Kobrynski et al. [1] showed gastrointestinal complaints in children can be an early sign of primary immunodeficiency disease (PIDD). Rotavirus infection associated with PIDD can be life-threatening. Rotavirus is a leading cause of childhood gastrointestinal disease worldwide [2]. Most rotavirus disease is caused by 5 G types (G1-G4 and G9) and 3 P types (P1A[8], P1B[4], and P2A[6]) [2]. Two live oral rotavirus vaccines, RotaTeq® (5 different human-bovine reassortant rotavirus strains; Merck and Co, Whitehouse Station, NJ) and Rotarix® (1 human rotavirus strain; GlaxoSmithKline, Rixensart, Belgium) are recommended for routine immunization of US infants [2]. Disease caused by emerging worldwide rotavirus type, G9P[8] may be prevented by both vaccines although this strain is not included in either vaccine.
Severe combined immunodeficiency (SCID) is characterized by lack of T cells and life-threatening infections [3]. Treatment of viral infections prior to hematopoietic stem cell transplantation (HSCT) with intravenous immunoglobulin (IVIG) and antivirals has been attempted, but persistence of viral disease has been reported [3–8]. We report a 7 month-old male infant with SCID who had persistent, nonvaccine-associated rotavirus gastroenteritis and viremia despite oral and IVIG administration. T-cell engraftment following HSCT possibly helped by oral and IVIG was necessary to eliminate rotavirus infection.
The full-term, formula-fed infant received RotaTeq at 2 and 4 months of age. The patient developed chronic intermittent diarrhea at 2 months of age and was hospitalized at 7 months of age with respiratory distress, diarrhea, and failure to thrive. A peripheral white blood cell count was 16,140 cells/μl with 59% neutrophils, 17% lymphocytes (absolute lymphocyte count=2743cells/μl [normal range 3,900–9,000]), 7% monocytes, and 13% eosinophils. Bronchoscopy aspirate revealed Pneumocystis jiroveci. Stool for rotavirus was positive by electron microscopy (EM). Immunoglobulins were very low including IgG 77 mg/dL (normal range 184–974 mg/dL), IgA <6 mg/dL (normal range 9–107 mg/dL), and IgM 36 mg/dL (normal range 41–197 mg/dL). The CD3+T cells were severely low (32 cells/mm3, normal range 1919–5054 cells/mm3), CD19+B cells were elevated (2715 cells/mm3, normal range 566–2535 cells/mm3), and CD3−CD56+CD16+NK cells were low (28 cells/mm3, normal range 181–901 cells/mm3). T-cell proliferation to mitogens was markedly depressed. A hemizygous mutation (nucleotide substitution A for G at position 1451 in the polyA tail region) was present in the common gamma chain of interleukin-2 receptor consistent with X-linked SCID. Multipe doses of IVIG (Gamunex®, Talecris) were given before and after transplantation, including two doses of 300 mg/kg administered orally at 8 months of age (Fig. 1). Molecular analysis of stool and serum specimens identified a non-vaccine associated human rotavirus strain G9P[8]. The patient received a 10/10 matched unrelated donor unfractionated HSCT with pretransplant myeloablative conditioning at 9.5 months of age. Rotavirus-positive diarrhea persisted until 2 months post transplant (age 11.5 months), coincident with T-cell engraftment (Fig. 1). The patient, last tested at 14.5 months of age, had no detectable rotavirus.
Figure 1.
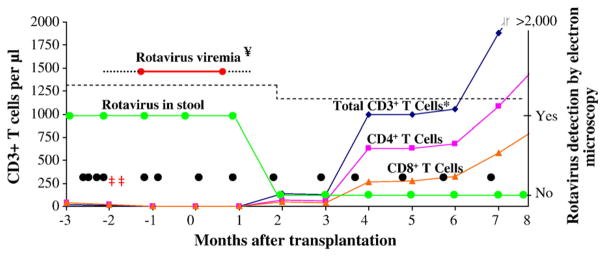
The detection of rotavirus in relation to the presence of CD3+,CD4+, and CD8+T-cell quantification before and after bone marrow transplantation. *=CD3+T cells were 100% donor origin calculated by short tandem repeat studies; ¥=Several serum samples were positive for rotavirus by RT-PCR prior to transplantation; ‡‡=Oral IG administered in 2 separate doses; • =IVIG dose; (···········)=negative rotavirus viremia. The 10th percentile normal values for age for CD3+T cells is represented by (– – – – – –) [11].
Reverse transcriptase polymerase chain reaction (RT-PCR) using rotavirus gene 9 and gene 4 primer sets resulted in cDNA products from stool and serum samples. Homology of gene 9 and gene 4 amplicon sequences to GenBank database sequences confirmed the patient’s stools contained rotavirus strain G9P[8]. There was 98% nucleotide homology between the stool rotavirus gene 4 sequence, which comprised 51% of the 2328 nt ORF, and two fully-sequenced P[8] rotaviruses but no significant homology with a partial RotaTeq vaccine gene 4 sequence. There was 98% nucleotide homology between the stool rotavirus gene 9 sequence, which comprised 85% of the 978 nt ORF, and two fully-sequenced G9 rotaviruses. There was a single nucleotide change in gene 9 (residue 595 C→T, resulting in a silent mutation) between two stools obtained 74 days apart. There was no change in gene 4 sequence between stools obtained 54 days apart.
Neutralizing antibodies to rotavirus G9 were present in the orally administered immunoglobulin product at a concentration of 1:1600. Neutralizing antibodies to serotypes G1(WA, 1:800; K8, 1:1600) and G3 (SA11, 1:3200) were present at similar concentrations.
CD3+T cells were very low (32 cells/ml, normal range 2500–6500 cells/ml) prior to transplantation (Fig. 1). Rotavirus became undetectable by EM two months post transplantation with CD3+, CD4+, and CD8+T-cell engraftment as shown by return of lymphocytes by 65 days post transplantation (CD3+T cells=138/mm3 at 2 months post transplantation). T-cell proliferation, as assessed by response to mitogens, was <3% of normal range and became present at five months post transplantation (data not shown). Chimerism analysis showed presence of donor T cells (100%) and absence of donor B cells (0%) at two and seven months post transplantation.
1. Discussion
We report a SCID infant with persistent rotavirus infection for whom HSCT resulted in T-cell engraftment and clearance of rotavirus despite absent donor B cells. Prior to transplantation, rotavirus infection persisted despite oral administration of immunoglobulin containing neutralizing antibodies to G9 serotype. The presence of neutralizing antibodies to G9 serotype in immunoglobulin preparations suggests exposure to G9 rotavirus serotype among the donor pool, or cross-reactivity among antibodies of rotavirus serotypes other than G9. Oral administration of immunoglobulin for treatment of rotavirus gastroenteritis has been reported with some success [9,10].
Lessons to be learned from this case are 1) gastrointestinal problems can be a presenting sign of PIDD as shown by Kobrynski et al. [1], 2) clearance of rotavirus was associated with T-cell engraftment and function, 3) oral IG despite containing neutralizing antibodies to G9, and IVIG prior to transplant did not eliminate infection, 4) rotavirus infection was associated with an emerging serotype, and 5) rotavirus infection did not arise from the vaccine possibly due to protection from maternal antibody.
In summary, this case highlights that T-cell engraftment and function appear to have been necessary for clearance of rotavirus and that oral IG, despite containing neutralizing antibodies to G9, and IVIG, did not eliminate the chronic infection prior to transplant. Secondly, rotavirus infection was associated with emerging serotype G9, so protective maternal antibody may not have been present, making this infant particularly vulnerable to this strain. Infection did not arise from the live rotavirus vaccine, however, possibly due to protection from maternal antibody.
Acknowledgments
We thank the Texas Children’s Hospital Allergy and Immunology Laboratory for immunologic analysis. The research contained in this report was supported in part by an unrestricted fund (to M.K.E.), by NIH K12 HD41648 (to P.M.H.), and by NIH P30 DK 56338.
This is a commentary on article Kobrynski LJ, Mayer L. Diagnosis and treatment of primary immunodeficiency disease in patients with gastrointestinal symptoms. Clin Immunol. 2011 Jun;139(3):238-48.
Footnotes
Conflict of interest
All listed authors for this study have no conflicts of interest.
Contributor Information
Niraj C. Patel, Department of Pediatrics, Section of Infectious Disease and Immunology, Levine Children’s Hospital at Carolinas Medical Center, Charlotte, NC
Paula M. Hertel, Department of Pediatrics, Section of Gastroenterology, Baylor College of Medicine, Houston, TX, USA
Imelda C. Hanson, Department of Pediatrics, Section of Allergy and Immunology, Baylor College of Medicine, Houston, TX, USA
Robert A. Krance, Department of Pediatrics, Section of Hematology and Oncology, Baylor College of Medicine, Houston, TX, USA
Sue E. Crawford, Department of Virology and Microbiology, Baylor College of Medicine, Houston, TX, USA
Mary Estes, Department of Virology and Microbiology, Baylor College of Medicine, Houston, TX, USA.
Mary E. Paul, Department of Pediatrics, Section of Retrovirology and Global Health, Baylor College of Medicine, Houston, TX, USA
References
- 1.Kobrynski LJ, Mayer L. Diagnosis and treatment of primary immunodeficiency disease in patients with gastrointestinal symptoms. Clin Immunol. 2011;139:238–248. doi: 10.1016/j.clim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg HB, Estes MK. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitiution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 4.Patel NC, Hertel PM, Estes MK, et al. Vaccine-acquired rotavirus disease in infants with severe combined immunodeficiency. N Engl J Med. 2010;362:314–319. doi: 10.1056/NEJMoa0904485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saulsbury FT, Winkelstein JA, Yolken RH. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980;97:61–65. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- 6.Werther RL, Crawford NW, Bonifice K, Kirkwood CD, Smart JM. Rotavirus vaccine induced diarrhea in a child with severe combined immune deficiency. J Allergy Clin Immunol. 2009;124:600. doi: 10.1016/j.jaci.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Uygungil B, Bleesing JJ, Risma KA, McNeal MM, Rothenberg ME. Persistent rotavirus vaccine-shedding in a new case of severe combined immunodeficiency: A reason to screen. J Allergy Clin Immunol. 2010;125:270–271. doi: 10.1016/j.jaci.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Eiden J, Losonsky GA, Johnson J, Yolken RH. Rotavirus RNA variation during chronic infection of immunocompromised children. Pediatr Infect Dis. 1985;4:632–637. doi: 10.1097/00006454-198511000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Guarino A, Canani RB, Russo S, et al. Oral immmunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics. 1994;93:12–16. [PubMed] [Google Scholar]
- 10.Sarker SA, Casswall TH, Mahalanabis D, et al. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrums. Pediatr Infect Dis J. 1998;17:1149–1154. doi: 10.1097/00006454-199812000-00010. [DOI] [PubMed] [Google Scholar]


