Abstract
Type 1 diabetes (T1D) results from autoimmune destruction of pancreatic β-cells. Although Th1 cells are key orchestrators of T1D, the function(s) of the more recently identified Th17 subset are unclear due to inherent plasticity. Herein we analyzed Th17 cells for stability and diabetogenicity in NOD mice. We found that like Th1 cells, Th17 are a distinct population throughout the pre-diabetic phase. At diabetes onset there were marked increases in IL-17-producing Th17 cells and IFNγ-producing Th1 cells in the pancreas as well as in the serum levels of these cytokines, indicating that these proinflammatory mediators serve as biomarkers of advanced autoimmunity. Although naturally occurring Th17 cells in diabetic mice did not contribute to diabetes development in transfer models, islet-specific Th17 cells were diabetogenic independently of IL-17 and displayed inflammation-induced Th17-to-Th1 reprogramming that could be elicited by Th1 cells. However, an inability to generate Th1 cells because of Stat4, Ifngr, and Ifng deficiencies did not prevent diabetes. Instead, TNFα could mediate diabetes in response to either Th17 cells or Th1 cells. The results identify a previously unknown mechanism by which Th17 cells can contribute to T1D. Our studies also suggest that when developing interventions for T1D, it will be potentially advantageous to focus on mechanisms common to T cell effectors than on the signature cytokines of various subsets.
Keywords: Type 1 diabetes (T1D), autoimmune disease, Th17, reprogramming, TNFα
Introduction
In type 1 diabetes (T1D), tolerance to pancreatic islet β-cells is lost due to genetic and environmental factors (1). Although multiple cell types participate in the pathogenesis of T1D, CD4+ T cells, Th1 cells, which produce the signature cytokine IFNγ, play a key role in orchestrating the autoimmune response in both rodent models and human patients (2). However, in the non-obese diabetic (NOD) mouse model of T1D, genetic deficiencies in the expression of IFNγ or IFNγR did not prevent diabetes onset (3–5), suggesting that Th1 cells may be dispensable. In other autoimmune diseases, such as the EAE model of multiple sclerosis, IFNγ deficiency not only failed to prevent disease, but exacerbated the pathology (6). Instead, the more recently identified Th17 subset, that produces IL-17A as a signature cytokine (7), is thought to play a dominant role (8, 9). Whether Th17 cells contribute to T1D is unclear and controversial.
Early studies implying pathogenicity of Th17 cells in T1D showed that treatment of NOD mice with neutralizing anti-IL-17 antibody or IL-25, which antagonizes Th17 differentiation in vivo, prevented development of diabetes (10). Furthermore, studies of T1D patient samples showed an elevated population of peripheral blood monocytes that could promote Th17 cell differentiation (11). Elevated levels of IL-17 and/or IL-17-producing CD4+ T cells in the peripheral blood and pancreatic lymph nodes (PLN) of T1D patients have also been reported (12–15). Conversely, under certain conditions, the in vivo induction of Th17 cells has been associated with protecting NOD mice from diabetes progression (16, 17). A complicating issue is the inherent plasticity of Th17 cells. Th17 cells can be reprogrammed into IFNγ-producing Th1-like cells (18), and in some systems, especially with human Th17 cells, the co-expression of IL-17 and IFNγ appears to mark the most pathogenic cells (19, 20). Both Th1 driving IL-12 and Th17-promoting IL-23 can be important for this coexpression (21, 22). The plasticity of Th17 cells has confounded efforts to elucidate their function(s) in T1D in part because the induction of diabetes in NOD.Scid recipients by in vitro differentiated islet antigen-specific Th17 cells coincided with their acquisition of a Th1 phenotype (23, 24). It is not yet clear if this reprogramming is required for disease induction or if it is instead a byproduct of the immune/inflammatory response.
To complicate the issue further, each known Th subset produces multiple cytokines, and their functions may not necessarily depend only on the respective “signature cytokine(s)”. For instance, recent studies identified GM-CSF as a key effector cytokine of Th17 cells in EAE (25, 26). It is thus possible that although IL-17, like IFNγ, may contribute to the inflammatory processes in T1D, other cytokines could ultimately be more critical for the pathogenesis leading to islet damage and β-cell death.
In this study, we analyzed both Th1 and Th17 populations, defined by the production of IFNγ and IL-17, respectively, during the spontaneous progression to diabetes in NOD mice. In parallel, we analyzed both in vivo and in vitro developed Th17 cells, including two different islet antigen-specific TCR Tg Th17 cells, for their diabetogenic potential, stability, and the requirements for IFNγ and IL-17 for diabetes induction. Our results show that discrete subsets of IL-17 or IFNγ producing CD4+ T cells are found early in the autoimmune process and that these cytokines can serve as biomarkers of advanced disease. However, IL-17 is not required for progression to diabetes and inflammation could support reprogramming of Th17 cells to Th1 cells to a differing extent, depending upon the TCR. When Th1 development was prevented, TNFα, but not IL-17 could mediate the pathogenicity of islet-specific Th17 cells. For Th1 cells, blocking TNFα was also sufficient to prevent development of diabetes. The data indicate that although both Th1 and Th17 cells can elicit T1D independently of their signature cytokines, the impact of Th17 cells to T1D onset can be limited by the overwhelming presence of Th1 cells in the pancreas as well as by a potentially more constrained overall pathogenicity in vivo.
Materials and methods
Mice
NOD, NOD.Scid, NOD.Thy1.1, NOD.CD45.2, and NOD.Stat4−/− mice were obtained from the Jackson Laboratory. NOD.BDC2.5 TCR transgenic, NOD.Ifng−/−, NOD.Ifngr−/− mice were from the Genetically Modified NOD Mouse Core at Harvard Medical School. NOD.BDC6.9 TCR transgenic mice were a gift from Dr. Kathryn Haskins (University of Colorado, Denver, CO). The TCR transgenic lines were crossed to NOD.Thy1.1 mice. NOD.Stat4−/−, NOD.Ifng−/−, and NOD.Ifngr−/− mice were crossed with NOD.BDC2.5 mice. NOD.Stat4−/− and NOD.Ifngr−/− mice were crossed to generate a double gene-deficient line. All animals were maintained in a specific pathogen free facility at Sanford-Burnham Medical Research Institute (SBMRI). Only female mice were used. All experiments were approved by the Institutional Animal Care and Use Committee of SBMRI.
Differentiation of effector T cells in vitro
CD4+ T cells were isolated from the lymphoid tissues of 6–8 wk old mice using EasySep kits (StemCell Technologies) according to the manufacturer’s instructions, except that CD25+ nTregs and γδ T cells were also depleted during the process. Purified CD4+ T cells were cultured in 6-well plates coated with anti-CD3 (5µg/ml, clone 2c11, BioLegend) and anti-CD28 (5µg/ml, clone 37.51, BioLegend) with complete RPMI-1640 medium for 5 days. For Th1 differentiation, the cultures were supplemented with anti-IL-4 (Frederick National Laboratory) (10µg/ml), rIL-12 (R&D Systems) (5ng/ml), and rIL-2 (Frederick National Laboratory) (200units/ml). For Th17 differentiation, the cultures were supplemented with anti-IL-4 (10µg/ml), anti-IFNγ (10µg/ml, purified in house), rTGFβ1 (2ng/ml), rIL-6 (20ng/ml), rIL-1β (10ng/ml), and rIL-23(5ng/ml) (BioLegend). After 5 days of culture, the cells were rested in complete medium containing rIL-7 (10ng/ml, Frederick National Laboratory) for 2 days before purification and cell transfer.
Th17 cell purification
Th17 cells were identified by surface staining of IL-17A and purified by FACS sorting, using a method modified from a recent report (27). Briefly, in vitro differentiated Th17 cells or enriched ex vivo CD4+ T cells were stimulated with PMA (Sigma-Aldrich) (25ng/ml) and ionomycin (0.5µg/ml, Sigma-Aldrich) in complete medium for 3 hours. After washing, the cells were stained with APC-cojugated anti-CD4 antibody (clone RM4-5, BioLegend) and PE-conjugated anti-IL-17 antibody (Clone TC11-18H10.1, BioLegend). The anti-IL-17 antibody was used at 2µg/ml concentration. CD4+IL-17surface+ and CD4+IL-17surface− cells were then sorted by FACS (Supplemental Fig.1).
Adoptive transfer
In vitro differentiated effector CD4+ T cells or ex vivo cells isolated from diabetic NOD mice were transferred into NOD, NOD.Scid, or NOD.Ifng−/− recipients by i.v. injection in doses of 0.2-1×106 as indicated for the individual experiments. In some experiments, anti-IL-17, anti-TNFα, or anti-IFNγ (BioXcell or purified in house), were injected i.p. at indicated doses and times. Control mouse and rat IgG were purchased from Jackson ImmunoResearch Laboratories Inc. Diabetes incidence was monitored by blood glucose test using Bayer’s Contour meters and strips (Bayer); two consecutive readings of higher than 300mg/dl were considered indicative of diabetes.
Flow cytometry
The conjugated antibodies for FACS were purchased from BioLegend except for anti-T-Bet (clone 4B10) and RORγ (clone B2D), which were purchased from eBioscience. To recover cells from the pancreas, the whole organ was minced and treated with colagenase P (Roche) followed by mechanic disruption and filtering through a cell strainer to obtain a single cell suspension. Lymphocytes were then harvested by gradient centrifugation over Histopique-1077 (Sigma). For intracellular cytokine staining, cells were restimulated with PMA (50ng/ml) and ionomycin (1µg/ml) with GolgiPlug™ (BD Biosciences) for 4 hours. Cells were stained for surface markers, fixed and permeabilized, and finally stained with anti-cytokine antibodies. In some experiments, anti-T-Bet and/or anti-RORγ were included to stain these transcription factors. FACS data were collected on FACS Calibur or LSR-Fortessa (BD). FACS data were analyzed using FlowJo software (Tree Star).
Cytokine assays
Blood samples were collected when the animals were dissected. Serum was separated after natural blood coagulation in vitro, and stored at −80°C. Cytokine levels were determined by Luminex assays with multiplex reagent kits from Millipore according to manufacturer’s instructions.
Histology
After dissection, pancreata were fixed with 4% paraformaldehyde at 4°C for overnight. The fixed samples were then embedded with paraffin, sectioned, and stained with haematoxylin and eosin (H&E).
Statistical analysis
All statistic analyses (one-way ANOVA, t-Test, and survival analysis) were done using the Prism program (GraphPad Software, Inc).
Results
The association of Th17 cells with T1D
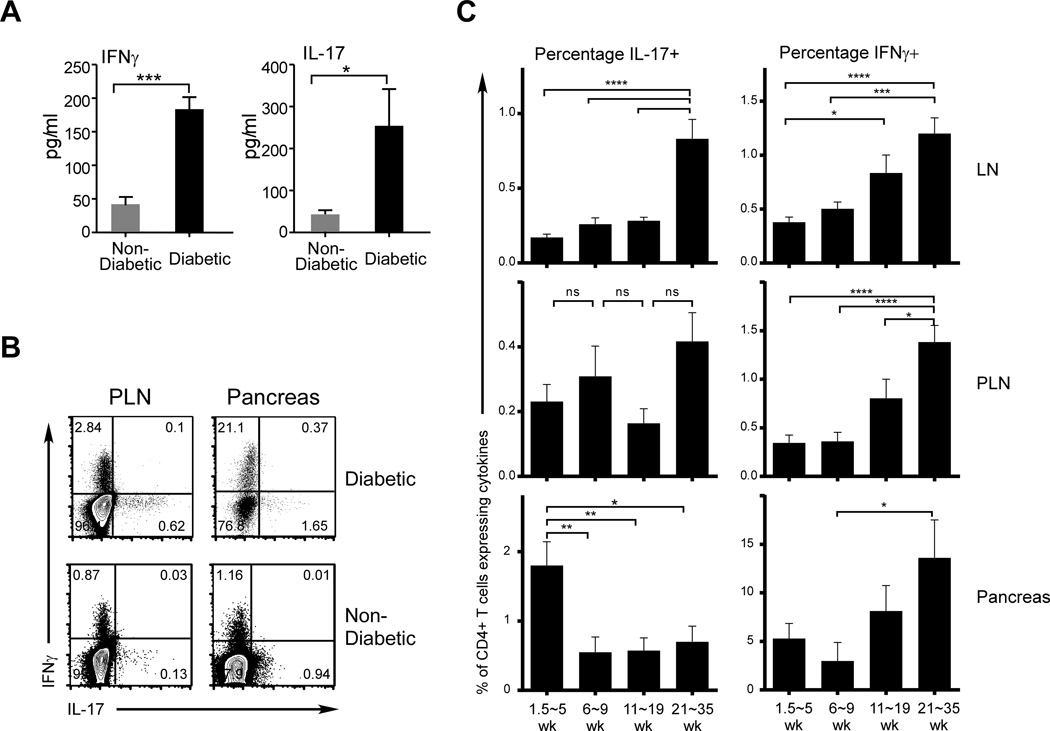
To address the potential role of Th17 cells in T1D, we analyzed the serum levels of the effector cytokines of Th17 and Th1 cells, IL-17 and IFNγ, respectively. We compared samples from a group of recently identified diabetic NOD mice with those from normoglycemic animals, and found a dramatic increase in the serum levels of both cytokines upon diabetes onset (Fig. 1A). We then analyzed the CD4+ T cell compartment of diabetic and non-diabetic mice for the presence of cells with the capacity to produce these cytokines, and found that cells producing either IL-17 or IFNγ were readily detected in the PLN and pancreas of both groups of animals, with increased frequencies particularly in the PLN of the diabetic animals (Fig. 1B). Importantly, there were few IL-17/IFNγ double producers. Our results parallel recent studies of T1D patients that showed elevated Th17 cells and Th17 activities at T1D onset (12–15).
FIGURE 1. Both Th1 and Th17 are stable populations, increased activities of which correlate with diabetes onset.
Female NOD mice were analyzed for serum cytokine levels and cytokine producing CD4+ T cells. (A) Serum samples were collected from normoglycemic (gray bars) and newly diagnosed diabetic NOD mice (black bars). Levels of IL-17 and IFNγ were analyzed by Luminex cytokine assays. Data shown were from one test with 4 samples per group. (B) Cells from PLN and pancreas of newly diagnosed diabetic mice, as well as those from non-diabetic age-matched controls, were analyzed for cytokine production by intracellular cytokine staining. Shown are examples of IL-17 and IFNγ staining of CD4-gated cells, from 1 each of 10 diabetic and non-diabetic mice at 20 weeks of age. (C) IL-17- and IFNγ-producing CD4+ T cells from LN, PLN, and pancreata of pre-diabetic NOD mice at different ages. Percentages of both populations were calculated and grouped according to the ages indicated. Shown are percentages of IL17- and IFNγ-producing cells (left and right panels, respectively). Each age group consists of 22 to 26 mice.
We then asked when Th1 and Th17 cells develop with respect to the progression of autoimmunity. We analyzed both effector populations over a wide range of ages in NOD mice (1.5–35 weeks) in the peripheral lymphoid organs and pancreata of non-diabetic mice. Surprisingly, we found CD4+ T cells producing IL-17 or IFNγ in these tissues at 1.5 weeks of age (Fig. 1C), suggesting that both effector populations can develop in the early stages of the autoimmune process. In the peripheral lymphoid compartment (LN), IL-17 producers were maintained at relatively stable frequencies until the immediate pre-diabetic phase at 21–35 weeks when IL-17+ cells increased (Fig. 1C). This change was not observed in the pancreas, and Th17 cell levels were highest in younger animals (≤5 weeks). In contrast, the frequencies of IFNγ producers increased in all tissues from 11 wks of age. These data show that although there is an increase in Th17 cell activity associated with disease progression in the lymphoid compartment, in the target organ, the Th17 population is typically considerably less frequent than the Th1 population. This finding raises the possibility that, although Th17 cells arise in response to inflammatory processes, they may be less important contributors to the autoimmune response against β-cells than Th1 cells until the acute phase leading to diabetes onset.
Pathogenicity and stability of Th17 cells in T1D
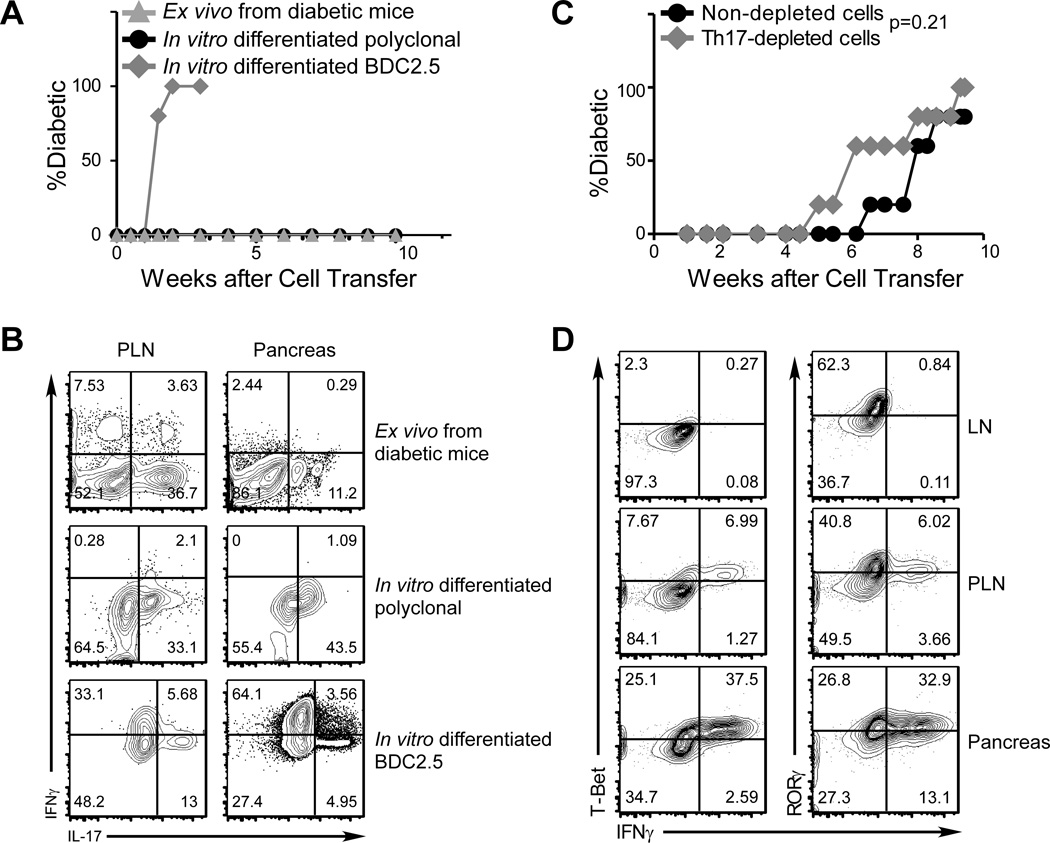
To investigate diabetogenic potential of Th17 cells, we used an adoptive transfer model of T1D wherein cells are assayed for their ability to elicit diabetes after transfer into NOD.Scid recipients, which have no preexisting autoimmunity. To ensure that we transferred a pure population of Th17 cells, we sorted IL-17 surface-staining positive CD4+ T cells from the lymphoid tissues of newly diabetic mice. Previous reports indicate that surface IL-17+ cells represent true Th17 cells in both human and mouse CD4+ T cells (27). In our hands, this method routinely yields over 90% purity of IL-17 producing cells with no IFNγ-producing contaminants, verified by intracellular cytokine staining (Supplemental Fig. 1).
As expected, transferring total splenocytes from diabetic NOD mice induced diabetes in all NOD.Scid recipients (not depicted). In contrast, CD4+IL-17+ cells sorted from spleens of diabetic donor mice did not induce diabetes (Fig. 2A), even after extended periods of observation (up to 7 months). Similarly, when NOD.Scid mice were given purified, in vitro differentiated WT polyclonal Th17 cells, no diabetes induction was observed (Fig. 2A). Interestingly, in both cases, donor cells were readily recovered from the lymphoid organs as well as the pancreas, and many of these cells maintained their IL-17-producing capacity with very few acquiring an IFNγ-producing phenotype (Fig. 2B, upper and middle panels). These results demonstrate that both in vitro and in vivo generated polyclonal Th17 cells can be stable in vivo, and that a lymphopenic environment per se does not drive an apparent phenotypical change with respect to cytokine production. To further test the contribution of in vivo-developed Th17 cells to the pathogenesis of diabetes, Th17 cells were depleted from total splenocytes of diabetic donor mice prior to transfer. As shown in Fig. 2C, this depletion did not delay diabetes induction, and if anything, somewhat accelerated disease onset. Together, these data suggest that islet antigen-specific Th17 cells may not have sufficient representation to play a major role in pathogenesis during progression to T1D.
FIGURE 2. Only in vitro differentiated islet antigen-specific Th17 cells elicit diabetes in adoptive recipients.
(A and B) IL-17 producing cells were purified by FACS sorting from in vitro Th17-differentiated WT NOD CD4+ T cells (polyclonal, black circles) or BDC2.5 CD4+ T cells (gray diamonds), or from PLN and SP of spontaneous diabetic NOD mice (Ex vivo Th17 cells, gray triangles). Purified cells were transferred into NOD.Scid recipients by i.v. injection (0.5×106 per recipient). Blood glucose levels were monitored for diabetes incidence (A). Donor cells were recovered from PLN and pancreas of recipients and analyzed for cytokine production at disease onset (for BDC2.5 Th17 cell recipients) or 10-week to 7-month after cell transfer (for polyclonal or ex vivo Th17 cell recipients, representative plots from a 10-week post cell transfer mouse is shown) (B). Shown are representative FACS plots of 5 to 10 recipients/group from different experiments with gating on Thy1.2+ CD4+ cells for polyclonal cells, Thy1.1+ CD4+ cells for BDC2.5 cells; gates for the cytokine analyses were determined from the controls in each respective experiment (B). (C) Total cells from SP and PLN of diabetic mice were pooled. IL-17-producing CD4+ T cells were depleted by FACS sorting (Th17-depleted). Th17-depleted (gray diamonds), or non-depleted (black circles) cells were transferred into NOD.Scid recipients (4×106 per recipient, n=5/group) that were then monitored for development of diabetes. (D) Purified in vitro differentiated BDC2.5 Th17 cells were transferred into NOD.Scid recipients. After 7 days, Thy1.1+ CD4+ donor cells recovered from LN, PLN, and pancreas of recipients were analyzed for the expression of IFNγ and the transcription factor T-bet by intracellular staining and FACS. Shown are plots from 1 recipient representative of 6.
To increase the number of islet-specific Th17 cells in adoptive transfer experiments, we differentiated Th17 cells in vitro from BDC2.5 CD4+ T cells. Purified BDC2.5 Th17 cells efficiently induced diabetes in NOD.Scid recipients (Fig. 2A). Furthermore, these cells could also induce diabetes in WT NOD recipients (not depicted and Fig. 4C), suggesting that proliferation in a lymphopenic environment is not a prerequisite for the pathogenesis of islet antigen-specific Th17 cells. However, consistent with previous reports (23, 24), the donor cells recovered from PLN and particularly the pancreata of diabetic recipients had acquired the capacity to produce IFNγ (Fig. 2B, bottom panels). This phenotypical change was associated with upregulation of T-Bet and downregulation of RORγt expression (Fig. 2D), suggesting a bona fide lineage reprogramming of Th17 cells to Th1 cells. This reprogramming was not found in the CD4+ T cells recovered from non-draining peripheral LN, suggesting that antigen engagement and/or the local inflammatory environment are necessary for the process.
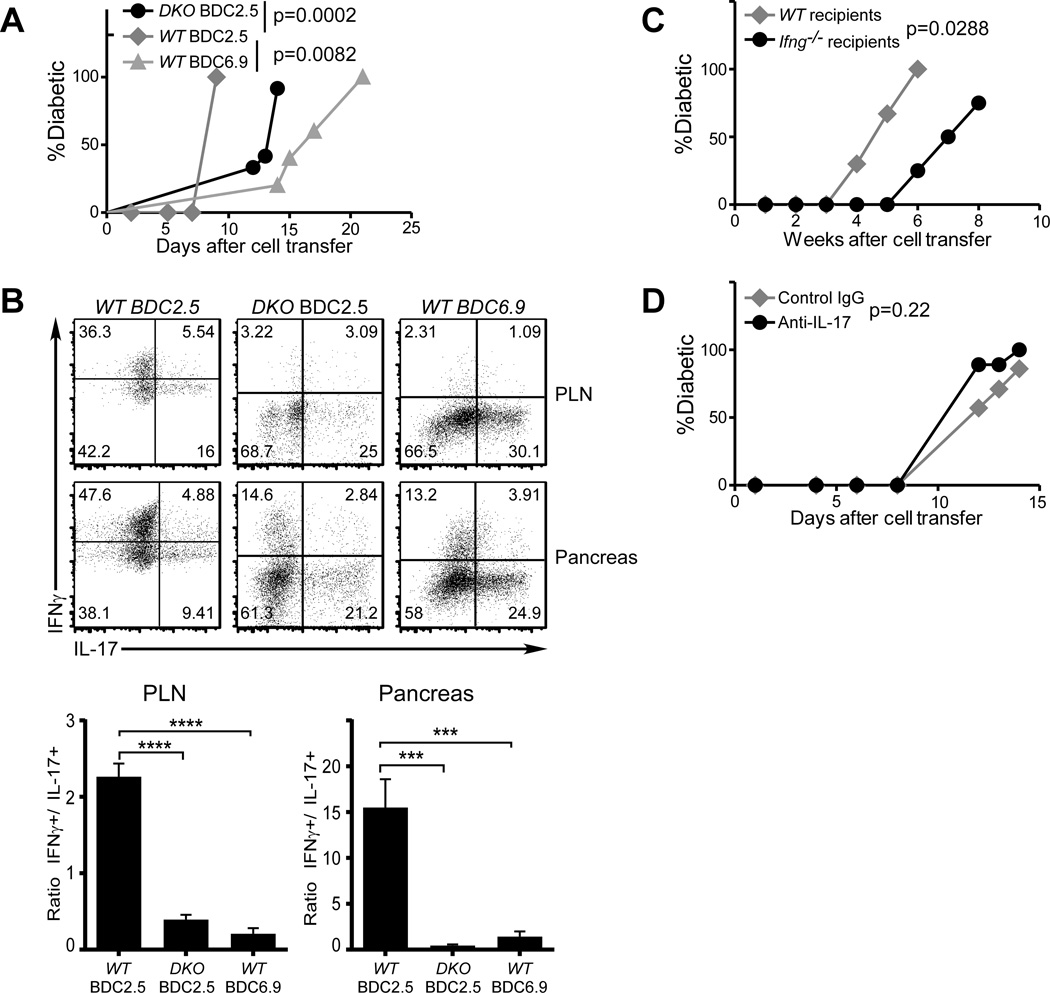
FIGURE 4. Neither Reprogramming nor IFNγ is required for the pathogenicity of islet antigen-specific Th17 cells.
(A and B) Purified in vitro differentiated WT BDC2.5 (gray diamonds), WT BDC6.9 (gray triangles), or Ifngr−/−Stat4−/− BDC2.5 (DKO, black circles) Th17 cells were transferred into NOD.Scid recipients (0.5×106/recipient, n=5/group). Diabetes incidence was monitored (A). Upon disease onset, the donor cells were analyzed for cytokine expression (B, upper panels, representative from each group). The ratios between IFNγ+ and IL-17+ donor cells were calculated (B lower panels). (C) Purified in vitro differentiated Ifng−/− BDC2.5 Th17 cells were transferred into WT NOD (gray diamonds) or NOD.Ifng−/− black circles) recipients (0.5×106 per recipient, n=3 for WT, n=4 for Ifng−/−). Diabetes incidence was monitored after cell transfer. (D) Purified in vitro differentiated BDC2.5 Th17 cells were transferred into NOD.Scid recipients (0.5×106 per recipient). The recipients were then treated with anti-IL-17 neutralizing antibody (black circles) or control mouse IgG (gray diamonds) (300µg/dose and 2 doses/week, 5 recipients per group). Diabetes incidence was monitored after cell transfer.
Reprogramming to Th1 cells and IFNγ are not essential for the pathogenicity of antigen-specific Th17 cells
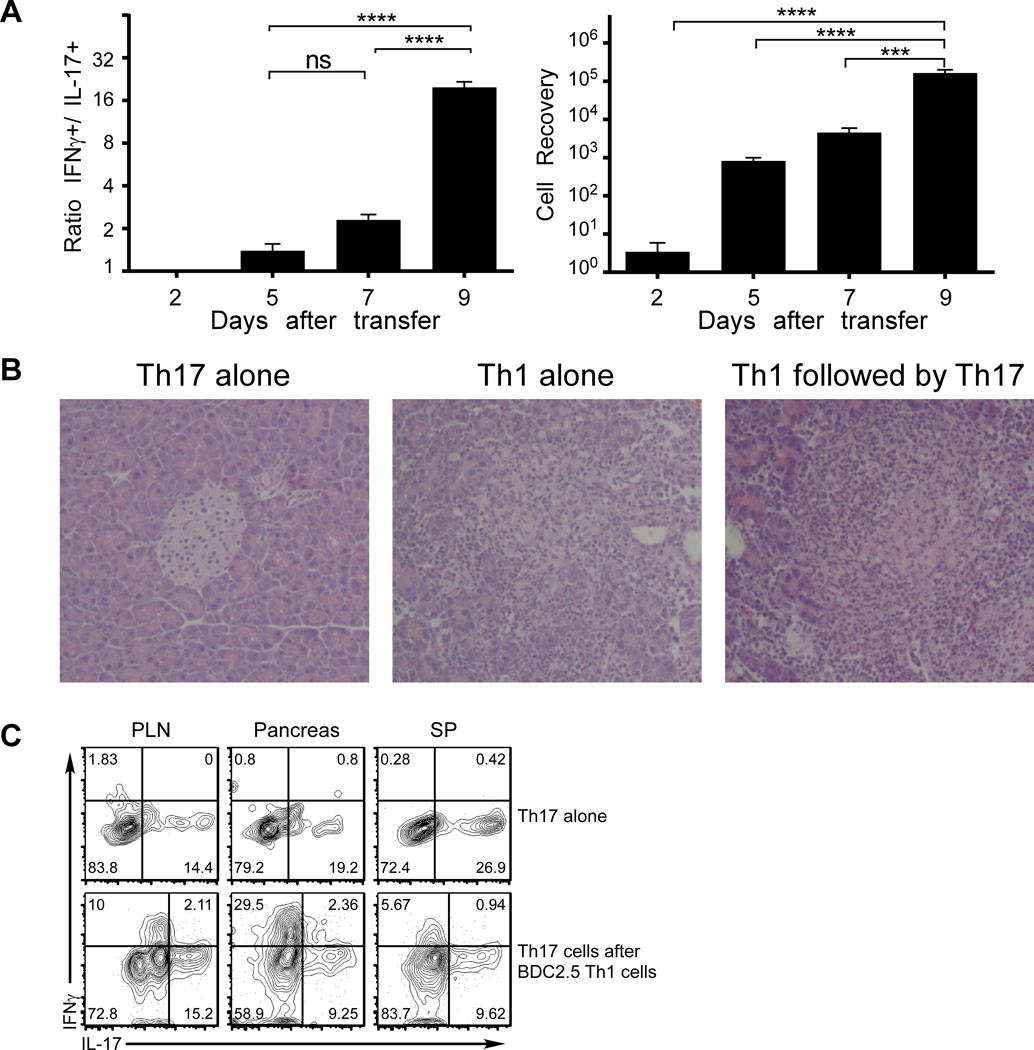
To further investigate the process of Th17-to-Th1 reprogramming, we transferred purified in vitro differentiated BDC2.5 Th17 cells into NOD.Scid mice that were analyzed at different times after cell transfer. As shown in Fig.3A, the increase in frequencies of reprogrammed cells was clearly paralleled by increasing levels of pancreatic infiltration. We then initiated the autoimmune response by transferring a small number of BDC2.5 Th1 cells two days before the transfer of purified in vitro differentiated polyclonal Th17 cells, which themselves did not induce diabetes. As shown in Fig. 3B, the transfer of antigen-specific Th1 cells alone but not Th17 cells alone led to the development of pancreatic infiltrates. Th1 cell transfer was sufficient to promote the acquisition of IFNγ-production by the polyclonal Th17 cells (Fig. 3C). These results indicate that reprogramming to a Th1 phenotype can be elicited by local inflammation orchestrated by Th1 cells.
FIGURE 3. Local inflammation in pancreas can induce the reprogramming of Th17 cells into Th1 cells.
(A) Purified in vitro differentiated BDC2.5 Th17 cells were transferred into NOD.Scid recipients (0.5×106/recipient). At different time points after cell transfer, mononuclear cells recovered from pancreata were enumerated (right panel). Cytokine production of donor cells was analyzed by intracellular staining and FACS; the ratios between IFNγ+ and IL-17+ cells were calculated (left panel). Three to four recipients were analyzed at each time point in the same experiment. (B) Purified in vitro differentiated WT (polyclonal, Thy1.1) Th17 cells were transferred into NOD.Scid recipients (0.5×106 per recipient) alone, or 2 days after a pre-transfer of BDC2.5 Th1 cells (Thy1.2, 0.2×106). Pancreas samples were harvested 5 days after Th17 cell transfer, fixed and analyzed by histology using H&E staining. Shown are representatives of 3 recipients per group. (C) Th17 cells and Th1 cells were generated and transferred to NOD.Scid recipients as in (B). Recipients of Th17 alone were analyzed 14 days after cell transfer. Recipients given Th17 cells after BDC2.5 Th1 cells were analyzed 11 days after Th1 transfer, when diabetes was diagnosed. Donor cells were recovered and analyzed for cytokine expression. Th17 and Th1 cells were distinguished by Thy1.1 vs Thy1.2 staining, respectively. Shown are representative FACS plots of 5 recipients per group in two experiments.
It is known that signals from IFNγ and from IL-12 via Stat4 are needed for Th1 differentiation (28). To test whether reprogramming of Th17 cells is similarly regulated, we crossed the Ifngr−/− and Stat4−/− NOD mice to BDC2.5 mice. Purified in vitro differentiated Th17 cells from CD4+ T cells from these doubly deficient mice induced diabetes in NOD.Scid recipients, although with delayed kinetics compared to WT Th17 cells (Fig. 4A). When the donor cells were recovered from diabetic recipients, the ratio of IFNγ+ vs IL-17+ donor cells was greatly reduced (Fig. 4B, left and middle plots), indicating a more limited reprogramming of Th17 cells. These results confirm that the reprogramming of Th17 cells to Th1 cells is to a large extent regulated similarly to classical Th1 differentiation. The conclusion that local inflammatory environment regulates the Th17-to-Th1 reprogramming is further supported by a recent report that showed a reduced reprogramming of BDC2.5 Th17 cells in IL-12p35 deficient NOD.Scid hosts (29). Nonetheless, acquisition of IFNγ production by Th17 cells was not fully abrogated, particularly in the pancreas suggesting that other mechanisms are also involved.
Although the BDC2.5 model is widely used to study antigen specific T cell responses in T1D, this clonotype may not fully reflect the diverse nature of T cell responses in this complex disease. Thus, we took advantage of another TCR Tg line, BDC6.9 (30), which recognizes a different antigen than BDC2.5 (31). Purified in vitro differentiated BDC6.9 Th17 cells also elicited diabetes in NOD.Scid recipients (Fig. 4A). Interestingly, however, the recovered donor cells showed considerably diminished Th1-reprogramming compared to BDC2.5 Th17 cells in both the draining LN and pancreas (Fig. 4B, left vs far-right panels). These results support the hypothesis that the TCR-antigen interaction can influence the extent of reprogramming of Th17 cells. Furthermore, together our data support the possibility that Th1-reprogramming may be unnecessary for antigen-specific Th17 cells to induce or contribute to the development of diabetes.
To further test the requirement of IFNγ in the induction of diabetes by antigen-specific Th17 cells, we differentiated Th17 cells from Ifng−/− BDC2.5 NOD mice. Purified Ifng−/− BDC2.5 Th17 cells elicited diabetes in Ifng−/− as well as WT recipients (Fig. 4C), although with delayed kinetics. This result demonstrates that the availability of IFNγ was not essential for the disease process. Interestingly, we confirmed that anti-IFNγ treatment of NOD.Scid mice that received BDC2.5 Th17 cells could prevent their conversion to Th1 cells as well as the development of diabetes (data not shown) (23, 24). This result suggests that when present, IFNγ can play a dominant role in diabetes induction. However, when recipients were treated with neutralizing anti-IL-17 antibody, the Th17-induced disease was not prevented or delayed (Fig. 4D), indicating that IL-17 does not directly contribute to the pathogenicity of Th17 cells.
TNFα is required for antigen-specific effector T cell induced diabetes
Since IL-17 was not essential for antigen-specific Th17 cells to induce diabetes, to determine potential mechanisms that underlie the development of disease, we analyzed the expression of a panel of cytokines by Th17 cells. We noted that TNFα is highly expressed by the majority of in vitro differentiated Th17 cells as well as Th1 cells (Fig. 5A). Moreover, the expression of TNFα was maintained in donor cells after disease induction by either subset (Fig. 5B and not depicted). To test the involvement of the Th1-specific transcription factor, T-Bet, in TNFα expressing Th17 cells, we transferred in vitro differentiated Ifngr−/−Stat4−/− BDC2.5 Th17 into NOD.Scid recipients. Upon diabetes onset, the expression of T-Bet and cytokines in donor cells were analyzed by intracellular staining. As shown in Fig 5C, TNFα producing cells did not express detectable levels of T-Bet, indicating that this transcription factor is not involved in the pathogenicity of Th17 cells. These results further confirmed that the Th17-to-Th1 reprogramming is blocked in the Ifngr−/−Stat4−/− BDC2.5 Th17 cells upon disease induction. The data are consistent with recent reports that showed that T-Bet is not required for Th17-induced pathology in EAE models (32, 33).
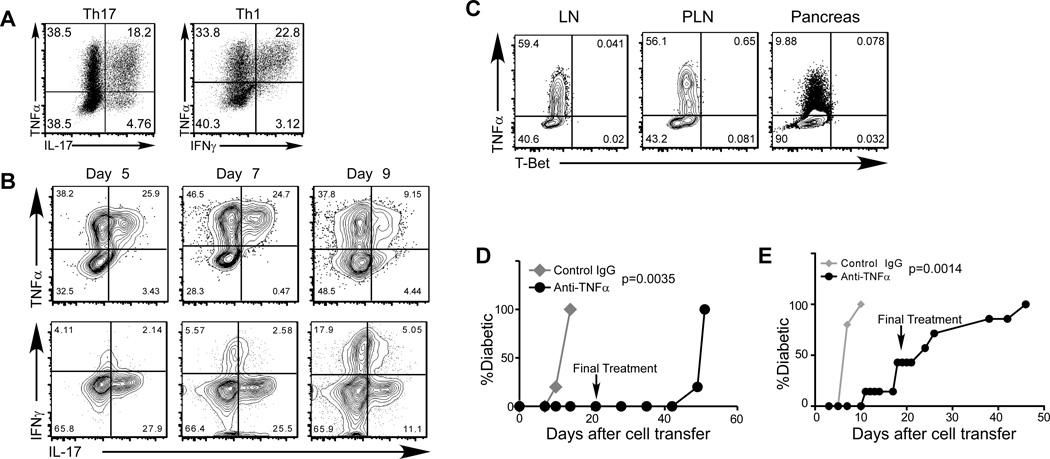
FIGURE 5. TNFα is expressed at high levels in both in vitro differentiated Th1 and Th17 cells, and is required for the induction of diabetes.
(A) CD4+ BDC2.5 T cells were activated under Th17 (left panel) or Th1 (right panel) differentiation conditions in vitro. The cells were analyzed for the expression of IL-17, IFNγ, and TNFα by intracellular staining and FACS. Shown are representative plots of 10 experiments. (B) Purified in vitro differentiated BDC2.5 Th17 cells were transferred into NOD.Scid recipients (0.5×106 per recipient). At different time points, recipient mice were dissected, and donor cells were analyzed for cytokine expression by intracellular staining. At day 9 after cell transfer, the recipients became diabetic. The data shown are representative of 3 mice per time point in one experiment. (C) Purified in vitro differentiated Ifngr−/−Stat4−/− BDC2.5 Th17 cells were transferred into NOD.Scid recipients (0.5×106 per recipient, n=5). Ten days after cell transfer, all recipients became diabetic. Recipient mice were dissected, and donor cells were analyzed for the expression of T-Bet and cytokines by intracellular staining. (D) Purified in vitro differentiated Ifngr−/−Stat4−/− BDC2.5 Th17 cells were transferred into NOD.Scid recipients (0.5×106 per recipient). The recipients were then treated with anti-TNFα neutralizing antibody (black circles) or control rat IgG (gray diamonds) (300µg/dose and 2 doses/wk, 5 recipients per group in one experiment). The last antibody treatment was administered at the 21-day after cell transfer (indicated by the arrow). Diabetes incidence was monitored after cell transfer. (E) In vitro differentiated BDC2.5 Th1 cells were transferred into NOD.Scid recipients (0.2×106 per recipient). The recipients were then treated with anti-TNFα (black circles) or control rat IgG (gray diamonds) (300µg/dose and 2 doses/week, 5 recipients per group in one experiment). The last antibody treatment was administered at the 21-day after cell transfer (indicated by the arrow). Diabetes incidence was monitored after cell transfer.
TNFα is known to be involved in the pathology of T1D as well as in many other autoimmune or inflammatory disorders (34). To test the potential involvement of this cytokine in Th17-induced diabetes, we treated recipients of BDC2.5 Th17 cells with neutralizing anti-TNFα antibody. The treatment resulted in a complete inhibition of diabetes development (Fig. 5D). Six weeks after the treatment was terminated, hyperglycemia emerged, consistent with a role for TNFα in diabetes development. Furthermore, when BDC2.5 Th1 cell recipients were treated with TNFα neutralizing antibody, diabetes induced by these cells was also significantly delayed (Fig. 5E). These results demonstrate that TNFα can play a crucial role in mediating the pathogenic autoimmune response of both islet antigen-specific Th17 and Th1 cells, and that this can occur independently of either IL-17 or IFNγ.
Discussion
By analyzing IL-17 producing CD4+ T cells in the context of T1D, we have clarified several aspects regarding the involvement of this subset of cells in this disease. We demonstrate that islet antigen-specific Th17 cells can mediate acute pathogenesis that can be dependent upon TNF without also requiring IL-17 or production of IFNγ, which is associated with reprogramming to a Th1 cell phenotype. Despite the potential plasticity, in vivo Th17 cells were a discrete population in the lymphoid compartment and pancreas throughout the development of spontaneous T1D. Although IFNγ/IL-17 double producers have been associated with pathogenicity in other autoimmune diseases (20), these cells were rare in spontaneously diabetic mice as well as after adoptive transfer of Th17 cells.
We find that there is a clear correlation between T1D onset and an increased representation of IL-17 and Th17 cells in NOD mice as has also been found with human patients (12–15). Further, our data indicate that during spontaneous disease progression, Th1 cells develop in greater frequencies, with Th17 cells remaining a lesser population, particularly in the draining PLN and pancreas. Our findings that depletion of Th17 cells does not diminish the ability of T cells from the lymphoid compartment of diabetic mice to transfer disease implies that during spontaneous development of T1D recruitment of this subset of cells to the pancreas may not be critical until the later stages of the autoimmune response that leads T1D onset. However, in addition to a potentially non-pathogenic role of Th17 cells during the prolonged pre-diabetic phase of T1D, it is also possible that these cells could have a protective role in diabetes in some contexts, particularly when they are present in elevated numbers due to gut colonization with segmented filamentous bacteria (17).
It is intriguing that higher percentages of IL-17 producing CD4 T cells are found in the earliest pancreatic infiltrates than at later times. These early effector cells are likely to be induced by the wave of pancreas remodeling that begins at 5 days of age and is accompanied by substantial β-cell apoptosis (35) that can lead to autoreactive T cell priming (36). It is possible that these conditions are supportive of Th17 cell differentiation. Since TNFα is thought to play an important role in initiating T1D (37), it is possible that IL-17 is not required. Alternatively, IL-17 could contribute to inflammation (38) in synergy with IL-1β and/or IL-6, which are upregulated with disease progression (39) along with TNFα. Furthermore, IL-17 could enhance the cytotoxic effect of IL-1β on β-cells (12, 15), as well as IL-1β/IFNγ-induced and TNFα/IFNγ-induced apoptosis in β-cells (15). However, neutralization of IL-17 did not prevent the development of diabetes in the context of the adoptive transfer model suggesting that in the absence of its activity, other proinflammatory cytokines can support disease development. Further studies with genetically modified NOD mice, such as inducible IL-17 deficiency and IL-17 reporters, will be needed to dissect the mechanisms.
At later stages leading to diabetes onset, our data support the concept that despite Th17-cell plasticity and the potential for Th1-reprogramming, TNFα can be a major factor in the development of T1D mediated by Th17 cells. TNFα has been shown to be a critical cytokine for antigen-specific Th1 and Th2 cells in diabetes induction in adoptive transfer models (40, 41). Thus, currently available data highlight the requirements for strong antigen reactivity and a common effector function, such as TNFα production, for islet antigen-specific helper CD4+ T cells to induce diabetes in adoptive recipients. This common effector mechanism can explain that, even under certain conditions where the Th17-to-Th1 reprogramming is inhibited, islet antigen-specific Th17 cells are still able to induce diabetes in adoptive hosts (29). The signature cytokines IFNγ or IL-17 are associated with inflammation and progression to diabetes, but can be dispensable. Our findings are consistent with a recent report that for CD4+ T cells, the killing of islet β-cells is dependent upon their production of TNFα in vivo. Our current ongoing studies are directed at determining which cell types contribute to TNFα production in our model. It will also be important to address whether TNFα exerts direct or indirect effects on β-cells themselves, or amplifies the magnitude of the pathology that culminates with β-cell destruction (42).
On the issue of Th17 cell stability, our data indicate that Th17 cells are not necessarily short-lived (43), and potentially can be maintained as a stable memory population in vivo. Our findings demonstrate that local inflammation driven by Th1 cells can induce the reprogramming of Th17 cells into the Th1 lineage, extending the findings of previous reports (21–23). Even in the absence of reprogramming to Th1 cells, it is clear that Th17 cells can lose IL-17 production, which could contribute to their more limited representation compared to Th1 cells during spontaneous T1D development. The loss of IL-17 and acquisition of IFNγ expression by Th17 cells, although dispensable, clearly can play a role under conditions that favor Th1 development, which are found in association with islet inflammation induced by Th1 cells themselves.
Our results suggest an interesting scenario, wherein stable Th17 cells arise during the early pre-diabetic phase of spontaneous autoimmunity and possibly persist as memory cells but do not necessarily contribute substantively to T1D pathogenesis. With progressive inflammation, the plasticity of Th17 cells becomes evident and they can be reprogrammed into Th1 cells particularly in the microenvironment of the pancreas. Importantly, however, neither this reprogramming nor its associated phenotypical changes are prerequisites for the pathogenicity of antigen-specific T cells that were once Th17 cells. An alternative possibility is that during the spontaneous progression of autoimmunity in T1D, autoreactive islet-specific CD4+ T cells may not naturally develop into the Th17 lineage in vivo. Instead, the Th17 cells we observed could be nonpathogenic bystander cells that may even counteract the pathogenic effector cell responses, possibly through a contribution of TNFα production to the maintenance of Tregs (44). The propensity of islet antigen-specific autoreactive CD4+ T cells to develop into Th1 cells might thus be determined by the nature of their antigen engagement including TCR signal strength, as well as the nature of the local environment in the pancreatic lymph nodes, the islets, or both. This concept is supported by our studies demonstrating the differential behavior of BDC2.5 and BDC6.9 Th17 cells that are specific for distinct islet antigens, as well as by previous studies of diabetogenic CD4+ T cell clones derived from diabetic NOD mice, which displayed a Th1 phenotype (45). Therefore, the contribution(s) of TNFα and IFNγ to pathogenesis could differ at the level of individual T cell clones.
On the basis of our findings we hypothesize that the potency of the reactivity of T cells to autoantigens rather than their phenotypes as either Th1 or Th17 cells per se is an underlying factor in the pathogenic potential of these subsets in the autoimmune response and disease progression. The identification of TNFα as a key mediator and cytokine of Th1- and Th17-induced diabetes supports the concept that a common pathway can underlie pathogenesis at the acute stage that precipitates T1D. Our studies therefore suggest that although the subset-associated cytokines IFNγ and IL-17 can serve as biomarkers of disease progression, a broader focus on shared T cell effector mechanisms could be critically important when considering potential therapeutic interventions.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Kathryn Haskins (University of Colorado, Denver) for the BDC6.9 TCR Tg line, Jennifer Nguyen (Sanford-Burnham Medical Research Institute) for overseeing the maintenance and screening the animals used in these studies, and the Sanford-Burnham Medical Research Institute Flow Cytometry Core for cell sorting.
This work was supported by grants from NIH (AI081238) and from JDRF (32-2008-353) to LMB.
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haskins K, Cooke A. CD4 T cells and their antigens in the pathogenesis of autoimmune diabetes. Curr Opin Immunol. 2011;23:739–745. doi: 10.1016/j.coi.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 4.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes. 2000;49:2007–2011. doi: 10.2337/diabetes.49.12.2007. [DOI] [PubMed] [Google Scholar]
- 5.Kanagawa O, Xu G, Tevaarwerk A, Vaupel BA. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. J Immunol. 2000;164:3919–3923. doi: 10.4049/jimmunol.164.7.3919. [DOI] [PubMed] [Google Scholar]
- 6.Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- 7.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 8.Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison PJ, Ballantyne SJ, Kullberg MC. Interleukin-23 and T helper 17- type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology. 2011;133:397–408. doi: 10.1111/j.1365-2567.2011.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. Journal of immunology. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O. IL-17 immunity in human type 1 diabetes. Journal of immunology. 2010;185:1959–1967. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 13.Marwaha AK, Crome SQ, Panagiotopoulos C, Berg KB, Qin H, Ouyang Q, Xu L, Priatel JJ, Levings MK, Tan R. Cutting edge: Increased IL-17-secreting T cells in children with new-onset type 1 diabetes. Journal of immunology. 2010;185:3814–3818. doi: 10.4049/jimmunol.1001860. [DOI] [PubMed] [Google Scholar]
- 14.Ferraro A, Socci C, Stabilini A, Valle A, Monti P, Piemonti L, Nano R, Olek S, Maffi P, Scavini M, Secchi A, Staudacher C, Bonifacio E, Battaglia M. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes. 2011;60:2903–2913. doi: 10.2337/db11-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R, Vives-Pi M, Powrie J, Tree T, Marchetti P, Huang GC, Gurzov EN, Pujol-Borrell R, Eizirik DL, Peakman M. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes. 2011;60:2112–2119. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikoopour E, Schwartz JA, Huszarik K, Sandrock C, Krougly O, Lee-Chan E, Singh B. Th17 polarized cells from nonobese diabetic mice following mycobacterial adjuvant immunotherapy delay type 1 diabetes. Journal of immunology. 2010;184:4779–4788. doi: 10.4049/jimmunol.0902822. [DOI] [PubMed] [Google Scholar]
- 17.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Annunziato F, Cosmi L, Romagnani S. Human and murine Th17. Curr Opin HIV AIDS. 2010;5:114–119. doi: 10.1097/COH.0b013e32833647c2. [DOI] [PubMed] [Google Scholar]
- 20.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bending D, De la Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 26.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23 induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brucklacher-Waldert V, Steinbach K, Lioznov M, Kolster M, Holscher C, Tolosa E. Phenotypical characterization of human Th17 cells unambiguously identified by surface IL-17A expression. J Immunol. 2009;183:5494–5501. doi: 10.4049/jimmunol.0901000. [DOI] [PubMed] [Google Scholar]
- 28.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 29.Wan X, Guloglu FB, VanMorlan AM, Rowland LM, Jain R, Haymaker CL, Cascio JA, Dhakal M, Hoeman CM, Tartar DM, Zaghouani H. Mechanisms underlying antigen-specific tolerance of stable and convertible Th17 cells during suppression of autoimmune diabetes. Diabetes. 2012;61:2054–2065. doi: 10.2337/db11-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauza ME, Dobbs CM, He J, Patterson T, Wagner S, Anobile BS, Bradley BJ, Lo D, Haskins K. T-cell receptor transgenic response to an endogenous polymorphic autoantigen determines susceptibility to diabetes. Diabetes. 2004;53:978–988. doi: 10.2337/diabetes.53.4.978. [DOI] [PubMed] [Google Scholar]
- 31.Delong T, Baker RL, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Barbour G, Bradley B, Haskins K. Islet amyloid polypeptide is a target antigen for diabetogenic CD4+ T cells. Diabetes. 2011;60:2325–2330. doi: 10.2337/db11-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor RA, Cambrook H, Huettner K, Anderton SM. T-bet is essential for Th1-mediated, but not Th17-mediated, CNS autoimmune disease. Eur J Immunol. 2013 doi: 10.1002/eji.201343689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting Edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J Immunol. 2013;190:4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XD, Tisch R, Singer SM, Cao ZA, Liblau RS, Schreiber RD, McDevitt HO. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes. 2000;49:1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee LF, Xu B, Michie SA, Beilhack GF, Warganich T, Turley S, McDevitt HO. The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: analysis of dendritic cell maturation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15995–16000. doi: 10.1073/pnas.0508122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis Rheum. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Koulmanda M, Bhasin M, Awdeh Z, Qipo A, Fan Z, Hanidziar D, Putheti P, Shi H, Csizuadia E, Libermann TA, Strom TB. The role of TNF-alpha in mice with type 1- and 2- diabetes. PLoS One. 2012;7:e33254. doi: 10.1371/journal.pone.0033254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantor J, Haskins K. Effector function of diabetogenic CD4 Th1 T cell clones: a central role for TNF-alpha. J Immunol. 2005;175:7738–7745. doi: 10.4049/jimmunol.175.11.7738. [DOI] [PubMed] [Google Scholar]
- 41.He J, Haskins K. Pathogenicity of T helper 2 T-cell clones from T-cell receptor transgenic non-obese diabetic mice is determined by tumour necrosis factor-alpha. Immunology. 2008;123:108–117. doi: 10.1111/j.1365-2567.2007.02715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varanasi V, Avanesyan L, Schumann DM, Chervonsky AV. Cytotoxic mechanisms employed by mouse T cells to destroy pancreatic beta-cells. Diabetes. 2012;61:2862–2870. doi: 10.2337/db11-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Gregoire S, Martin GH, Elhage R, Derian N, Carpentier W, Marodon G, Klatzmann D, Piaggio E, Salomon BL. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120:4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haskins K, Wegmann D. Diabetogenic T-cell clones. Diabetes. 1996;45:1299–1305. doi: 10.2337/diab.45.10.1299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.