Abstract
This prospective study examined the effect of widowhood on physical activity by comparing widowed elders to health status-, age-, and sex-matched married controls. Participants included 396 married controls and 396 widows/widowers age 64 to 91 (M age = 72.7 years) who experienced the death of their spouse while participating in the Cardiovascular Health Study (CHS). Compared to married controls, widowed men, but not women, were more likely to increase their physical activity following the death of their spouse. However, this increased level of activity was not sustained and declines as time since spousal death passes. Moreover, during the year before spousal death, soon-to-be widowed men, but not women, increase their physical activity. Our results suggest that widowed men experience significant changes in physical activity and that the transition to widowhood contribute to these changes.
Keywords: spousal loss, health behaviors, energy expenditure, depressive symptomatology, sex differences
Widowhood is one of the most stressful events a married adult can endure (Holmes & Rahe, 1967). Experiencing the loss of a spouse initiates multiple life changes, and is rated as the life transition requiring the most readjustment (Stroebe et al., 2007). Although the psychosocial correlates of late-life widowhood have been studied extensively, less research has examined the impact of this life transition on health behaviors. Given the substantial evidence indicating that physical activity is associated with physical and mental health benefits (Penedo & Dahn, 2005), assessing the effects of widowhood on physical activity is an important issue. The present study prospectively examined the effect of spousal loss on widowed elders’ physical activity.
Late-life bereavement is associated with multiple negative health outcomes including decreased physical well-being. Widowhood significantly elevates the mortality risk for surviving spouses (Elwert & Christakis, 2006; Schulz et al., 2000), including increased suicide (Erlangsen et al., 2004). Widowed elders also have a significantly higher incidence of physical health events, including stroke (Engström et al, 2004), cancer, and atherosclerosis (Chen et al., 1999) compared to married controls. They also have higher rates of disability, medication usage, and hospitalization compared to married controls (Lee & Carr, 2007).
Possible Mechanisms for Physical Health Outcomes
The mechanisms underlying the effect of widowhood on health outcomes are becoming increasingly well-documented. A range of explanations may account for this association, including the onset of depressive symptomatology, the loss of spousal support, and changes in routine health behaviors. Widowhood is associated with psychological symptoms, including the onset of depressive symptomatology (Carr et al., 2000), and the exacerbation of pre-existing levels of depression (Gilewski et al., 1991). Depression may in turn trigger physical symptoms including increased sleep disturbances (Monk et al., 2008) and physical ailments (headaches, chest pain) that impair bereaved elders’ daily functioning (Stroebe et al., 2007). Besides the emotional loss, older adults’ social environment is dramatically changed. One of the most difficult challenges may be the behavioral changes to compensate for their lost social partner (Utz et al., 2002). Changes in routine health behaviors may also help explain the negative health effects of widowhood. Behavioral changes following widowhood such as being sedentary, having poor sleep, gaining or losing weight, and smoking likely undermine health during the transition to widowhood (Lee et al., 2005; Schulz et al., 2001; Williams, 2004).
Effect of Widowhood on Physical Activity
Prior research has not resolved whether or to what extent physical activity changes following widowhood. Research has provided considerable evidence that physical activity offers one of the greatest opportunities to extend years of active life, reduce disability, and improve quality of life among older adults (Prohaska et al., 2006). Cross-sectional evidence reports both positive (Patterson, 1996) and negative (Fitzpatrick et al., 2001; Okun, et al., 2011) effects of bereavement status (versus married control) on physical activity. Prospective evidence is also mixed and reports both an increase (Janke et al., 2008a; Lee et al., 2005; Schul et al., 2001; Wilcox et al., 2003), decrease (Eng et al., 2005; Janke et al., 2008b, 2008c), and no change (Avis et al., 1991; Tran, 2007) in activity during the transition to widowhood. Post-bereavement longitudinal evidence reports both a decrease (Caserta, 2001; Nurriddin, 2008; Wilcox & King, 2004) and no change (Chen et al., 2005; McIntyre & Howie, 2002) in activity. It is important to note that each study defines physical activity somewhat differently. As a result, a variety of outcomes have been assessed including leisure activity, exercise activity, exercise status (yes/no), and class-based exercise attendance, among others.
Only three studies have examined change in physical activity measured by energy expenditure – the amount of energy (kilocalories) a person uses to perform bodily activities. These types of measures are a good source of information about the dimensions of physical activity (frequency, intensity, type, and time) in older adults. Two studies examined bereaved women, and one examined bereaved men. Among women, recent widows decreased their physical activity following spousal loss (Wilcox et al., 2003), but slightly increased their physical activity over time (Lee et al, 2005; Wilcox et al., 2003). However, the effect sizes in both studies were small. Among men, those who became widowed across a 4-year period increased their physical activity (Eng et al., 2004). However, the length of time since widowhood was not included in model testing, making it difficult to determine the variation in physical activity over time.
Research suggests that the more active one is following spousal death, the easier the adjustment process may be (Utz et al., 2002). Still, widowhood is a distressing experience that causes considerable disruption, and for some the disruption is intense and prolonged. Documenting the effects of widowhood on the physical activity trajectories of older adults is important because significant declines in activity may place individuals at risk for compromised health. It is important to note that persistent strains prior to spousal loss (caregiving responsibilities) affect the well-being of widowed elders (Schulz, 2001), and likely affects their willingness and ability to engage in activity. Therefore, understanding physical activity patterns prior to spousal death may be important in understanding physical activity after spousal death.
The evidence relating widowhood to physical activity is inconsistent. There are several limitations to note. First, most studies have not characterized changes in physical activity before, at the time of, and after spousal death while including a matched married control sample. A control group is necessary because individuals may be experiencing change in activity independent of spousal health or death. Second, few prospective studies include widows and widowers in the same analysis, which precludes examination of sex differences. Third, none of these studies explore the possibility that significant changes in physical activity may occur prior to the transition to widowhood because of caregiving responsibilities associated with health declines in one’s spouse.
Current Study
Our study had three objectives. First, we prospectively identified the effect of the transition to widowhood on physical activity by comparing widowed elders to health status-, age- and sex-matched married controls. Umberson (1987, 1992) has theorized that widowhood results in the disturbance of routine health behaviors that result from less frequent health reminders and assistance once provided by a spouse. Therefore, we hypothesized that becoming a widow/widower would be associated with lower levels of physical activity relative to remaining married. In addition, we expanded upon previous research by considering sex differences in physical activity both before and after the death of one’s spouse. Both men and women are vulnerable to the detrimental health effects of widowhood. However, accumulating evidence suggests that men benefit more from marriage and may experience more negative consequences following spousal loss when compared to women (Lee & Carr, 2007; Stroebe, 1998). For instance, spousal loss is a powerful predictor of depressive symptomology for men (Lee et al., 1998). Widowers are at an increased risk for developing an affective disorder and remain significantly depressed longer compared to widows (Umberson et al., 1992). Spousal loss also increases the risk of mortality more for men than women (Mineau et al., 2002). Because depression may affect physical activity and men are at increased risk for developing depression we also examined the interaction of sex and depression symptomatology on physical activity.
Next, we explored whether time since spousal death was related to widows’/widowers’ post-bereavement physical activity. We tested the extent to which the rate of physical activity change varied by sex as time passed following spousal loss. Third, we compared changes in physical activity prior to spousal death between the “soon-to-be” widowed and married control groups. We hypothesized that relative to married controls, the soon-to-be widowed group would show a significant decline in physical activity prior to spousal death due to the emotional, social, and physical effects associated with an end of life experience and anticipatory grief period. To our knowledge, this has not been examined.
Method
Design
Data from three waves of the Cardiovascular Health Study (CHS) were analyzed. The CHS is a prospective population-based cohort study of risk factors for cardiovascular disease in adults 65 years or older. Starting in 1989 and continuing through 2006, participants underwent annual extensive clinical examinations and structured interviews. Participants were recruited from four communities in the United States: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA. Further details of the CHS design, sampling procedures, data collection, and response rates are available in Fried and colleagues (1991). The present study includes CHS data collected between 1989 and 1997, spanning as many as 8 years. We used data from three assessments that collected information on respondent physical activity: baseline (1989–1990), wave 3 (1992–1993), and wave 7 (1996–1997).
Participants
The original sample included 5,201 individuals, of which 2,524 reported being married and had spousal data available (n=1,262 couples). The CHS supplemented the original cohort with an additional 687 African Americans during the third wave of the study (1992–1993). Eligible for this study were all CHS participants who had spousal data available and became widowed while participating in the study between baseline and wave 7. At wave 7, 484 spouses were deceased, including 44 spousal pairs (i.e. both members of the dyad were deceased). These 44 spousal pairs had baseline data only and were not included in the analysis. Few married participants from the African American cohort became widowed by wave 7 (n=9). The final sample included 396 widows/widowers. The 307 women and 89 men were equally representative across the four CHS sites χ2 (3, N=396) = 1.04, p=.79. Widows/widowers were matched for health status, age, and sex with 396 randomly selected married participants from the same study. Our married control group did not experience the death of their spouse.
Table 1 presents descriptive information for the 792 widows/widowers and married controls at the time of CHS enrollment. Widows/widowers and married controls were similar in age, sex, BMI, race, and education. Most participants were women, White, high school educated, and either overweight or normal weight. Significantly more widowed participants earned less income when compared to married controls. Most widows/widowers and married controls reported providing care to a family member at the time of enrollment. A majority of spousal deaths were related to non-cardiovascular disease (60%), followed by Atherosclerotic CHD (28%), cerebrovascular disease (9%) and other atherosclerotic or cardiovascular diseases (4%). The deceased spouses were primarily men (78%) and similar in age to their surviving spouses (M=73.98, SD=5.54).
Table 1.
Sociodemographic Characteristics of the Widows/Widowers and Married Controls
| Characteristic | Widowed Sample Married Sample | ||
|---|---|---|---|
| (n=396) | (n=396) | p value | |
| Age in years (SD) | 72.81 (5.32) | 71.13 (3.96) | 0.07 |
| Sex - no. (%) | 1.00 | ||
| Female | 307 (77.5) | 307 (77.5) | |
| Male | 89 (22.5) | 89 (22.5) | |
| Body mass indexa - no. (%) | 0.36 | ||
| Underweight | 4 (1.0) | 9 (2.3) | |
| Normal weight | 154 (39.2) | 166 (42.1) | |
| Overweight | 159 (40.5) | 153 (38.8) | |
| Obese | 76 (19.3) | 66 (16.8) | |
| Cognitive status | |||
| 3MS (SD) | 91.3 (6.9) | 92.1 (6.9) | .081 |
| Race or ethnic group - no. (%) | 0.33 | ||
| White | 385 (97.2) | 388 (98.2) | |
| Black | 9 (2.3) | 6 (1.5) | |
| Asian | 2 (0.5) | 1 (0.3) | |
| Educationb - no. (%) | 0.08 | ||
| High-school graduation | 238 (60.4) | 207 (52.4) | |
| College Education | 128 (32.5) | 156 (39.5) | |
| Graduate or Professional | 28 (7.1) | 32 (8.1) | |
| Incomec - no. (%) | 0.01 | ||
| < $25,000 | 214 (57.9) | 167 (46.4) | |
| $25,000–$49,999 | 107 (28.5) | 141 (39.2) | |
| ≥ $50,000 | 54 (13.6) | 52 (14.4) | |
| Providing cared - no. (%) | |||
| Yes | 234 (59.1) | 230 (58.4) | |
| No | 162 (40.9) | 164 (41.6) | |
| CHS site - no. (%) | 0.00 | ||
| Forsyth County, NC | 98 (24.7) | 174 (43.9) | |
| Sacramento County, CA | 96 (24.2) | 123 (31.1) | |
| Washington County, MD | 119 (30.1) | 94 (23.7) | |
| Allegheny County, PA | 83 (21.0) | 5 (1.3) | |
Notes. All variables are baseline characteristics at the time of enrollment. Statistical analyses included analysis of variance tests for continuous variables and contingency table analysis using the χ2 test for categorical variables.
n=393, 394,
n=394, 394,
n= 375, 370,
n=342
Measures
The following measures were collected at baseline, wave 3, and wave 7 of the CHS:
Demographics
A brief questionnaire assessed basic demographic information, including age, sex, race, education, and annual income.
Physical health and functioning variables
Body mass index. Participants’ height (inches) and weight (pounds) were measured by clinicians during clinic visits. We used the adult body mass index (BMI) calculation (kg/m2) recommended by the Centers for Disease Control (CDC, 2007) and their recommended cutoffs to determine BMI classifications [underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), and obese ≥ 30)]. Physical functioning ability. Physical functioning was assessed using the Health Interview Survey Supplement (HISS) on aging questionnaire (Wallace, 1992). The HISS items asked if, due to health problems, a person had difficulty performing 10 activities of daily living (e.g., walking, dressing, or bathing) and 7 instrumental activities of daily living (e.g., cooking, shopping, managing finances). Items were coded as 0 (no difficulty) and 1 (any difficulty) and summed to create a functional impairment index. Higher values indicate greater difficulty to function independently. General health status. General health status was assessed using a single item asking participants to self-report their health status using a Likert-type scale ranging from 1 (poor health) to 5 (excellent health).
Depressive symptomatology
Symptoms of depression were measured with the modified version of the Center for Epidemiology Studies Depression Scale (CES-D; Orme et al., 1986). The scale assessed self-reported depressive symptoms experienced during the previous week. The scale consists of 10 symptoms, each scored 0–3, for a maximum of 30 points. Higher scores indicate greater frequency of depressive symptoms and correlate with an increased risk of clinical depression. A total score of 10 or greater has been found to be an appropriate cutoff for identifying major depression among all adults (Andresen et al., 1994).
Physical activity
Energy in kilocalories expended weekly (kcal/week) was measured using the Minnesota Leisure-Time Physical Activity questionnaire (MN LTPA; Taylor et al., 1978). The MN LTPA questionnaire measures the frequency and duration of participation in 15 activities in which older adults are likely to engage, including walking, leisure activity, and sports/exercise. The MN LTPA algorithmically translates the frequency and average duration of each activity into an estimated weekly energy expenditure in kilocalories (kcal). Each activity was assigned a specific activity intensity code (MET value [metabolic equivalent of task]) that had been adjusted for use in older adult samples. MET values indicate the energy cost associated with performing an activity. Most items (11 out of 15) were of moderate-to vigorous intensity (MET≥3.0). Total kcal/week was the sum of kcal/week for all 15 activities. Higher kcal/week scores indicate greater energy expenditure. This questionnaire has been validated indirectly against duration of treadmill exercise (Jacobs et al., 1993) and correlates reasonably well with measured energy expenditure (Albanes et al., 1990).
Analytic Approach
For our first research question, our outcome measure was physical activity change, and was defined as the difference in kcal/week between the last interview visit prior to widowhood and the first interview visit following widowhood. As such, pre-bereavement values refer to the interview visit prior to spousal death while post-bereavement values refer to the interview visit following spousal death. The same physical activity measurements were used with the married controls, using the same distribution of yearly visits as their bereaved counterparts.
For comparisons between widows/widowers and married controls, statistical analyses included analysis of variance for continuous variables and contingency table analysis using the χ2 test for categorical variables. For our first research question, a hierarchical multiple regression model was constructed to test the effect of widowhood on physical activity change. Included in the model were physical health change variables that correlated with physical activity change, using a significance level of .1. We also entered participants’ CES-D change score. Due to consistent sex differences in the literature, we examined the moderating effect of sex with widowhood status (vs. married control) and change in depressive symptomatology in our model. We used Aiken & West (1991) methods to further understand any significant interaction effects.
We next tested the relationship between time since spousal death and longitudinal post-bereavement physical activity among widows/widowers. Time since spousal death was calculated as the number of days between spouses’ date of death and widows’/widowers’ next post-bereavement interview visit. Some individuals had more than one post-death assessment available. Therefore, Generalized Estimating Equations were used to adjust for within-subject correlations in the context of multiple regression models. The dependent variable was physical activity (kcal/week) at all available waves following spousal death; the independent variables were: days after spousal death, age, sex, and post-bereavement depressive symptomatology.
We then tested the hypothesis that the soon-to-be widowed group would engage in less physical activity compared to married controls prior to spousal bereavement. We used GEE to compare physical activity between married controls and widows/widowers at all available waves prior to spousal death. We also examined the moderating effect of sex. GEE was also used to examine whether time prior to spousal death was associated with widows’/widowers’ longitudinal pre-bereavement physical activity. Time prior to spousal death was calculated as the number of days between spouses’ date of death and widows’/widowers’ previous pre-bereavement interview visit. In this model, the dependent variable was physical activity (kcal/week) at all available waves prior to spousal death; the independent variables were: days before spousal death, age, sex, and pre-bereavement depressive symptomatology.
Finally, we generated plots to assess the functional form of the relation between physical activity and the days before and days after spousal death, for men and women. The average length of time between widows/widowers pre- and post bereavement interview visit was 1339 days (3.7 years). We plotted activity during this period (+1 SD before and after spousal death) to gain a detailed understanding of the change in activity directly before and after spousal death. We used a piecewise linear regression approach to graph the data and chose to plot physical activity across 180 day intervals in order to have adequate numbers of deaths within each interval and because 180 days represents an important transition point in the literature on bereavement effects (Forte et al., 2004; Prigerson et al., 1996; Schulz et al., 2006). Six intervals were created: 0–180 days, 181–360 days, 361–540 days, 541–720 days, 721–900 days, and more than 900 days before and after the death of the spouse. For the analyses mentioned, p values smaller than .05 were considered statistically significant. We calculated Cohen’s d effect sizes and used well-known standards for interpreting the effects (0.3 is considered small, 0.5 is medium, and > 0.8 is large) (Cohen, 1988). All analyses were performed using SPSS, version 18.0, statistical software for Windows.
Results
Descriptive statistics
Descriptive statistics for model variables pre- and post-bereavement between widows/widowers and married controls are presented in Table 2. The level of physical functioning and general health status was similar between the widows/widowers and married controls. Most participants were overweight, but did not report any difficulty with ADL or IADL tasks and reported to be in “good” physical health. The CES-D scores indicated that widowed elders experienced significantly more depressive symptoms pre- and post- bereavement relative to married controls, but were not at-risk for clinical depression. Pre-bereavement physical activity was significantly lower for widowed elders compared to married controls.
Table 2.
Descriptive Statistics of Model Variables at Pre- and Post-bereavement between Widow/Widowers and Married Controls
| Variable | Widowed Sample (n=396) | Married Sample (n=396) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (n=89) | Women (n=307) | Total | Men (n=89) | Women (n=307) | Total | ||||||||
|
|
|
|
|
|
|||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | p* | |
|
|
|
|
|
|
|||||||||
| Body mass index | |||||||||||||
| Pre-bereavementa | 26.33 | 3.61 | 26.70 | 4.59 | 26.61 | 4.39 | 25.79 | 3.26 | 26.71 | 4.49 | 26.48 | 4.23 | 0.69 |
| Post-bereavement | 26.22 | 3.81 | 26.08 | 4.20 | 26.11 | 4.40 | 26.48 | 5.51 | 26.73 | 4.35 | 26.37 | 4.67 | 0.15 |
| Change | −0.24 | 4.00 | −0.39 | 4.76 | −0.36 | 4.59 | 0.82 | 6.48 | −0.16 | 3.28 | 0.11 | 4.38 | 0.24 |
| Physical functioningb | |||||||||||||
| Pre-bereavement | 0.37 | 0.82 | 0.59 | 1.14 | 0.54 | 1.08 | 0.25 | 1.18 | 0.53 | 1.04 | 0.48 | 1.08 | 0.06 |
| Post-bereavement | 1.09 | 2.26 | 0.79 | 1.60 | 0.86 | 1.77 | 0.43 | 0.99 | 1.11 | 2.06 | 0.95 | 1.89 | 0.35 |
| Change | 0.53 | 1.75 | 0.25 | 1.52 | 0.31 | 1.57 | 0.05 | 0.71 | 0.50 | 1.75 | 0.39 | 1.58 | 0.56 |
| General health statusc | |||||||||||||
| Pre-bereavement | 2.87 | 1.16 | 2.94 | 1.09 | 2.92 | 1.11 | 2.67 | 0.99 | 2.82 | 1.07 | 2.79 | 1.06 | 0.07 |
| Post-bereavement | 2.96 | 0.77 | 3.04 | 1.27 | 3.02 | 1.18 | 2.75 | 0.85 | 2.91 | 0.89 | 2.87 | 0.88 | 0.44 |
| Change | 0.12 | 1.31 | 0.12 | 1.63 | 0.12 | 1.57 | 0.14 | 0.93 | 0.11 | 1.36 | 0.11 | 1.27 | 0.62 |
| Depression (CES-D) | |||||||||||||
| Pre-bereavement | 4.02 | 3.71 | 5.43 | 4.91 | 5.11 | 4.69 | 3.40 | 4.08 | 4.21 | 3.89 | 4.02 | 3.95 | <.01 |
| Post-bereavement | 7.54 | 6.18 | 7.53 | 5.81 | 7.53 | 5.88 | 4.02 | 3.93 | 5.08 | 4.34 | 4.83 | 4.26 | <.01 |
| Change | 3.55 | 6.93 | 2.45 | 5.58 | 2.70 | 5.92 | 0.72 | 4.22 | 0.80 | 3.97 | 0.78 | 4.03 | <.01 |
| Physical activity (kcal) | |||||||||||||
| Pre-bereavement | 1378.28 | 1809.11 | 1110.36 | 1496.31 | 1171.58 | 1574.70 | 2627.63 | 2610.75 | 1145.46 | 1385.00 | 1497.23 | 1861.23 | <.01 |
| Post-bereavement | 1532.03 | 2609.63 | 914.62 | 1162.54 | 4056.36 | 1629.32 | 1762.59 | 2069.09 | 862.39 | 1162.26 | 1071.32 | 1471.55 | 0.44 |
| Change | 154.79 | 2462.66 | −264.69 | 1684.39 | −168.00 | 1896.09 | −871.00 | 2438.58 | −248.86 | 1648.55 | −397.95 | 1766.55 | 0.98 |
Notes.
Pre- and post-bereavement refers to the nearest interview visit prior to and following spousal death. The same measurement points were used with the married controls. Among the widowed sample, the average length of time between the nearest prebereavement assessment and death was 761.5 days [2.08 years (SD=387.8); Mdn=762.0 days]. The average length of time between death and the first follow-up assessment was 578.7 days [1.58 years (SD=395.28); Mdn=541.0 days].
Physical functioning (ADL+IADL) was analyzed as a discrete variable because most participants had a value of 0 or 1. Lower ADL+IADL scores indicate greater functional independence.
Subjective, Likert-type scale ranging from 1 (Excellent) to 5 (Poor).
Higher CES-D scores indicate greater depressive symptoms; range = 0–30.
Significant test comparing bereaved and married samples.
The effect of widowhood on physical activity change
Table 3 presents a hierarchical multiple regression model using change in physical activity pre-to post-bereavement (kcal/week) as the dependent variable. In the first step of the regression, physical activity change was regressed onto widowhood status (vs. married control), age, sex, and baseline physical activity. In the second step, we added BMI change, physical functioning change, health status change, and depressive symptomatology change. In the third step, we added into the model the interactions of widowed status (vs. married control) with sex and change in depressive symptomatology with sex.
Table 3.
Hierarchical Multiple Regression Model to Predict Physical Activity Change (kcal/week)a Pre to Post-bereavement
| Predictor Variables | B | SE(B) | β | p | R2 | Δ R2 |
|---|---|---|---|---|---|---|
| Widowed/Married Control (Widowed = 0, Control = 1) | −344.076 | 170.364 | −.087 | .04 | ||
| Age | −28.061 | 18.436 | −.068 | .13 | ||
| Sex (Female=0, Male=1) | 266.02 | 200.092 | .059 | .18 | ||
| Baseline physical activity | −0.478 | .041 | −.500 | .00 | ||
| Step 1, F (4, 415)=34.57, p<.01, d=.33. | .25 | |||||
| Widowed/Married Control | −360.195 | 174.197 | −.091 | .04 | ||
| Age | −27.540 | 18.315 | −.067 | .13 | ||
| Sex | 301.039 | 197.653 | .067 | .13 | ||
| Baseline physical activity | −0.475 | .040 | −.502 | .00 | ||
| Change in BMI | −57.391 | 27.411 | −.088 | .04 | ||
| Change in physical functioning | −67.224 | 58.751 | −.049 | .25 | ||
| Change in general health status | 19.477 | 73.162 | .011 | .79 | ||
| Change in depressive symptomatology | −9.988 | 17.045 | −.026 | .558 | ||
| Step 2, F (8, 424)=18.53, p<.01, d=.37. | .27 | .02 | ||||
| Widowed/Married Control | 653.721 | 526.721 | 0.164 | .22 | ||
| Age | −31.743 | 18.186 | −0.077 | .08 | ||
| Sex | 729.201 | 291.037 | 0.162 | .01 | ||
| Baseline physical activity | −0.464 | 0.040 | −0.491 | .00 | ||
| Change in BMI | −53.769 | 27.156 | −0.083 | .06 | ||
| Change in physical functioning | −78.219 | 58.393 | −0.057 | .18 | ||
| Change in general health status | 5.141 | 72.600 | 0.003 | .94 | ||
| Change in depressive symptomatology | −32.355 | 20.349 | −0.083 | .11 | ||
| Widowed/Married Control X Sex | −805.895 | 395.134 | −0.287 | .04 | ||
| Change in depressive symptomatology X Sex | 68.483 | 36.172 | 0.100 | .05 | ||
| Step 3, F(10, 432) = 16.20, p<.01, d=.39. | .28 | .01 |
Notes.
To reduce the effects of outlier bias, we used a trimmed mean on physical activity change. The left-most [lowest; (25th percentile − 3*(SD))] and right-most [highest; (75th percentile + 3*(SD))] data were excluded from analyses (n=7).
Results indicate that female sex and baseline activity were associated with a significant decline in physical activity. There was no significant effect for widowhood status (vs. married control) on physical activity change. However, the widowhood status (vs. married control) X sex interaction was significantly related to physical activity change. All other main effects, including age, BMI, physical functioning, general health status and depressive symptomology did not reach significance. Our final model accounted for a significant proportion of variance in physical activity change (28%, d=.38).
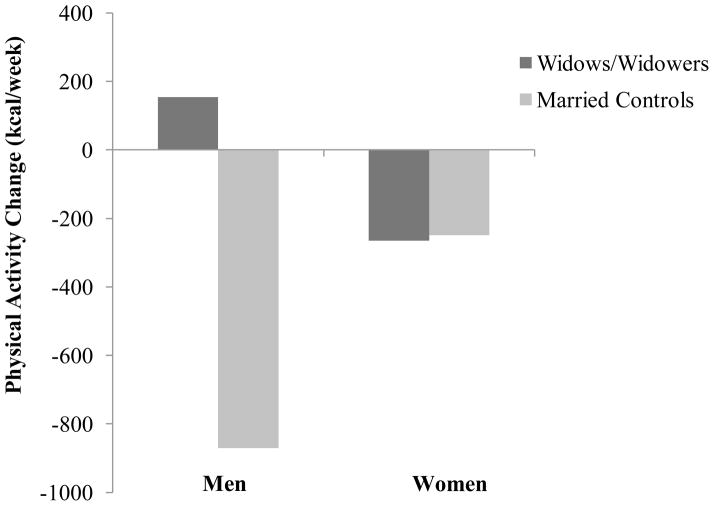
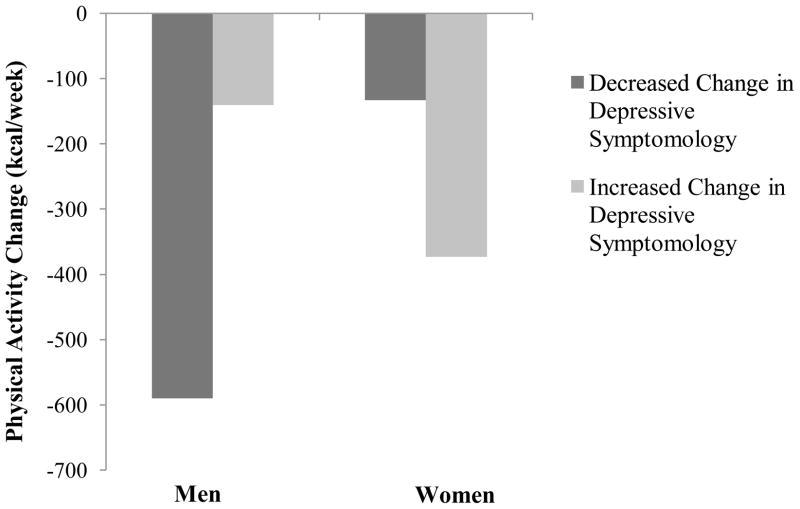
To further understand the significant interactions, simple slopes of widowhood status (vs. married control) on physical activity change for men and women were examined (Figure 1). Results indicate that widowhood status was significantly associated with physical activity change only for men, β = −.29, p < .05, d=.60. Widowhood status was not significantly associated with physical activity change for women, β = .01, p = .91, d=.02. Compared to married controls, widowed men, but not women, were more likely to increase their physical activity following spousal death. Simple slopes of depressive symptomatology change on physical activity change for men and women were also examined (Figure 2). Results show that depressive symptomatology change was not significantly associated with physical activity change for men (β = .15, p =.06, d=.31) or women (β = −.07, p =.11, d=.14). However, effect size estimates suggest that compared to women, men experienced a smaller decline in physical activity following spousal loss when they experienced increases in depressive symptomatology.
Figure 1.
Effects of widowed/married control status and sex on physical activity change among 792 older adults.
Figure 2.
Effects of depressive symptomatology change and sex on physical activity change among 792 older adults.
We also ran separate models using change in low intensity (MET < 3.0; walking, gardening) and moderate- to vigorous intensity activity (MET 3.0; jogging, aerobics) as dependent variables, but found no statistically significant effects. This could be due to the small sample of adults who reported engagement in these activities only.
Time since spousal death and post-bereavement physical activity (Time-span 18–2517 days)
GEE was used to examine the independent effect of time since spousal death on widows’/widowers’ post-bereavement physical activity (see Table 4). Days following spousal death (M=902 days, SD=663 days, d=.61) had a significant effect on longitudinal post-bereavement physical activity. Older age, female sex, and longitudinal increases in depressive symptoms were all significantly associated with decreased physical activity. Greater baseline physical activity had a positive effect on longitudinal post-bereavement physical activity. Compared to widows, widowers increased physical activity during the transition to widowhood, but were more likely to decrease their level of activity as time passed following spousal death. Widows’ level of physical activity remained relatively stable following spousal death.
Table 4.
Generalized Estimating Equations Predicting Longitudinal Physical Activity (kcal/week) Pre- and Post-bereavement
| Outcomea | Variables | B | SE | C.I. (95%) | Wald χ2 | p |
|---|---|---|---|---|---|---|
| Post-bereavement physical activity | Time since spousal death (days) | −0.29 | 0.10 | (−.47; −.10) | 8.87 | .00 |
| Age | −41.12 | 10.63 | (61.96; −20.28) | 14.96 | .00 | |
| Sex (Female=0) | −500.54 | 188.74 | (−870.46; −130.61) | 7.03 | .01 | |
| Depressive symptomatology | −22.32 | 10.45 | (−42.81; −1.85) | 4.57 | .03 | |
| Baseline physical activity | 0.31 | 0.07 | (.17; .44) | 20.70 | .00 | |
|
| ||||||
| Pre-bereavement physical activity | Time prior to spousal death (days) | 0.45 | 0.10 | (.24; .65) | 18.13 | .00 |
| Age | −33.37 | 15.44 | (−63.66; −3.13) | 4.68 | .03 | |
| Sex (Female=0) | −388.79 | 192.06 | (−765.22; −12.36) | 4.10 | .04 | |
| Depressive symptomatology | −15.06 | 17.42 | (−49.21; 19.09) | 0.75 | .39 | |
Notes.
Physical activity at all available waves pre- and post-bereavement; n = 139 widows/widowers had multiple waves of data after spousal death; n = 257 widows/widowers had multiple waves of data before spousal death.
Physical activity prior to spousal death (Long term trends, 12–2686 days)
GEE was used to compare physical activity prior to spousal death among widows/widowers and married controls. There was no significant effect for widowhood status (vs. married control) on physical activity. However, the widowhood status (vs. married control) X sex interaction was significant (Wald χ2 = 4.5, p<.05, d=.15). Contrasts revealed that widowed men (B= −491.3, p <.05, d=−.27), but not women (B = 72.4, p =.45, d=.04), had significantly lower levels of physical activity prior to spousal death when compared to married men. Next, GEE was used to examine the independent effect of time prior to spousal death on pre-bereavement physical activity among the widowed sample only (see Table 4). Days prior to spousal death (M= 1207 days, SD=685 days, range=12–2686 days) had a significant effect on pre-bereavement physical activity, indicating that widows/widowers were more likely to engage in less activity the closer their spouses’ death approached. Older age and female sex were significantly associated with less physical activity. Unlike our GEE model that predicted post-bereavement physical activity, depressive symptomatology was not significant in our pre-bereavement model.
Physical activity directly before and after spousal death (Short term trends, 12–900 days)
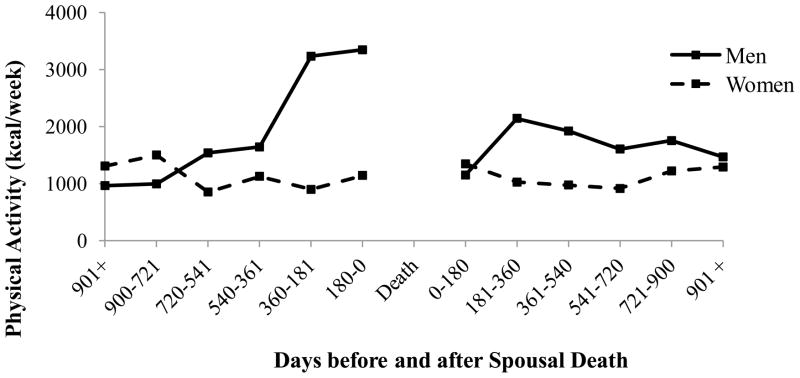
Figure 3 presents physical activity aggregated across 180 day intervals directly before and after spousal death, for men and women. GEE models examined linear change at all available waves before and after spousal death, spanning as many as seven years pre- and post death. To better understand the nature of physical activity at the end of life and the response of surviving spouses to the death of their spouse in the short term, we examined the functional form of physical activity in the two and a half years directly before and after spousal death. We used piecewise linear regression because it permitted the slopes to change across intervals but to remain constant within intervals. Estimates of the slopes of the lines in the model relating days before spousal death and physical activity showed that physical activity significantly increased in the 180–360 days prior to spousal death, but among men only (β=2.03, p<.05). Prior to those 360 days and through 900 days, physical activity increases, but the slopes of the line did not differ significantly from zero. Prior to those 900 days, men continuously decrease their physical activity (β= −.67, p<.05). In the model examining days after spousal death, physical activity increased among men in the 181–360 days immediately after spousal death (β=.56, p=.06). After 360 days, physical activity gradually decreases to day 900, but the slopes of the line did not differ significantly from zero. Among women, physical activity across each 180 day interval was relatively stable before (1103 kcal/week) and after spousal death (1094 kcal/week), and did not differ significantly from zero (p=.08 – .88).
Figure 3.
Changes in levels of physical activity (kcal/week) among 396 widows/widowers in the 900 days (2.5 years) before and after spousal death. Each point represents the average activity for elders whose spouse died during that time interval.
Post-hoc analyses
The unexpected finding that widowed men increase their activity following spousal death raised questions about the types of physical activity widowed men engage in post spousal death. We compared kcal/week spent in aerobic activity (e.g., jogging, biking, exercising), leisure-time activity (e.g., gardening, hiking, household activities), and walking pre- and post-bereavement among the 89 widowers. Paired-sample t-tests revealed that widowers reported, on average, more leisure-time activity post-bereavement (M=251.26kcal/week, SD=414.53kcal/week) than pre-bereavement (M=137.94kcal/week, SD=271.93.kcal/week), t(88) = −2.55, p<.05, d=.32. No significant differences emerged for aerobic activity or walking.
We also examined the specific types of activity men engage in during the 360 days in which they increased their activity prior to spousal death. Men spent more time engaged in household chores (M=216.87 min/week, SD=156.68 min/week) compared to the previous year (361–720 days) (M=55.74 min/week, SD=113.66 min/week), t (33) = −3.92, p<.01, d=1.17.
Discussion
The results of the current study indicate that experiencing the death of one’s spouse has a significant impact on husbands’ physical activity (kilocalories/week). Compared to married controls, widowed men increase their total energy expenditure following the death of their spouse. Medium effect sizes were calculated for men, whereas zero effect sizes were calculated for women. These findings are not consistent with those who suggest that physical activity declines following spousal loss (Umberson, 1987, 1992). However, our results further indicate that this increased level of activity is not sustained and declines as time since death passes. Our analyses show that within six months after the death, widows’ and widowers’ energy expenditure was similar, but within one year, widowers had significant increases in their levels activity. After one year postloss, this level of activity starts to decline, and total energy expenditure remains relatively stable among both men and women. The fact that activity levels do not change among widows could be due to the generally lower levels of physical activity among women, or the notion that the transition to widowhood affects men and women differently. Indeed, research by Lee et al. (2004) suggests that the effect of marital transitions on the health behaviors of women is not as intense as those reported by men.
Multiple studies have consistently shown an inverse association between depressive symptomatology and physical activity (Penedo & Dahn, 2005). Our findings indicate that within the context of widowhood, men may experience a smaller decline in their total energy expenditure when they experience an increase in depressive symptomatology following spousal loss. Although not statistically significant (p=.06), the effect sizes were larger for men than women and offer practical significance. Given the body of research that shows that men become more depressed than women following spousal loss, this finding provides useful direction for future research on the association between depression and physical activity among widowed men.
As time passes following spousal loss, depressive symptomatology has a negative effect on post-bereavement energy expenditure. Perhaps, the initial increase in activity post-death among men is a coping strategy aimed at alleviating depression symptoms associated with the transition to widowhood. This interpretation is consistent with the follow-up analysis showing that the increase in physical activity occurs predominantly in leisure activities (small effects calculated). Widowers may be more likely to engage in distracting activities following spousal loss, especially when experiencing depressive symptoms (Stroebe et al., 2001). These data suggest that men experience significant fluctuations in physical activity, particularly during the first year postloss.
The current study also shows that compared to married men, widowed men have significantly lower levels of physical activity prior to spousal death when we include all deaths occurring over a seven year period prior to death. Small effect sizes were calculated for men, whereas zero effect sizes were calculated for women. A more fine-grained analysis of activity levels in the two and a half years prior to death shows that men significantly increase their physical activity within one year before the death. It is likely that physical activity increases due to increased caregiving responsibilities related to the needs of a physically declining spouse. Our follow-up analyses show that men primarily engage in household activities and chores (large effects calculated). Among women, energy expenditure remains relatively stable prior to the death. Future studies should examine how the emotional and social reactions associated with an anticipatory grief period are associated with activity changes, particularly among men who may be providing care to spouse.
There are several limitations to note. Because widowhood is associated with increased morbidity and mortality (Elwert & Christakis, 2006; Schulz et al., 2000) it is possible that our sample was biased toward healthier participants who were more likely to attend their clinic/interview visits. Bereaved elders who were more depressed and more disabled may have been less likely to continue study participation. Due to limitations in the available data, we focused primarily on the association of physical health and depression with physical activity change among widows/widowers. Future research should include other psychological components such as loneliness, guilt, and grief. We were also not able to address the social changes associated with bereavement, including, social networks, and level of social support received from others. In addition, the average length of time between spousal death and the next assessment was one and a half years. Although widowhood may be sufficiently devastating to have lasting effects on physical activity, widows/widowers could have experienced additional events and/or transitions that were also associated with physical activity change. Finally, we could not control for the possible confound of being single. The number of other marital (divorce, separation) transitions in the CHS was too low to examine the effect of other life-changing events on physical activity.
Another limitation relates to the use of self-report questionnaires to assess older adults’ physical activity. Older adults typically engage in lighter activities at lower energy expenditures and perform these activities on an irregular basis (Harada et al., 2001). Unless designed for older populations, self-report measures are unable to capture these dimensions and underestimate older adults’ physical activity (Kowalski et al., 2012). In addition, older adults may have problems with cognition and short- and long-term memory, which affects their ability to accurately recall past behaviors. Future research should address these shortcomings by including objective measures of physical activity, such as the use of accelerometers.
Despite these limitations, the CHS dataset provided an excellent opportunity to prospectively examine the effect of the transition to widowhood on physical activity and the unique contributions of participant gender and depressive symptomatology. A major strength of this study is that it is the first to prospectively examine physical activity among both widows and widowers, and physical activity change prior to spousal bereavement. However, additional work is needed to examine the clinical significance of the identified relationships, including the role of post-bereavement physical activity on mental and physical health outcomes.
Acknowledgments
Funding
The research reported was supported by contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Preparation of this manuscript was also supported in part by grants from NIH L30 MH098406, P30 MH090333-01A1, MHO19986, NR009573, NR013450, AG026010, AG032370, and NSF 0540865.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Albanes D, Conway JM, Taylor PR, Moe PW, Judd J. Validation and comparison of eight physical activity questionnaires. Epidemiology. 1990;1:65–71. doi: 10.1097/00001648-199001000-00014. [DOI] [PubMed] [Google Scholar]
- Avis NE, Brambilla DJ, Vass K, McKinlay JB. The effect of widowhood on health: A prospective analysis from the Massachusetts women’s health study. Social Science Medicine. 1991;33:1063–1070. doi: 10.1016/0277-9536(91)90011-z. [DOI] [PubMed] [Google Scholar]
- Caserta MS, Lund DA, Rice SJ. Participants’ Attendance at a health promotion program for older widows and widowers. American Journal of Health Education. 2001;32:229–236. [Google Scholar]
- Carr D. A good death for whom? Quality of spouse’s death and psychological distress among older widowed persons. Journal of Health and Social Behavior. 2003;44:215–232. [PubMed] [Google Scholar]
- Carr D, House JS, Wortman C, Neese R, Kessler RC. Psychological adjustment to sudden and anticipated spousal loss among older widowed persons. Journals of Gerontology: Psychological Sciences. 2000;56:237–248. doi: 10.1093/geronb/56.4.s237. [DOI] [PubMed] [Google Scholar]
- Chen JH, Gill TM, Prigerson HG. Health behaviors associated with better quality of life for older bereaved persons. Journal of Palliative Medicine. 2005;8:96–106. doi: 10.1089/jpm.2005.8.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Elwert F, Christakis N. Widowhood and race. American Sociological Review. 2006;71:16–41. [Google Scholar]
- Eng PM, Kawachi I, Fitzmaurice G, Rimm EB. Effects of marital transitions on changes in dietary and other health behaviours in US male health professionals. Journal of Epidemiological Community Health. 2005;59:56–62. doi: 10.1136/jech.2004.020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström G, Khan FA, Ziab E, Ijerntorpa I, Pessag-Rasmussen H, Norrving B, Janzona L. Marital dissolution is followed by an increased incidence of stroke. Cerebrovascular Diseases. 2004;18:318–324. doi: 10.1159/000080770. [DOI] [PubMed] [Google Scholar]
- Erlangsen A, Jeune B, Bille-Brahe U, Vaupel JW. Loss of partner and suicide risk among oldest old: A population-based register study. Age and Ageing. 2004;33:378–383. doi: 10.1093/ageing/afh128. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick T, Spiro A, Kressin NR, Greene E, Bosse R. Leisure activities, stress, and health among bereaved and non-bereaved elderly men: The Normative Aging Study. Omega. 2001;43:217–245. [Google Scholar]
- Forte AL, Hill M, Pazder R, Feudtner C. Bereavement care interventions: A systematic review. BMC Palliative Care. 2004;3:3–17. doi: 10.1186/1472-684X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Newman A. The cardiovascular health study: Design and rationale. Annals of Epidemiology. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Medicine & Science in Sports & Exercise. 2001;33:962–70. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The social readjustment scale. Journal of Psychosomatic Research. 1967;11:213–228. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Janke MC, Nimrod G, Kleiber DA. Leisure activity and depressive symptoms of widowed and married women in later life. Journal of Leisure Research. 2008a;40:250–266. [Google Scholar]
- Janke MC, Nimrod G, Kleiber DA. Leisure patterns and health among recently widowed adults. Activities, Adaptation & Aging. 2008c;32:19–39. [Google Scholar]
- Janke MC, Nimrod G, Kleiber DA. Reduction in leisure activity and well-being during the transition to widowhood. Journal of Women & Aging. 2008b;20:83–98. doi: 10.1300/J074v20n01_07. [DOI] [PubMed] [Google Scholar]
- Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine & Science in Sports & Exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Kowalski K, Rhodes R, Naylor P, Tuokko H, MacDonald S. Direct and indirect measurement of physical activity in older adults: A systematic review of the literature. International Journal of Behavioral Nutrition and Physical Activity. 2012;9:148–169. doi: 10.1186/1479-5868-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Carr D. Does the context of spousal loss affect the physical functioning of older widowed persons? A longitudinal analysis. Research on Aging. 2007;29:454–487. [Google Scholar]
- Lee S, Cho E, Grodstein F, Kawachi I, Hu FB, Colditz GA. Effects of marital transitions on changes in dietary and other health behaviours in US women. International Journal of Epidemiology. 2005;34:69–78. doi: 10.1093/ije/dyh258. [DOI] [PubMed] [Google Scholar]
- Monk TH, Germain A, Reynolds CF., III Sleep disturbances in bereavement. Psychiatric Annals. 2008;38:671–678. doi: 10.3928/00485713-20081001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Reynolds CF, III, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosomatic Medicine. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiologic Studies Depression (CES-D) Scale. Journal of Clinical Psychology. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Patterson I. Participation in leisure activities by older adults after a stressful life event: The loss of a spouse. Journal of Aging and Human Development. 1996;42:123–142. doi: 10.2190/TG1M-75CB-PL27-R6G3. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Bierhals AJ, Kasl SV, Reynolds CF, III, Shear MK, Newson JT, Jacobs S. Complicated Grief as a disorder distinct from bereavement-related depression and anxiety A replication study. American Journal of Psychiatry. 1996;11:1484–1490. doi: 10.1176/ajp.153.11.1484. [DOI] [PubMed] [Google Scholar]
- Prohaska T, Belansky E, Belza B, Buchner D, Marshall V, McTigue K, Satariano W, Wilcox S. Physical activity, public health, and aging: Critical issues and research priorities. Journal of Gerontology, Social Sciences. 2006;61:267–273. doi: 10.1093/geronb/61.5.s267. [DOI] [PubMed] [Google Scholar]
- Schone BS, Weinick RM. Health-related behaviors and the benefits of marriage for elderly persons. The Gerontologist. 1998;38:618–627. doi: 10.1093/geront/38.5.618. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: The Cardiovascular Health Study. Archives of Internal Medicine. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Ling B, Martire LM, Zdaniuk B, Hirsch C, Burton L. Involvement in caregiving and adjustment to death of a Spouse: Findings from the caregiver health effects study. Journal of the American Medical Association. 2001;285:3123–3129. doi: 10.1001/jama.285.24.3123. [DOI] [PubMed] [Google Scholar]
- Schulz R, Boerner K, Shear K, Zhang S, Gitlin LN. Predictors of complicated grief among dementia caregivers: A prospective study of bereavement. The American Journal of Geriatric Psychiatry. 2006;14:650–658. doi: 10.1097/01.JGP.0000203178.44894.db. [DOI] [PubMed] [Google Scholar]
- Stroebe MS. New directions in bereavement research: Exploration of gender differences. Palliative Medicine. 1998;12:5–12. doi: 10.1191/026921698668142811. [DOI] [PubMed] [Google Scholar]
- Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- Stroebe M, Stroebe W, Schut H. Gender differences in adjustment to bereavement: An empirical and theoretical review. Review of General Psychology. 2001;5:62–83. [Google Scholar]
- Taylor HL, Jocobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activity. Journal of Chronic Diseases. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Tran MHB. Doctoral dissertation. 2007. The caregiving stress process: Examining the influence of the nature of the care-recipient illness, stress-buffering variables, mediating factors, and caregiving transitions on caregiver health. Retrieved from ProQuest Information & Learning. (AAINR31877) [Google Scholar]
- Umberson D. Family status and health behaviors: Social control as a dimension of social integration. Journal of Health and Social Behavior. 1987;28:306–319. [PubMed] [Google Scholar]
- Umberson D. Gender, marital status, and the social control of health behavior. Social Science and Medicine. 1992;34:907–917. doi: 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- Umberson D, Wortman CB, Kessler RC. Widowhood and depression: Explaining long-term gender differences in vulnerability. Journal of Health and Social Behavior. 1992;33:10–24. [PubMed] [Google Scholar]
- Utz RL, Carr D, Nesse R, Wortman CB. The effect of widowhood on older adults’ social participation. An evaluation of activity, disengagement, and continuity theories. The Gerontologist. 2002;42:522–533. doi: 10.1093/geront/42.4.522. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Evenson KR, Aragaki A, Wassertheil-Smoller S, Mouton CP, Loevinger BL. The effects of widowhood on physical and mental health, health behaviors, and health outcomes: The women’s health initiative. Health Psychology. 2003;22:513–522. doi: 10.1037/0278-6133.22.5.513. [DOI] [PubMed] [Google Scholar]
- Wilcox S, King AC. The effects of life events and interpersonal loss on exercise adherence in older adults. Journal of Aging and Physical Activity. 2004;12:117–130. doi: 10.1123/japa.12.2.117. [DOI] [PubMed] [Google Scholar]