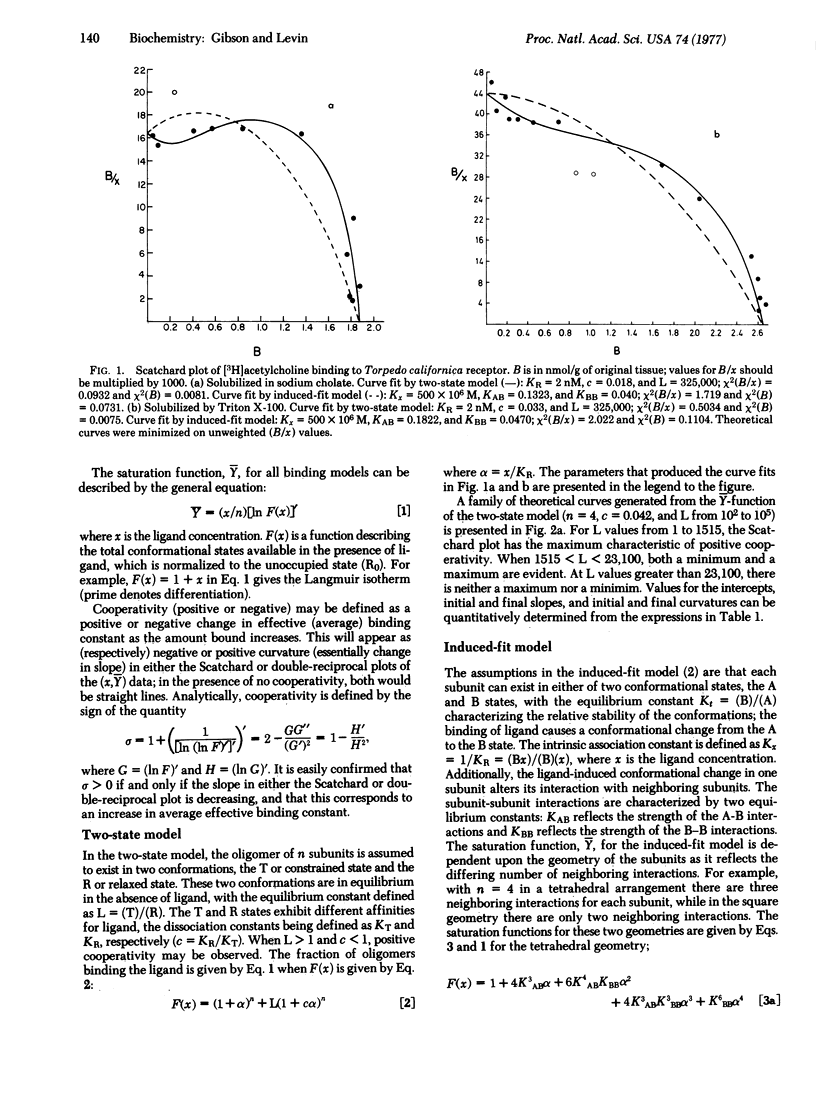
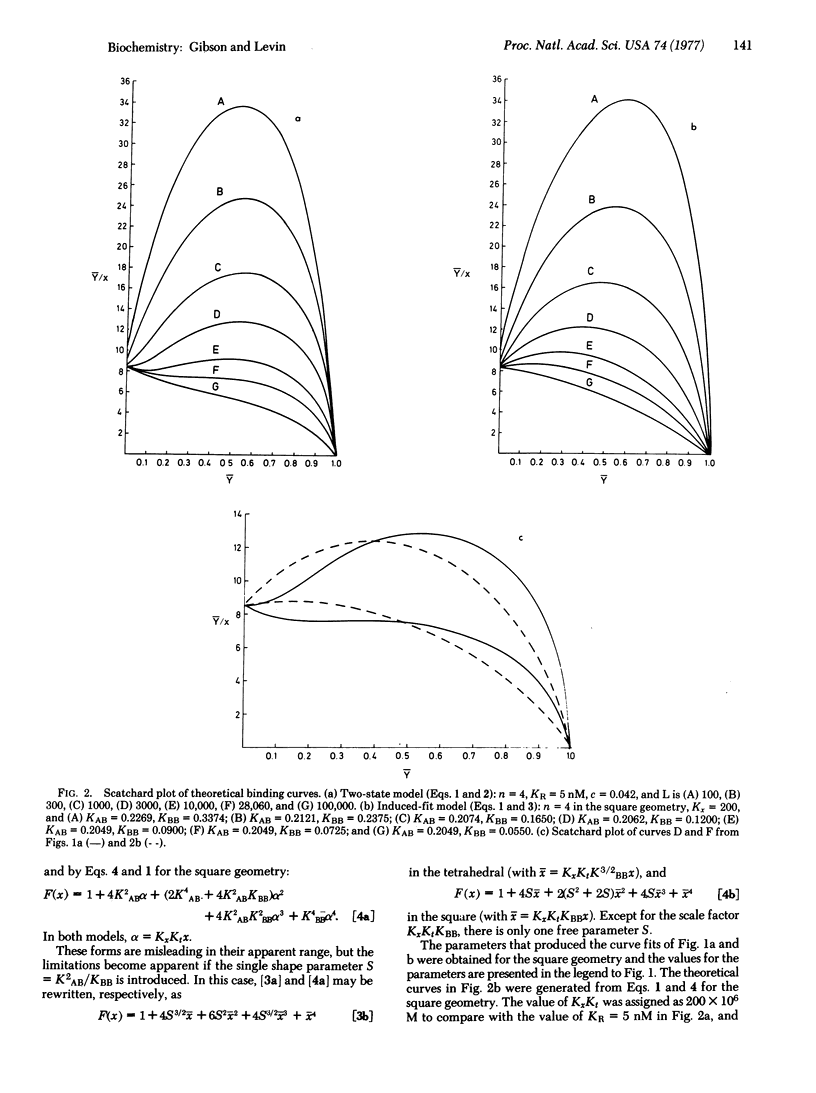
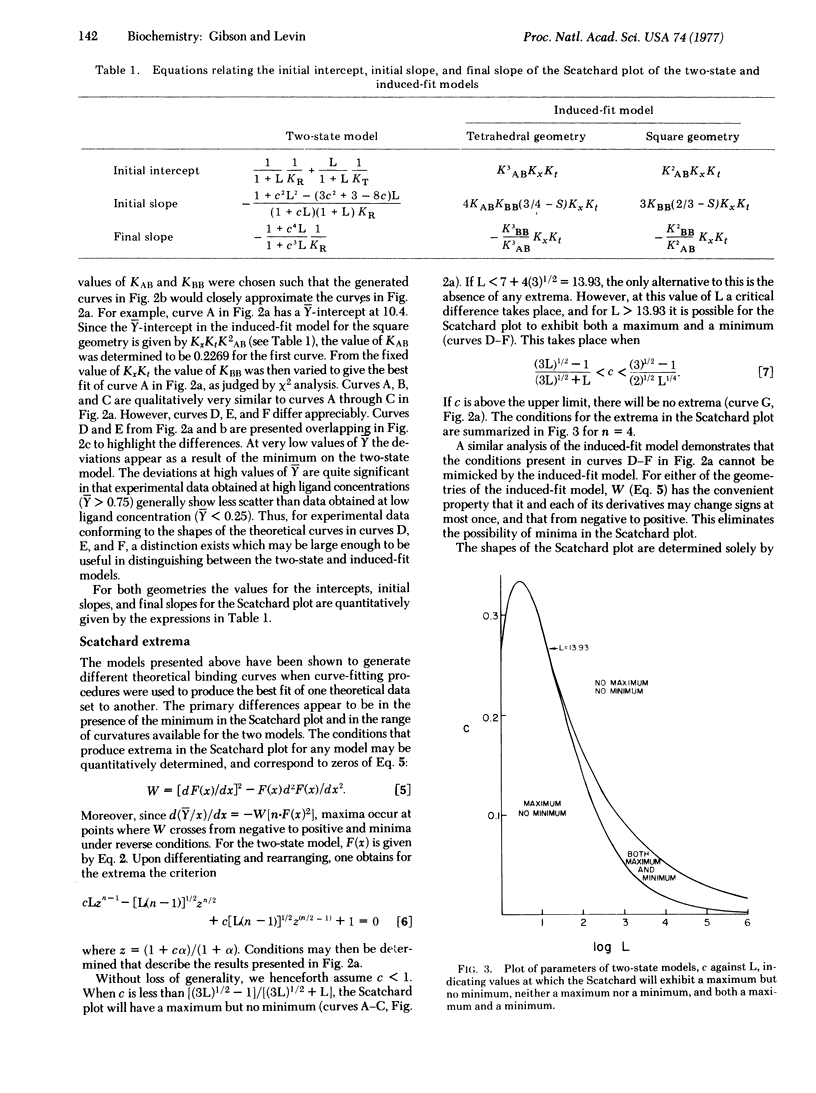
Abstract
The two-state and sequential models for positive cooperativity in ligand binding can produce significantly different theoretical binding curves when presented in a Scatchard plot. The conditions that produce the greatest differences have been examined. The theoretical differences have been used to select the two-state model as the best model for describing the binding of acetylcholine to acetylcholine receptors that have been solubilized by Triton X-100 and sodium cholate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish-Bowden A., Koshland D. E., Jr A general method for the quantitative determination of saturation curves for multisubunit proteins. Biochemistry. 1970 Aug 18;9(17):3325–3336. doi: 10.1021/bi00819a006. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J. Cooperative interactions of hemoglobin. Annu Rev Biochem. 1975;44:209–232. doi: 10.1146/annurev.bi.44.070175.001233. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J. Extensions of the allosteric model for hemoglobin. II. Consequences of functional nonequivalence of the alpha and beta chains. Biochemistry. 1974 Nov 19;13(24):4998–5002. doi: 10.1021/bi00721a020. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Wu C. W. Kinetics of allosteric enzymes. Annu Rev Biophys Bioeng. 1974;3(0):1–33. doi: 10.1146/annurev.bb.03.060174.000245. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mockrin S. C., Byers L. D., Koshland D. E., Jr Subunit interactions in yeast glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Dec 16;14(25):5428–5437. doi: 10.1021/bi00696a008. [DOI] [PubMed] [Google Scholar]
- O'Brien R. D., Gibson R. E. Conversion of high affinity acetylcholine receptor from Torpedo californica electroplax to an altered form. Arch Biochem Biophys. 1975 Aug;169(2):458–463. doi: 10.1016/0003-9861(75)90188-5. [DOI] [PubMed] [Google Scholar]
- Pauling L. The Oxygen Equilibrium of Hemoglobin and Its Structural Interpretation. Proc Natl Acad Sci U S A. 1935 Apr;21(4):186–191. doi: 10.1073/pnas.21.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waygood E. B., Mort J. S., Sanwal B. D. The control of pyruvate kinase of Escherichia coli. Binding of substrate and allosteric effectors to the enzyme activated by fructose 1,6-bisphosphate. Biochemistry. 1976 Jan 27;15(2):277–282. doi: 10.1021/bi00647a006. [DOI] [PubMed] [Google Scholar]


