Miyata J, Hirao K, Namiki C, et al. Reduced white matter integrity correlated with cortico-subcortical gray matter deficits in schizophrenia. Schizophr Res. 2009; 111: 78–85 [DOI] [PubMed] [Google Scholar]
- 24. Spoletini I, Cherubini A, Di Paola M, et al. Reduced fronto-temporal connectivity is associated with frontal gray matter density reduction and neuropsychological deficit in schizophrenia. Schizophr Res. 2009; 108: 57–68 [DOI] [PubMed] [Google Scholar]
- 25. Koch K, Schultz CC, Wagner G, et al. Disrupted white matter connectivity is associated with reduced cortical thickness in the cingulate cortex in schizophrenia. Cortex. 2012;. 10.1016/j.cortex.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 26. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13: 261–276 [DOI] [PubMed] [Google Scholar]
- 27. Lançon C, Aghababian V, Llorca PM, Auquier P. Factorial structure of the Positive and Negative Syndrome Scale (PANSS): a forced five-dimensional factor analysis. Acta Psychiatr Scand. 1998; 98: 369–376 [DOI] [PubMed] [Google Scholar]
- 28. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999; 9: 179–194 [DOI] [PubMed] [Google Scholar]
- 29. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999; 9: 195–207 [DOI] [PubMed] [Google Scholar]
- 30. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23: Suppl 1 S208–S219 [DOI] [PubMed] [Google Scholar]
- 31. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002; 15: 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009; 44: 83–98 [DOI] [PubMed] [Google Scholar]
- 33. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006; 31: 968–980 [DOI] [PubMed] [Google Scholar]
- 34. Lewis DA. Neuroplasticity of excitatory and inhibitory cortical circuits in schizophrenia. Dialogues Clin Neurosci. 2009; 11: 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006; 81: 47–63 [DOI] [PubMed] [Google Scholar]
- 36. Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005; 162: 1200–1202 [DOI] [PubMed] [Google Scholar]
- 37. Walter H, Ciaramidaro A, Adenzato M, et al. Dysfunction of the social brain in schizophrenia is modulated by intention type: an fMRI study. Soc Cogn Affect Neurosci. 2009; 4: 166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roy K, Murtie JC, El-Khodor BF, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci USA. 2007; 104: 8131–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Höistad M, Segal D, Takahashi N, Sakurai T, Buxbaum JD, Hof PR. Linking white and grey matter in schizophrenia: oligodendrocyte and neuron pathology in the prefrontal cortex. Front Neuroanat. 2009; 3: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011; 93: 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karlsgodt KH, Sun D, Jimenez AM, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008; 20: 1297–1327 [DOI] [PubMed] [Google Scholar]
- 42. Qiu A, Tuan TA, Woon PS, Abdul-Rahman MF, Graham S, Sim K. Hippocampal-cortical structural connectivity disruptions in schizophrenia: an integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. Neuroimage. 2010; 52: 1181–1189 [DOI] [PubMed] [Google Scholar]
- 43. Sui J, Pearlson G, Caprihan A, et al. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage. 2011; 57: 839–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schurov IL, Handford EJ, Brandon NJ, Whiting PJ. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry. 2004; 9: 1100–1110 [DOI] [PubMed] [Google Scholar]
- 45. Chen MS, Bermingham-McDonogh O, Danehy FT, Jr, et al. Expression of multiple neuregulin transcripts in postnatal rat brains. J Comp Neurol. 1994; 349: 389–400 [DOI] [PubMed] [Google Scholar]
- 46. James R, Adams RR, Christie S, Buchanan SR, Porteous DJ, Millar JK. Disrupted in Schizophrenia 1 (DISC1) is a multicompartmentalized protein that predominantly localizes to mitochondria. Mol Cell Neurosci. 2004; 26: 112–122 [DOI] [PubMed] [Google Scholar]
- 47. Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006; 497: 436–450 [DOI] [PubMed] [Google Scholar]
- 48. Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004; 127: 125–136 [DOI] [PubMed] [Google Scholar]
- 49. Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009; 66: 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schultz CC, Koch K, Wagner G, et al. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010; 116: 204–209 [DOI] [PubMed] [Google Scholar]
- 51. Crespo-Facorro B, Roiz-Santiáñez R, Pérez-Iglesias R, et al. Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med. 2011; 41: 1449–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White T, Magnotta VA, Bockholt HJ, et al. Global white matter abnormalities in schizophrenia: a multisite diffusion tensor imaging study. Schizophr Bull. 2011; 37: 222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Inagaki A, Inada T. Dose equivalence of psychotropic drugs. Part xxi. Dose equivalence of novel antipsychotics: blonanserin. Jpn J Clin Psychopharmacol. 2008; 11: 887–890 [Google Scholar]
- 54. Inagaki A, Inada T. Dose equivalence of psychotropic drugs. Part xxii. Dose equivalence of depot antipsychotics iii: risperidone long-acting injection. Jpn J Clin Psychopharmacol. 2010; 13: 1349–1353 [Google Scholar]
- 55. Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004; 161: 1–56 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.