Abstract
The first molecular adducts of platinum and arsenic based anticancer drugs - arsenoplatins - show unanticipated structure, substitution chemistry, and cellular cytotoxicity. The PtII-AsIII bonds in these complexes are stable in aqueous solution and strongly influence the lability of the trans ligand.
Keywords: arsenic, drug design, antitumor agents, platinum, X-ray diffraction
Two inorganic drugs, the widely used cis-diamminedichloroplatinum(II)[1], and anti-leukemia agent arsenic trioxide, are highly successful agents for treatment of cancer. Cisplatin is used in combination chemotherapy to treat ovarian, testicular, head, neck, and bladder cancers.[2] Unfortunately, these and other cancers frequently develop resistance to this drug and there are intensive efforts to develop new agents that overcome cisplatin resistance.[3] As2O3, discovered as a traditional Chinese medicine, is a front line treatment for acute promyelocytic leukemia[4] and has also shown preliminary efficacy in the treatment of blood cancers such as multiple myeloma and myelodysplastic syndromes[4a]. Both compounds induce apoptotic cell death, but through different pathways: cisplatin reacts with DNA and causes intra- and inter-strand DNA cross-links[2a, 2d], whereas at low concentrations arsenous acid, the principle component of aqueous solutions of As2O3 at pH=7, can react with and trigger degradation of key zinc-dependent regulatory proteins and also inhibit angiogenesis, migration, and invasion. At higher concentrations it triggers apoptosis[5], [6] through pathways that involve elevated levels of ROS in mitochondria.[4a, 5, 7] Synergistic activity of these drugs has been reported[8] supporting the idea that compounds combining both species may have advantages as anticancer therapeutics. The only example of a platinum adduct with arsenous acid in the literature emerged in efforts to develop efficient systems for loading As2O3 into liposomes with aquated forms of cisplatin: EXAFS spectroscopy suggested a new type of PtII-AsIII center was stabilized in the nanocrystalline formulation.[9] Given the absence of structural precedents in the literature, it was not clear that such complexes could exist or would be stable in aqueous solution. Herein we report synthetic routes to a novel family of small molecule complexes of the aqueous form of As2O3 bound directly to PtII as an As(OH)2 moiety and demonstrate their robust molecular structures involves an AsIII center that acts simultaneously as a Lewis acid and a Lewis base. These arsenoplatins are stable in solution and exhibit chemical bonding, ligand substitution chemistry, and biological activities that are distinct from the parent compounds and show promising activity in drug-resistant cancer cell lines.
Arsenoplatin 1 (Figure 1) is synthesized by heating cisplatin with As2O3 in an acetonitrile-water mixture (9:1, v/v) at 90 °C for three days. The yield increases from 23% to 75% when the starting material is K2PtCl4] (see the Supporting Information for syntheses and characterizations of 1-3).
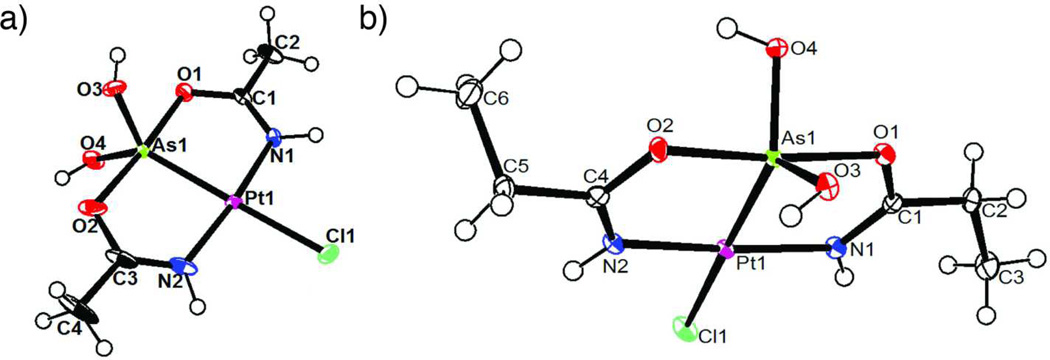
Figure 1.
a) Thermal ellipsoid plots of complex 1a (1 crystallizes in two crystal systems) and b) complex 2. Solvent molecules have been omitted for clarity. The plots are drawn at 50 % probability level.[10]
Variations on this Pt-As core complex are accessible by varying substituent on the nitrile. For instance, arsenoplatin 2 is obtained from the reaction of K2PtCl4] with As2O3 in the presence of propionitrile (Figure 1). Conditions for synthesis of 2 were different from 1 because of the different miscibility of propionitrile in water (1:9, v/v). Complex 2 is obtained at room temperature after 4 days, whereas 1 is obtained at elevated temperatures. In 1 and 2, the PtII center adopts a square planar geometry, with arsenic, chloride, and two nitrogen donors in a trans configuration. The nitrogen donors are derived from acetamide (propanamide) formed via Pt-assisted acetonitrile (propionitrile) hydrolysis in situ[11]. The Pt-N bond lengths in 1a (Pt1-N1 = 2.000(3) Å and Pt1-N2 = 2.004(3) Å are consistent with the Pt-N bond lengths obtained in other PtII complexes with the deprotonated form of acetamide[11–12]. The N1-C1 and O1-C1 bond lengths (both 1.302(4) Å) and N2-C3 and O2-C3 (1.289(6) and 1.297(6) Å) in 1a are indicative of a high degree of delocalization[11] present in the chelate rings formed by bridging N,O acetylamido ligands.
The closest precedents for these arsenous acid/platinum complexes are found in hetereometallic clusters where the NiII[13 and PdII[14 centers bind directly to arsenous acid. In PdII and NiII complexes, As(OH)3 is bound to the metal as a Lewis base with arsenic in a distorted tetrahedral and pyramidal environment, respectively. In arsenoplatins, the geometry at the AsIII is best described as trigonal bipyramidal (TBP), with the PtII and two hydroxides binding in the trigonal plane. In 1 and 2, arsenic retains a formal oxidation state of three, and displays distorted TBP geometry with a PtO4 coordination sphere. The TBP geometry around AsIII in metal complexes is uncommon, however, it has been observed in complexes of an organoarsenic ligand with PtII[15, and in complexes of AgI[16 and FeII[17. While AsIII can act as either a Lewis base or a Lewis acid[18], in 1 and 2 AsIII acts simultaneously as a Lewis base (As→Pt) and as a Lewis acid (O→As). These multiple intramolecular interactions explain in part the strong Pt-As interaction in arsenoplatins: 2.2687(4) Å in 2, 2.2729(2) Å in 1b, and 2.2732(3) Å in 1a (the shortest Pt-As bond length found in the CSD is 2.267(2) Å in one organoarsenical compound).[19]
Heteronuclear NMR reveals that the short Pt-As interaction observed in the solid state is stable in solution (Figures S2-S7). Due to symmetry the 1H NMR spectrum of 1 contains only one NH signal at 8.16 ppm and one OH signal at 8.92 ppm – the first chemical shifts reported for an M-As-OH moiety. The 195Pt chemical shift for 1 (-3589 ppm) lies between the signals for PtII-diamines (e.g., cisplatin at -2097 ppm)[20] and those of mixed PtII-arsine halides (e.g., [Pt(o-C6H4(AsMe2)2)Cl2 at -4556 ppm)[21], consistent with a PtIIN2ClAs coordination sphere.
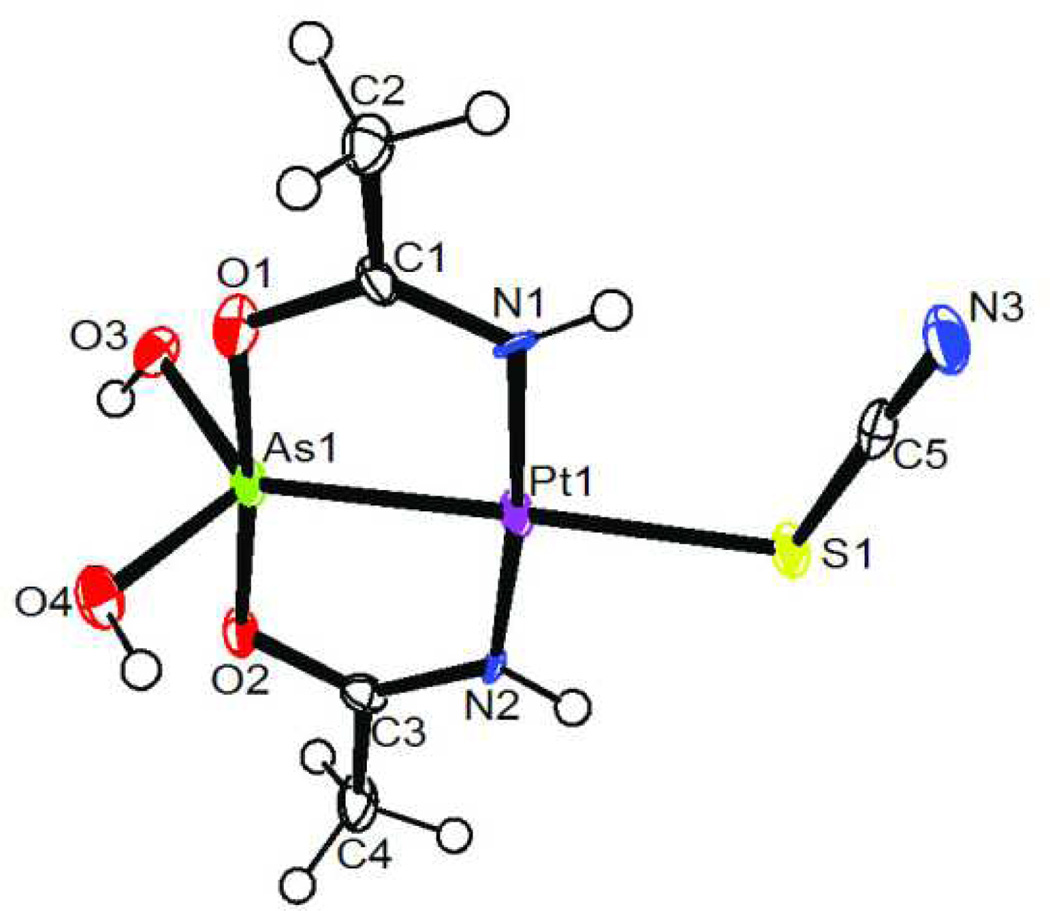
This Pt-As core is also stable to ligand substitution reactions. In general, hydrolysis of Pt-Cl bonds is slow (t1/2 = 2h at 37°C and 4 mM Cl−)[22] and rapid substitution usually requires addition of reagents such as AgNO3. The substitution of the Cl− ligand in 1 with SCN− in water occurs immediately at room temperature likely driven by the trans effect of the arsenic moiety[23]. Solution NMR and X-ray crystallography confirm that the Pt-As bond remained intact. Crystals suitable for a single crystal X-ray analysis were obtained when this complex (3) was synthesized in a 1:1 water/methanol mixture (Figure 2). NMR spectroscopy reveals facile linkage isomerization of 3 in solution at room temperature (Figures S8-S12). Specifically, upon dissolving 3 in [D6]DMSO solution equilibrium mixture of 64 ± 1.2 % of S-isomer and 36 % ± 1.5 of N-isomer is quickly established, i.e., the 1H NMR spectrum obtained after 5 min upon dissolution of 3 does not change over time. Assignments of chemical shifts for the S- and N-bound isomers signals are based on multidimensional 195Pt (Figure 3) and 15N NMR spectroscopy on sample of 3 which is synthesized using thiocyanate enriched in 13C and 15N at 99 % (see the Supporting Information).
Figure 2.
Thermal ellipsoid plot of 3. The plot is drawn at 50 % probability level.
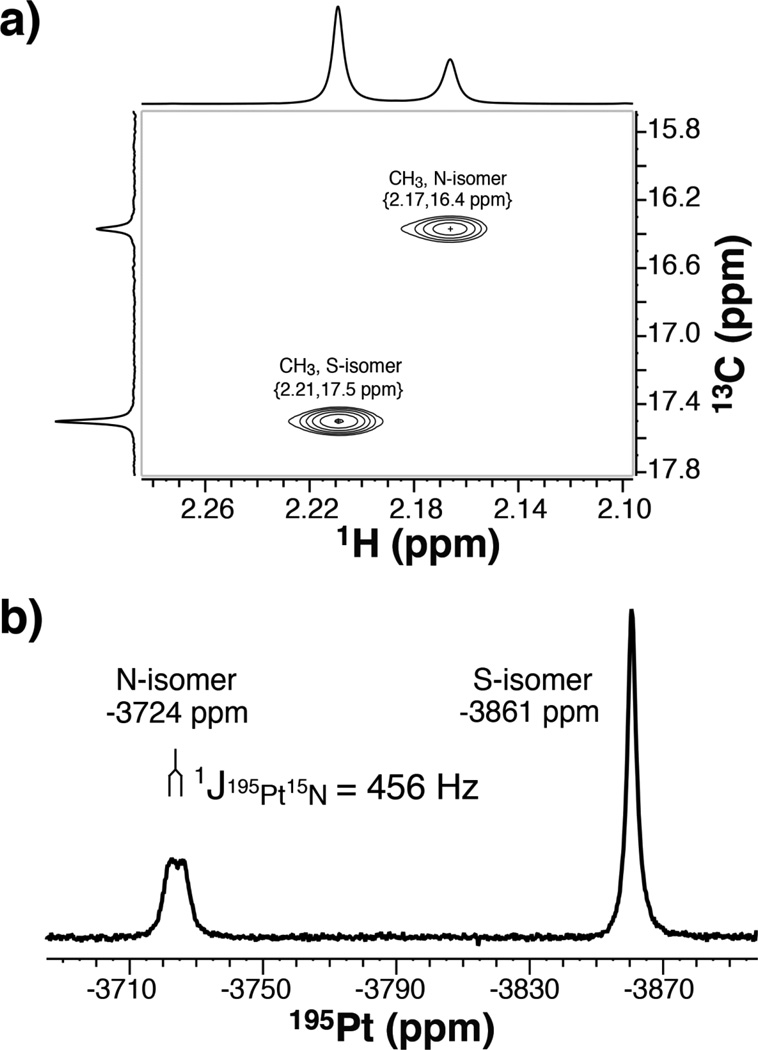
Figure 3.
a) 1H-13C HSQC NMR spectrum of 3 (with S13C15N) in [D6]DMSO, acquired at 25 °C at 600 MHz 1H with high resolution in the indirect (13C) dimension to distinguish the methyl resonances of the N- and S-isomers; b) 195Pt NMR spectrum of 3 (with S13C15N) in [D6]DMSO, referenced indirectly to 1H TMS such that Na2195PtCl6 resonates at 0.0 ppm. The 456 Hz splitting of the 195Pt peak at -3724 ppm arises from scalar coupling to the SCN 15N.
Initial formation of 3 with S-bound thiocyanate can be kinetically or thermodynamically controlled, but both isomers [(Eq. 1)] are sufficiently stable in [D6]DMSO solution to be observed using NMR spectroscopy. Analysis of VT NMR experiments reveals the thermodynamics of this facile linkage isomer equilibrium. A Van’t Hoff plot of the temperature dependent NMR spectra (Figure S13) reveals an equilibrium constant Keq for isomerization of 0.563, ΔH° = −15.7 kJmol−1, ΔS°= −57.5 Jmol−1K−1, and ΔG° = 1.42 kJmol−1 ([D6]DMSO solution, 25°C). These parameters indicate a low barrier to substitution at the PtII site trans to the AsIII ligand, consistent with a very strong trans effect of the AsIII(OH)2 moiety. On the basis of our thermodynamic data, the N-isomer is enthalpically favored in solution by 15.7 kJmol−1. Interestingly, only the S-linked complex could be isolated in the solid state, which may be the result of both rapid equilibration and a lower solubility for the S-isomer.
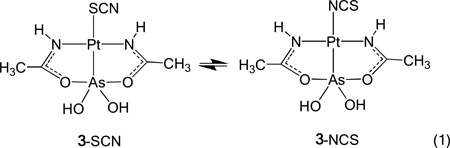 |
Equation 1 |
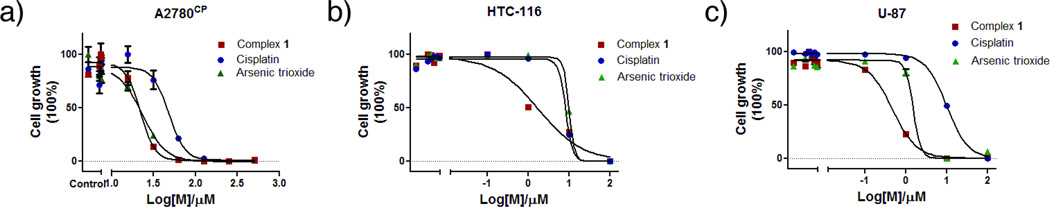
Complex 1 demonstrates significant anticancer activity in a panel of human cancer cell lines (Table S10) and also overcomes one of the most significant limitations of platinum drugs, namely tumor-based drug resistance mechanisms. The ovarian cisplatin resistant A2780CP cancer cell line is of special interest since it encompasses all of the known major mechanisms of resistance to cisplatin (reduced uptake, increased level of glutathione, increased DNA repair, and tolerance to PtII-induced lesions)[24]. The results show that 1 exhibited more than twice the cytotoxicity of cisplatin against the cisplatin resistant cell line A2780CP (IC50 21.4 ± 1.8 versus 47.3 ± 2.1), Figure 4. The ability of 1 to circumvent cisplatin-acquired resistance was determined from the resistance factor (RF), and an RF value of < 2 denotes no cross-resistance[25]. In the case of ovarian A2780 and A2780CP cell lines all approved platinum drugs have RFs between 6.1 and 16.0[24–25]. The RF of 1.1 for 1 indicates that it is far more effective at killing this cisplatin resistant cancer cell line and may be able to bypass drug resistance mechanism(s) that lower cisplatin cytotoxicity.
Figure 4.
Dose response curves for a) ovarian cisplatin resistant A2780CP, b) colon HCT-116, and c) glioblastoma U-87 cancer cell lines after exposure to 1 cisplatin, and As2O3.
Complex 1 has showed better cytotoxic activity than either cisplatin or As2O3 in colon HCT-116 (IC50 = 1.6±0.4 µM vs. 5.5±1.3 µM and 9.4± 0.9 µM) and glioblastoma U-87 (IC50 = 0.37±0.11 µM vs. 9.6±0.8 µM and 1.6±2.9µM) cancer cell linesFigure 4. Additionally, 1 showed twice the cytotoxicity of cisplatin against MDA-MB-231-mCherry cells (IC50 = 9.5±0.1 µM vs. 22.3±2.8 µM), as well as improved cytotoxicity compared with As2O3 in RPMI 8226 multiple myeloma cells (IC50 = 4.5±1.0 µM vs. 7.1±0.2 µM).
Trans-platinum compounds in comparison with cis-compounds display different patterns of ligand substitution, which contributes to the potency of trans-platinum compounds in cisplatin-resistant cell lines[26]. Although we do not have evidence that arsenoplatin compounds target DNA, the distinct biological activity of 1 in vitro may be the result of the strong trans effect of the As(OH)2 moiety combined with the trans stereochemistry of the N-atoms at the platinum center.
In conclusion, the first compounds containing a Pt-As(OH)2 core (arsenoplatins 1 and 2) have been synthesized and characterized as robust complexes that are stable in aqueous solution. Single crystal X-ray structure characterization reveals that these unprecedented compounds contain very short Pt-As bond with the expected square planar PtII coordination but an atypical five coordinate AsIII geometry. Intriguingly, the arsenic atom in these complexes exhibits both Lewis acid and Lewis base behavior upon binding to the platinum-acetylamido moiety. The Pt-As core in these complexes readily undergo ligand exchange reactions at the PtII center with retention of core bonding and stereochemistry. Both, the rapid substitution of chloride in 1 and isomerism in 3 demonstrate a strong trans effect of the arsenic moiety. Complex 1 has significant biological activity in several cancer cell lines and preliminary data are consistent with the ability of arsenoplatins to overcome drug resistance mechanisms. These results are promising and future work with mouse xenograft models should help shed more light on the real potential of this unique class of compounds.
Supplementary Material
Footnotes
We thank the NIH (NCI Cancer Nanotechnology Platform Partnerships Grant U01CA151461 and PSOC grant U54CA143869, Center of Cancer Nanotechnology Excellence Grants U54CA119341, and Core Grant P30CA060553 to the Robert H. Lurie Comprehensive Cancer Center) and the CDMRP Breast Cancer Research Program BC076723 for funding. R.W. Ahn thanks the CDMRP Breast Cancer Research Program Predoctoral Fellowship (BC073413). We thank Prof. M. Djuran, Dr. E. Que, and E. Swindell for valuable discussions.
References
- 1.Rosenberg B, Vancamp L, Trosko JE, Mansour VH. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 2.a) Siddik ZH. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]; b) Kelland L. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]; c) Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113–8127. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]; d) Wang D, Lippard SJ. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 3.a) Graf N, Lippard SJ. Advanced Drug Delivery Reviews. 2012;64:993–1004. doi: 10.1016/j.addr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Casini A, Reedijk J. Chemical Science. 2012;3:3135–3144. [Google Scholar]
- 4.a) Emadi A, Gore SD. Blood Reviews. 2010;24:191–199. doi: 10.1016/j.blre.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang X-W, Yan X-J, Zhou Z-R, Yang F-F, Wu Z-Y, Sun H-B, Liang W-X, Song A-X, Lallemand-Breitenbach V, Jeanne M, Zhang Q-Y, Yang H-Y, Huang Q-H, Zhou G-B, Tong J-H, Zhang Y, Wu J-H, Hu H-Y, de The H, Chen S-J, Chen Z. Science. 2010;328:240–243. doi: 10.1126/science.1183424. [DOI] [PubMed] [Google Scholar]
- 5.Platanias LC. J. Biol. Chem. 2009;284:18583–18587. doi: 10.1074/jbc.R900003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Ralph SJ. Metal-Based Drugs. 2008 doi: 10.1155/2008/260146. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Litzow MR. Expert Opinion on Pharmacotherapy. 2008;9:1773–1785. doi: 10.1517/14656566.9.10.1773. [DOI] [PubMed] [Google Scholar]
- 7.Fulda S, Galluzzi L, Kroemer G. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 8.a) Zhang N, Wu Z-M, McGowan E, Shi J, Hong Z-B, Ding C-W, Xia P, Di W. Cancer Sci. 2009;100:2459–2464. doi: 10.1111/j.1349-7006.2009.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li H, Zhu X, Zhang Y, Xiang J, Chen H. J. Exp. Clin. Canc. Res. 2009;28:110. doi: 10.1186/1756-9966-28-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Pazicni S, Krett Nancy L, Ahn Richard W, Penner-Hahn James E, Rosen Steven T, O'Halloran Thomas V. Angew. Chem. Int. Ed. 2009;48:9295–9299. doi: 10.1002/anie.200903655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CCDC-831785 (1a)`, CCDC-831786 (1b)`, CCDC-831788 (2) and CCDC-831787 (3) contain the supplementary crystallography data for this paper. These data can be obtained free of charge from CCDC via www.ccdc.cam.uk/data_request/cif
- 11.Erxleben A, Mutikainen I, Lippert B. J. Chem. Soc. Dalton Trans. 1994:3667–3675. [Google Scholar]
- 12.Ziolkowski EJ, Turner P, Rendina LM. Inorg. Chem. Commun. 2006;9:53–56. [Google Scholar]
- 13.Hernández-Molina R, Sokolov MN, Clausen M, Clegg W. Inorg. Chem. 2006;45:10567–10575. doi: 10.1021/ic061146n. [DOI] [PubMed] [Google Scholar]
- 14.Sokolov MN, Virovets AV, Dybtsev DN, Chubarova EV, Fedin VP, Fenske D. Inorg. Chem. 2001;40:4816–4817. doi: 10.1021/ic015520p. [DOI] [PubMed] [Google Scholar]
- 15.Müller IM, Sheldrick WS. Eur. J. Inorg. Chem. 1998;1998:1999–2003. [Google Scholar]
- 16.Bott RC, Smith G, Sagatys DS, Lynch DE, Kennard CHL. Aust. J. Chem. 2000;53:917–924. [Google Scholar]
- 17.Yamamoto Y, Toyota K, Wakisaka Y, Akiba K-y. Heteroatom Chemistry. 2000;11:42–47. [Google Scholar]
- 18.a) Klapötke TM, Nöth H, Schütt T, Suter M. Eur. J. Inorg. Chem. 2002:2511–2517. doi: 10.1021/ic010463l. [DOI] [PubMed] [Google Scholar]; b) Carter TG, Vickaryous WJ, Cangelosi VM, Johnson DW. Comment Inorg. Chem. 2007;28:97–122. [Google Scholar]; c) Hill NJ, Levason W, Reid G. J. Chem. Soc. Dalton Trans. 2002:1188–1192. [Google Scholar]
- 19.Cooper MK, Guerney PJ, Goodwin HJ, McPartlin M. J. Chem. Soc. Dalton Trans. 1982:757–764. [Google Scholar]
- 20.Still BM, Kumar PGA, Aldrich-Wright JR, Price WS. Chemical Society Reviews. 2007;36:665–686. doi: 10.1039/b606190g. [DOI] [PubMed] [Google Scholar]
- 21.Hope EG, Levason W, Powel NA. Inorg. Chim. Acta. 1986;115:187–182. [Google Scholar]
- 22.a) Appleton SJB-PaTG. Platinum-Based Drugs in Cancer Therapy. Totowa, N.J.: Humana Press; 2000. [Google Scholar]; b) Zutphen S, Pantoja E, Soriano R, Soro C, Tooke D, Spek A, Dulk H, Brouwer J, Reedijk J. Dalton Trans. 2006:1020–1023. doi: 10.1039/b512357g. [DOI] [PubMed] [Google Scholar]
- 23.Kuznik N, Wendt OF. J. Chem. Soc. Dalton Trans. 2002:3074–3078. [Google Scholar]
- 24.Helleman HBJ, Hamelers IHL, Boersma AWM, de Kroon AIPM, Stoter G, Nooter K. Cancer Biol. Ther. 2006;5:943. doi: 10.4161/cbt.5.8.2876. [DOI] [PubMed] [Google Scholar]
- 25.Intini FP, Pellicani RZ, Boccarelli A, Sasanelli R, Coluccia M, Natile G. Eur. J. Inorg. Chem. 2008:4555–4561. [Google Scholar]
- 26.a) Kalinowska-Lis U, Ochocki J, Matlawska-Wasowska K. Coordin. Chem. Rev. 2008;252:1328–1345. [Google Scholar]; b) Reedijk J. Eur. J. Inorg. Chem. 2009:1303–1312. [Google Scholar]; b) Chen S, Xu D, Jiang H, Xi Z, Zhu P, Liu Y. Angew. Chem. Int. Ed. 2012;51:12258–12262. doi: 10.1002/anie.201206596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.