Abstract
G protein-coupled receptor kinase 4 (GRK4) gene variants, via impairment of renal dopamine receptor and enhancement of renin-angiotensin system functions, cause sodium retention and increase blood pressure. Whether or not GRK4 and the angiotensin type 1 receptor (AT1R) interact in the aorta is not known. We report that GRK4 is expressed in vascular smooth muscle cells (VSMCs) of the aorta. Heterologous expression of the GRK4γ variant 142V in A10 cells increased AT1R protein expression and AT1R-mediated increase in intracellular calcium concentration. The increase in AT1R expression was related to an increase in AT1R mRNA expression via the NF-κB pathway. As compared with control, cells expressing GRK4γ 142V had greater NF-κB activity with more NF-κB bound to the AT1R promoter. The increased AT1R expression in cells expressing GRK4γ 142V was also associated with decreased AT1R degradation, which may be ascribed to lower AT1R phosphorylation. There was a direct interaction between GRK4γ wild-type (WT) and AT1R that was decreased by GRK4γ 142V. The regulation of AT1R expression by GRK4γ 142V in A10 cells was confirmed in GRK4γ 142V transgenic mice; AT1R expression was higher in the aorta of GRK4γ 142V transgenic mice than control GRK4γ wild-type (WT) mice. Angiotensin II-mediated vasoconstriction of the aorta was also higher in GRK4γ 142V than WT transgenic mice. This study provides a mechanism by which GRK4, via regulation of arterial AT1R expression and function, participates in the pathogenesis of conduit vessel abnormalities in hypertension.
Keywords: GRK4, AT1R, artery, hypertension
Introduction
Essential hypertension, which affects 25% of the middle-aged adult population, constitutes a major risk factor for stroke, myocardial infarction, and heart and kidney failure 1, 2. The kidney, vasculature, and nervous system govern the long-term control of blood pressure by regulating sodium homeostasis, peripheral resistance, and central arterial stiffness 3-5; they, in turn, are influenced by numerous hormones and neural and humoral factors. Hypertension may be caused not only by increased activity of pro-hypertensive systems but also by defects in anti-hypertensive systems that serve as counter-regulatory mechanisms 4, 6-8. Most hormones and humoral factors regulate blood pressure via their receptors, including G protein-coupled receptors (GPCRs). GPCRs comprise the largest family of cell surface receptors 6-8; abnormal G protein-coupled receptor kinase (GRK) function has the potential to affect receptor-regulated biological responses in many physiological and pathological conditions, including hypertension 4, 5, 7.
The GRK family plays an important role in the regulation of blood pressure9. GRK4 is distinguished from other members of the GRK family by its constitutive activity 10, 11 and limited tissue expression 6, 7. The GRK4 variants 65L, 142V, and 486V are associated with essential hypertension in ethnically distinct populations 8, 11, 12. Over-expression of human (h) GRK4γ 142V or hGRK4γ 486V in mice produces hypertension 8, 13. The hypertension of spontaneously hypertensive rats (SHRs) may also be explained, in part, by increased renal GRK4 expression 14. Our previous study found that increased renal GRK4 expression causes the attenuated renal D1 dopamine receptor-mediated natriuresis and diuresis that play a role in the pathogenesis of the hypertension in SHRs14.
Increased activity of the renin-angiotensin system is important in the pathogenesis of hypertension 5, 15. GRK4 interacts not only with the dopaminergic but also with the renin-angiotensin system to regulate blood pressure 8. Increased renal expressions of both GRK4 and angiotensin type 1 receptor (AT1R) contribute to the increased blood pressure in SHRs because selective renal silencing of both GRK4 and AT1R increases sodium excretion and decreases blood pressure to a greater extent than silencing of either GRK4 or AT1R16.
Conduit and resistance arterial vessels are important in the regulation of blood pressure and myocardial function17. Increased aortic stiffness, a risk factor in cardiovascular disease, may be related to increased activity of the renin-angiotensin system 3, 18. Whether or not GRK4 and the AT1R interact in the aorta and other arteries in regulating vascular smooth muscle function is not known. Our present study found expression of GRK4 in the tunica media of arteries; vascular smooth muscle cells (VSMCs), transduced with the GRK4γ variant 142V, increased AT1R expression and function. The regulation of AT1R by GRK4 is of physiological significance because AT1R expression and angiotensin II (Ang II)-mediated vasoconstriction in the aorta were greater in hGRK4γ 142V than hGRK4γ wild-type (WT) transgenic mice. Infusion of the AT1R antagonist, candesartan, lowered blood pressure to a greater and longer extent in hGRK4γ 142V than hGRK4γ WT transgenic mice. Our present study provides a mechanism by which GRK4, via regulation of arterial AT1R expression and function, participates in the pathogenesis of hypertension.
Methods
1. Transgenic mice
hGRK4γ WT and hGRK4γ 142V transgenic mice were generated as previously described11, 13 in Supplemental Materials. As previously reported 11, 12, 19, the genetic variation is GCC to GTC (amino acid 142V, rs1024323). (Supplementary Figure S1)
This study was approved by the Third Military Medical University Animal Use and Care Committee. All experiments conformed to the guidelines of the ethical use of animals, and all efforts were made to minimize animal suffering and to reduce the number of animals used.
2. Cell culture and GRK4 transduction
Embryonic thoracic aortic smooth muscle cells (passage 10-20) from normotensive Berlin-Druckrey IX (A10; CRL 1476, ATCC) were homogenized in ice-cold lysis buffer (5 ml/gm tissue), sonicated, kept on ice for 1 hr, and centrifuged at 16,000 g for 30 min. All samples were stored at −70°C until use.
The lentivirus-based pLenti6.3-hGRK4γ-IRES2-EGFP plasmid (Invitrogen Life Technologies Corporation, Shanghai, China) (Supplementary Figure S2A), was transiently transduced into 293TN cells. The A10 cells (1.5×106 /ml) were cultured in 2 ml DMEM medium containing 2% FBS, 8 μg/ml polybrene and virus (MOI=100). The medium was replaced 48h after transduction, and then 5 μg/ml blasticidin was added and incubated for another 48h. The transduced cells were identified by GFP expression (Supplementary Figure S2B).
3. Small interfering RNA
Small interfering RNA (siRNA) against GRK4 mRNA and its control scrambled RNA were synthesized and purified with reverse-phase high-performance liquid chromatography as 25-mer phosphorothioate-modified oligodeoxynucleotides (GRK4 siRNA sequence: #1 5’-AUCUAAAGAGGUGCAUUGAAUUCUUdTdT-3’, #2 5’-AAGGACCUCAAUGAAUAUGAAGAUAdTdT-3’; scrambled RNA sequence: 5′-TGACGATAAGAACAATAACdTdT-3′), from nucleotides 412 to 436 and 1752 to 1776 of the rat GRK4 cDNA.
The effects of 50 nM siRNA were compared with scrambled RNA (control). Briefly, cells were grown in 6-well plates until 60% confluence, and 50 nM siRNA or control RNA were mixed with 6 μL of oligofectamine in Optimem medium (Invitrogen Life Technologies) and incubated for 24 hr, then switched to growth medium and incubated for another 24 hr. The cells were collected and processed for RT-PCR for GRK4 to determine the efficiency of siRNA-induced GRK4 gene silencing (Supplementary Figure S3).
4. Immunoblotting
After subjecting the cell lysates to centrifugation at 12,000 g for 15 min, the supernatants of A10 cells were collected and their protein concentrations were measured using a bicinchoninic acid (BCA) protein assay kit (Hyclone Pierce, Logan, USA). Immunoblotting was performed as previously reported 20, 21, except that the transblots were probed with the rabbit anti-GRK4 antibody (1:400) and rabbit anti-AT1R antibody (1:500) (Santa Cruz Biotechnology, CA). The amount of protein transferred onto the membranes was verified by immunoblotting for β-actin.
5. Confocal microscopy of double-stained transduced A10 cells and artery
The aortae from Sprague-Dawley (SD) rats, cleared of blood with ice-cold oxygenated saline and kept in Histochoice (Amresco, Solon, OH) for 1-2 days at 4°C, were sectioned (4 μm), embedded in paraffin, and mounted on slides. Reactions with antibodies were performed as described previously22-26. in Supplemental Materials
Transduced A10 cells, grown on coverslips, were fixed and permeabilized with 100% methanol (30 min). Reactions with antibodies were performed as described previously27 in Supplemental Materials.
6. Immunoprecipitation
Equal amounts of cell lysates (300 μg protein/ml supernatant) were incubated with affinity-purified anti-GRK4 receptor antibody (3μl/ml) (GRK4/AT1R co-immunoprecipitation) or polyclonal antiphosphoserine antibody (Zymed Laboratory, San Francisco, CA) (AT1R phosphorylation) (1μg/ml) for 1 hr and protein-G agarose at 4°C for 12 hr. The immunoprecipitates were subjected to immunoblotting with the AT1R antibody. To determine the specificity of the bands found on the immunoblots, IgG (negative control) and AT1R antibody (positive control) were used as the immunoprecipitants, instead of the GRK4 antibody.
7. RT-PCR of GRK4 and AT1R
A total of 2 μg of total RNA extracted from hGRK4γ WT or hGRK4γ 142V transduced cells was used to synthesize cDNA and served as a template for amplification of AT1R, GRK4, and β-actin, which served as the house-keeping gene control. The AT1R and GRK4 mRNA expressions were normalized by β-actin mRNA.
The GRK4 bands, cut from the gels, were extracted by DNA gel extraction kit (Omega, US). After purification, the DNA was sequenced and aligned by DNAMAN software (Lynnon Biosoft, USA)
8. Electrophoretic mobility shift assay (EMSA)
EMSA was performed with the Light-shift Chemiluminescent EMSA Kit (Pierce Chemical Co., Rockford, IL), according to the manufacturer's recommendations28, 29. A synthetic DNA double-stranded oligonucleotide probe (5’-biotin-AGTTGAGGGGACTTTCCCAGGC-5’) containing the sequence of the rat AT1R gene promoter between nucleotides -350 bp and -363 bp (5’-AAGGGAGTTCCCTA-3’) was labeled with biotin and incubated with the nuclear extracts.
9. Intracellular calcium measurement
Intracellular calcium was measured, as previously described with some modifications30, 31 in Supplemental Materials. The free Ca2+ concentration [Ca2+]free was calculated from the equation32: [Ca2+]free = Kd[(R-Rmin)/(Rmax-R)](F380max/F380min); The Kd is the dissociation constant of Fura-2 to calcium. R is the ratio of each 340 nm/380 nm. Minimum and maximum are the fluorescence values of cells treated by Triton X-100 (saturating Ca2+ concentration) or by EGTA (zero Ca2+ concentration).
10. Artery ring study
Thoracic aortae were obtained from the hGRK4γ WT and hGRK4γ 142V transgenic mice. Each artery was cut into a ring of 2- to 3-mm long for the experiments, which was used to measure the vascular reactivity to Ang II (Sigma-Aldrich, St. Louis, MO), in the presence or absence of the endothelium, as described in Supplemental Materials.
An intact functional endothelium in all preparations was assessed by determining a vasodilatory response to acetylcholine (Ach) (10−6 M; Sigma). If Ach (10−6 M) induced the relaxation of artery rings preconstricted with norepinephrine (10−6 M) by more than 75%, the arterial endothelium can be considered intact 33.
11. Statistical analysis
The data are expressed as mean ± SEM. Comparison within groups was made by repeated measures ANOVA (or paired t test when only 2 groups were compared), and comparison among groups (or t test when only 2 groups were compared) was made by factorial ANOVA with Holm-Sidak test. A value of P<0.05 was considered significant.
Results
1. Expression of GRK4 in artery
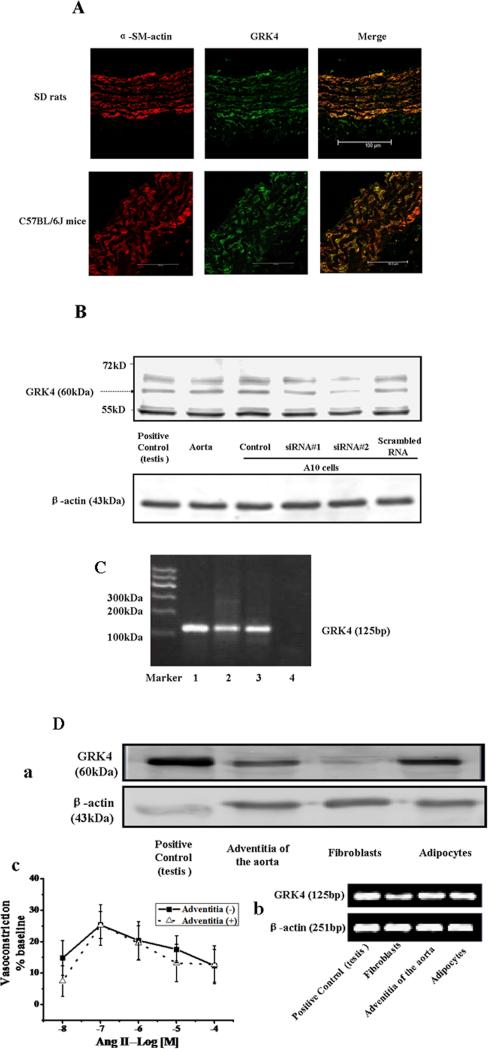
We first determined if GRK4 is expressed in the aorta by immunofluorescence, immunoblotting, and RT-PCR. Immunofluorescence microscopy showed GRK4 staining in the tunica media and adventitia of aortae from SD rats and C57BL/6J mice (Figure 1A). GRK4 expression was also found with immunoblotting; specific GRK4 (54 kDa, 60 kDa, 65 kDa) bands were found in A10 cells, which were attenuated, especially the 60 kDa band, after transduction with the specific GRK4 siRNA (Figure 1B). The specificity of this GRK4 antibody was reported in our published study14. RT-PCR showed the expected 125 bp GRK4 band, based on the primers, which was not observed when RNA was omitted in the RT period (Figure 1C). The gel containing the 125bp band was cut, sequenced, and aligned by DNAMAN software (Lynnon Biosoft, USA) (Supplemental Figure S4).
Figure 1. GRK4 expression in aorta.
A: Immunofluorescence staining of GRK4 in aorta from SD rats and C57BL/6J mice. The aorta was washed, fixed, and immunostained for GRK4 and α-smooth muscle (SM)-actin, as described in the Methods. Colocalization appears as yellow after merging the images of α-smooth muscle (SM)-actin (red) and GRK4 (green). These studies were repeated at least three times.
B: GRK4 protein expression in A10 cells. Protein (100μg) from A10 cells were subjected to immunoblotting with anti-GRK4 antibody (1:400). The band was attenuated after transfection with the specific GRK4 siRNA into A10 cells (GRK4 siRNA sequence: #1: 5’-AUCUAAAGAGGUGCAUUGAAUUCUUdTdT-3’; #2: 5’-AAGGACCUCAAUGAAUAUGAAGAUAdTdT-3’) compared with the band of A10 cells without siRNA transfection (scrambled RNA sequence: 5′-TGACGATAAGAACAATAACdTdT-3′). The 54, 60, and 65 kDa bands were found in the aorta and A10 cells, as well as in testis which was used as positive control. These bands are specific GRK4 proteins, as previously published, using the same GRK4 antibody 14.
C: GRK4 mRNA expression in aorta from SD rat and A10 cells. GRK4 RT-PCR products from testis (lane 1, positive control), aorta (lane 2) and A10 cells (lane 3) were analyzed in 10% polyacrylamide gel stained with ethidium bromide. An amplification product of the predicted size (125 bp) is seen in RT-PCR reaction using RNA (1μg). No amplification is seen in the absence of RNA (lane 4). D: GRK4 expression and function in the adventitia of the aorta. GRK4 expressions were checked in the fibroblasts and adipocytes by immunoblotting (D-a) and RT-PCR (D-b), samples from testis of SD rats were taken as positive control. Removal of the adventitia did not affect the Ang II (10−8-10−M)-mediated vasoconstriction (D-c) (n=4, P=NS).
To confirm the GRK4 expression in the adventitia, we checked the GRK4 expression in fibroblasts and adipocytes by immunoblotting and RT-PCR. We found that both fibroblasts and adipocytes expressed GRK4 (Figures 1D-a and 1D-b). Removal of the adventitia did not affect the Ang II-mediated vasoconstriction, indicating that the GRK4 in the adventitia did not take participate in the Ang II-mediated vasoconstriction (Figure 1D-c). The physiological significance of GRK4 in the adventitia remains to be determined.
We measured the GRK4 expression in large and small vessels, including the thoracic aorta, superior mesenteric artery, carotid arteries and renal artery, and found there was no difference for the GRK4 expression in those vessels (supplemental Figure S5).
2. Regulation by GRK4 of AT1R expression and function in A10 cells
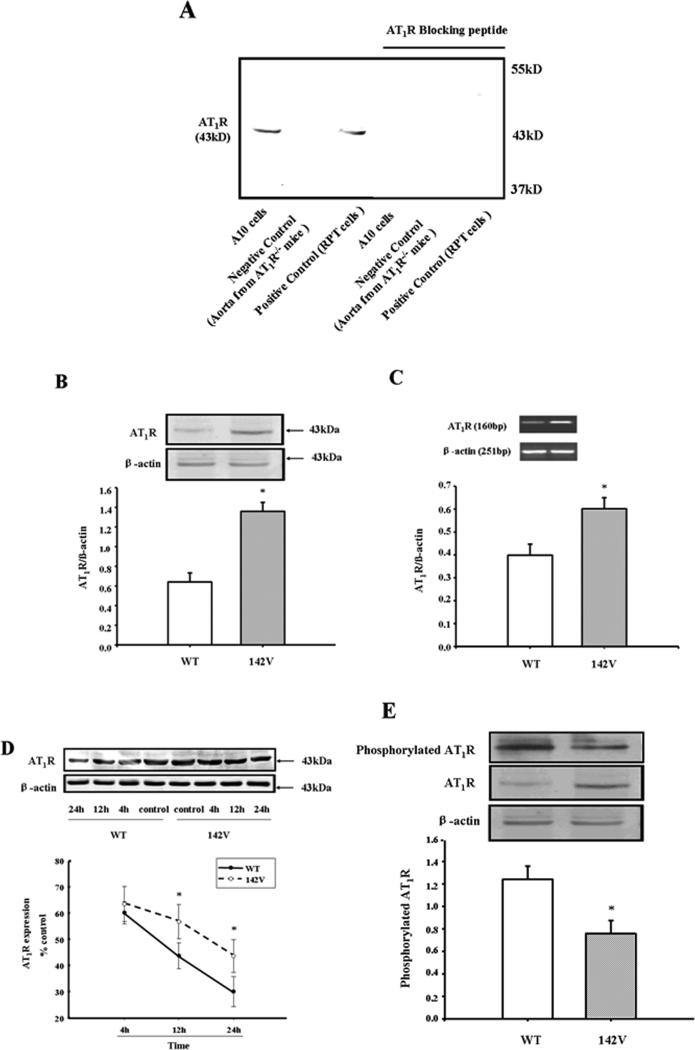
AT1R antibody specificity was determined by immunoblotting, the 43kDa band was absence in the aorta from AT1R−/− mice and no longer visible in A10 and renal proximal tubule cells (positive control) when the antibody was pre-adsorbed with the immunizing peptide (Figure 2A).
Figure 2. Expression of AT1R in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells.
A: Specificity of AT1R antibody. Protein (100μg) from A10 cells, renal proximal tubule (RPT) cells from SD rats, and aortae from AT1R−/− mice were subjected to immunoblotting with anti-AT1R antibody (1:500) with or without pre-incubation with the AT1R antibody immunizing peptide (Santa Cruz, 1:10 w/w incubation for 12 hr). These studies were repeated at least three times.
B and C: AT1R protein (B n=7) and mRNA (C, n=6) expressions in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells. Results are expressed as the ratio of AT1R receptor and α-actin (*P<0.05 vs. WT)
D: AT1R protein degradation in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells. The cells were incubated with cycloheximide (10−5 M) for the indicated times. Results are expressed as percent change of control in each group (n = 8, *P < 0.05 vs. WT).
E: AT1R phosphorylation in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells. The A10 cell lysate protein was immunoprecipitated with anti-phosphoserine antibody and immunoblotted with anti-AT1R antibody (n = 3, *P < 0.05 vs. WT).
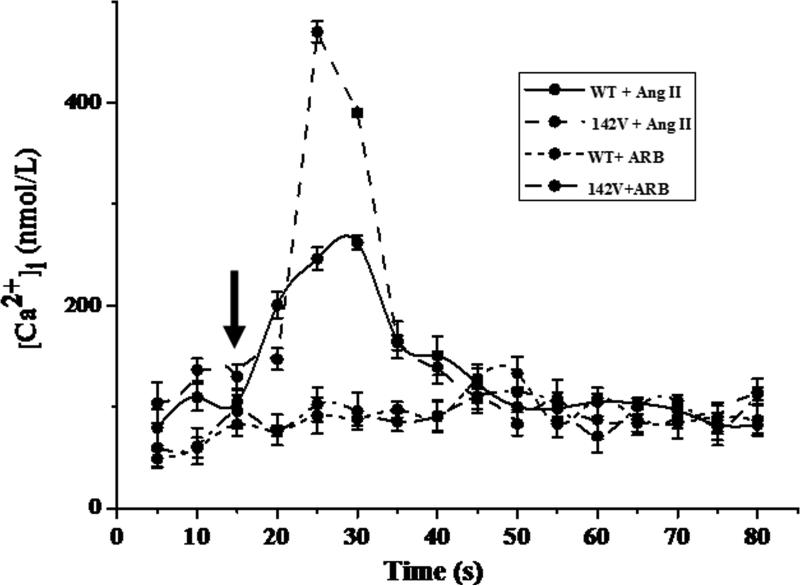
To determine the effect of hGRK4 on AT1R expression, we used A10 cells transduced with hGRK4γ 142V. We found, although the GRK4 expression was not different between hGRK4γ 142V and control (GRK4γ WT) cells (Supplemental Figure S6). However, AT1R protein and mRNA expressions were higher in hGRK4γ 142V than GRK4γ WT cells (Figures 2B and 2C); AT1R protein degradation was lower in hGRK4γ 142V- than GRK4γ WT-transduced cells (Figure 2D), indicating that the regulation of AT1R expression by hGRK4γ occurred at both post-translational and transcriptional levels. In addition, AT1R phosphorylation was lower in hGRK4γ 142V- than hGRK4 WT-transduced cells (Figure 2E), indicating that the decreased AT1R protein degradation may be ascribed to decreased AT1R phosphorylation. The increased AT1R expression is physiologically relevant because the intracellular calcium concentration after stimulation with Ang II (10−7 M) was higher in hGRK4γ 142V- than hGRK4γ WT-transduced cells (Figure 3).
Figure 3.
Intracellular calcium concentration in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells. Representative tracing of the effect of Ang II (10−7M) on intracellular free calcium in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells. Ang II was added 15s after the start of the experiment, shown as the arrow in the figure (n=8).
To investigate whether or not Ang II was involved in the regulation of GRK4 on AT1R expression, we measured the concentration of Ang II in the A10 cell culture supernatant and cell lysate; Ang II concentrations were not different between hGRK4γ 142V- and hGRK4γ WT-transduced cells (culture supernatant: 113.87± 13.07 v.s. 108.73±12.76; cell lysate: 237.3±23.7 v.s. 217±20, n=5, P=NS). The angiotensin converting enzyme inhibitor, captopril (10-4 M, Sigma-Aldrich, St. Louis, MO), also had no effect on the AT1R expression in both cell types (Supplemental Figure S7).
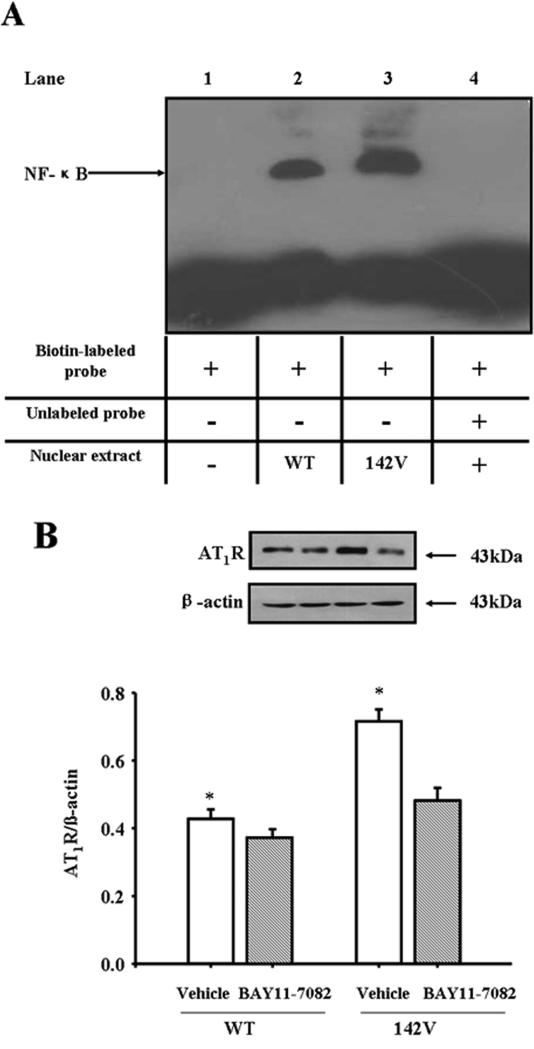
As a regulator of AT1R promoter activity, we measured NF-κB binding to the AT1R promoter and found it higher in hGRK4γ 142V- than hGRK4γ WT-transduced cells (Figure 4A). Blockade of NF-κB with an NF-κB inhibitor, BAY11-7082, inhibited the increase in AT1R expression in hGRK4γ 142V-transduced cells (Figure 4B), indicating that NF-κB was involved in the positive regulation of AT1R expression by hGRK4γ 142V.
Figure 4. Role of NF-κB on AT1R expression in GRK4γ WT- and hGRK4γ 142V-transduced A10 cells.
A. EMSA of nuclear protein from A10 cells. Binding activity of AT1R gene promoter (-350 bp and -363 bp), containing an NF-κB site, was examined in nuclear proteins from hGRK4γ WT- (lane 2) and hGRK4γ 142V (lane 3)-transduced A10 cells by EMSA. No nuclear extracts (lane 1) or fifty times unlabeled probe (lane 4) were added to the reaction mixture and served as negative controls. B. Effect of NF-κB on GRK4-mediated regulation of AT1R protein expression in A10 cells. hGRK4γ WT- or hGRK4γ 142V-transduced cells were treated with or without the NF-κB inhibitor BAY11-7082 (20 μM) for 24hr. Results are expressed as the ratio of AT1R and α-actin (n = 5, *P<0.05 vs. vehicle).
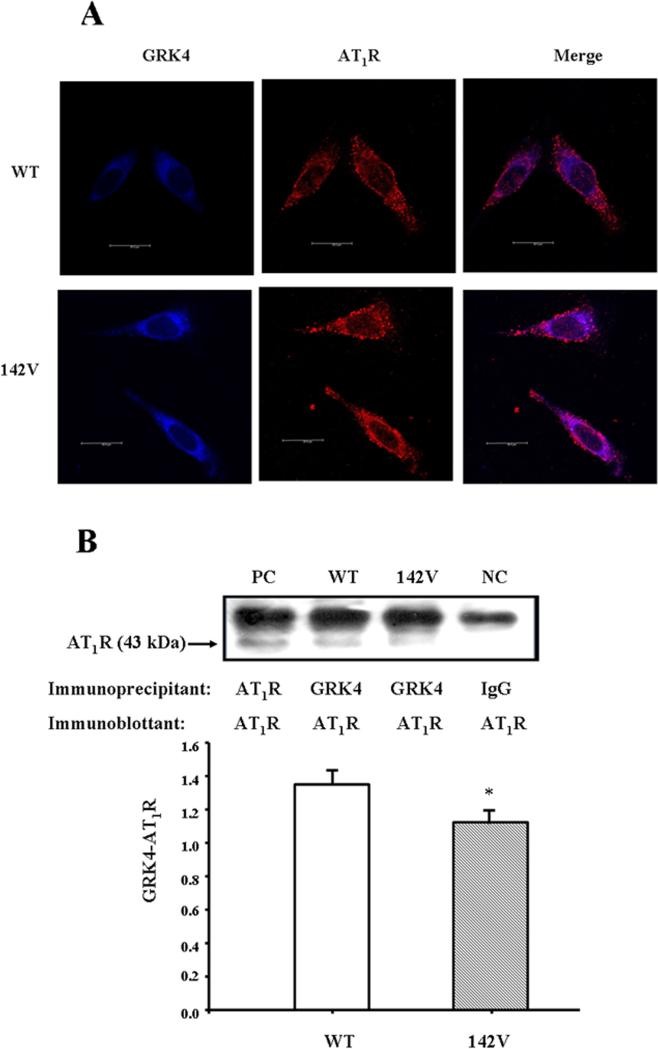
As aforementioned, the decreased AT1R degradation could be ascribed to the decrease in AT1R phosphorylation in GRK4γ 142V-transduced cells. An additional study found a co-localization (Figure 5A) and co-immunoprecipitation (Figure 5B) between GRK4 and AT1R; the co-immunoprecipitation of GRK4 and AT1R was less in hGRK4γ 142V- than hGRK4γ WT-transduced cells (Figure 5B), which could be a factor in the decreased phosphorylation of AT1R in hGRK4γ 142V-transduced cells.
Figure 5. Colocalization and co-immunoprecipitation of GRK4 and AT1R in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells.
A: Colocalization of GRK4 and AT1R in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells. The cells were washed, fixed, and immunostained for GRK4 and AT1R, as described in the Methods. Colocalization appears as purple after merging the images of AMCA-tagged GRK4 (blue) and rhodamine-tagged AT1R (red). B: Co-immunoprecipitation of GRK4 and AT1R in hGRK4γ WT- and hGRK4γ 142V-transduced A10 cells. The cells were immunoprecipitated with GRK4 antibodies and immunoblotted with AT1R antibodies (*P<0.05 vs. control, n=4, ANOVA, Holm-Sidak test). One immunoblot (43 kDa) is depicted in the inset: (PC = positive control, WT = hGRK4γ WT-transduced A10 cells, 142V = hGRK4γ 142V-transduced A10 cells, NC = negative control. For the positive control, AT1R antibody was used and for the negative control, IgG was used instead of GRK4 antibody as the immunoprecipitants.
3. AT1R expression and function in hGRK4γ 142V transgenic mice
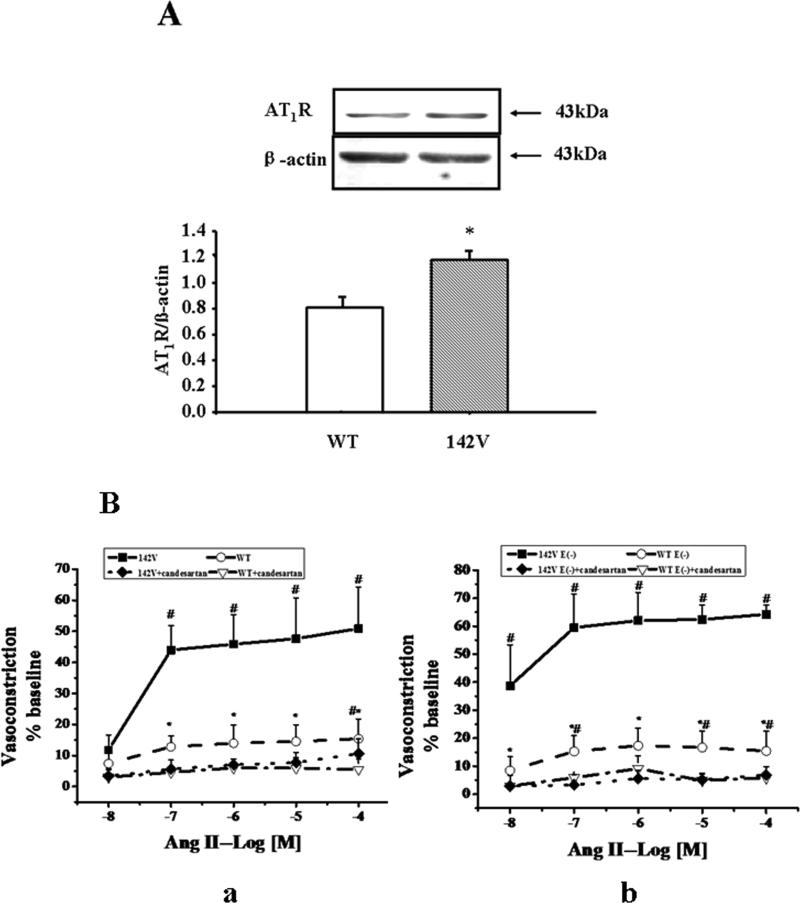
To further investigate the physiological role of the GRK4-regulated AT1R expression, we studied AT1R expression and function in hGRK4γ WT and hGRK4γ 142V transgenic mice. Consistent with previous reports6, 8, 11, 13, anesthetized hGRK4γ 142V transgenic mice had higher systolic (S), diastolic (D) and mean (M) blood pressures (SBP =123.37 ± 8.19, DBP = 96.37 ± 4.78 mmHg, MBP = 104.54 ± 3.99, n = 11) than anesthetized hGRK4γ WT transgenic mice (SBP = 98.38 ± 5.42, DBP = 83.00 ± 4.54 mmHg, MBP = 88.21 ± 3.63, n = 11, P<0.001). Although GRK4 expression was not different between hGRK4γ WT 142V and hGRK4γ 142V transgenic mice (Supplemental Figure S5), AT1R expression in aorta was higher in hGRK4γ 142V than hGRK4γ WT transgenic mice (Figure 6A). We also studied the vasoconstrictor effect of Ang II on the aorta from hGRK4γ 142V and hGRK4γ WT transgenic mice. The vasoconstriction caused by Ang II was greater in hGRK4γ 142V than hGRK4γ WT transgenic mice in the presence or absence of the endothelium. The AT1R blocker, candesartan (10−6M) blocked the vasoconstrictor effect of Ang II, in both transgenic mice such that there was no longer any difference between the two mouse strains (Figure 6B).
Figure 6. AT1R expression and function in hGRK4γ 142V transgenic mice.
A: AT1R expression in aortae from hGRK4γ 142V transgenic mice. Aortic homogenates (50μg) from hGRK4γ 142V and hGRK4γ WT transgenic mice were subjected to immunoblotting with anti-AT1R antibody (1:400). Results are expressed as the ratio of AT1R to ß-actin densities (n =5, *P<0.05 vs. control, t-test). B: Aortic rings with (B-a) or without (B-b) endothelium (E-) from hGRK4γ WT and hGRK4γ 142V transgenic mice were exposed to varying concentrations of Ang II (10−8-10−4M). To determine the specificity of the Ang II effect on the AT1R, candesartan (ARB, 10−6M) was added 15 min before the Ang II treatment. The results are expressed as percent change from baseline. (*P<0.05 vs. 142V control, #P<0.05 vs. corresponding group with ARB treatment, n = 12).
Consistent with a previous report34, the intravenous infusion of Ang II (1 μg/kg/min at rate of 10 μl/h) caused a greater increase in SBP in hGRK4γ 142V than hGRK4γ WT transgenic mice while the intravenous infusion of candesartan (0.139 μg/kg/min at a rate of 10 μl/h) caused a greater decrease in blood pressure in hGRK4γ 142V than hGRK4γ WT transgenic mice(Supplemental Figure S8).
Discussion
GRK4, as with the other members of the GRK family, is predominantly localized at the plasma membrane, as a result of palmitoylation of its C-terminal cysteine residues 35. GRK4 differs from the other GRKs in tissue distribution; GRKs 2, 3, 5, and 6 are ubiquitously expressed, whereas GRK4 is abundantly expressed in the testis, myometrium, and kidney 7, 8, 11. We now show for the first time the expression of GRK4 in the aorta, determined by immunoblotting, immunohistochemistry (tunica media) and RT-PCR, implying that GRK4 could be involved in the regulation of vascular smooth muscle function.
There is increasing evidence that GRK4 plays an important role in the pathogenesis of hypertension 6-8, 11, 13, 14, 16, 34. The GRK4 locus (4p16.3) is linked to and GRK4 gene variants are associated with human essential hypertension 4, 6, 8, 11, 12, 36-38. In Ghanaians, the two-locus model of angiotensin converting enzyme I/D and GRK4 65L predicts the hypertensive phenotype 70.5% of the time 37. GRK4 variants, including 65L, 142V, and 486V, by themselves, or interaction with other variants of other genes are associated with hypertension in American Caucasians39, Australian Caucasians12, Italians36 and northern Han Chinese40. We have reported that hGRK4γ 142V transgenic mice on 98% C57BL/6J background are hypertensive relative to non-transgenic littermates and hGRK4γ WT transgenic mice 6, 8, 11, 13, 34. To further investigate the role of GRK4 variants on the hypertension, we generated hGRK4γ 142V and GRK4γ WT transgenic mice on C57BL/6J and SJL/J background. C57BL/6 mice are salt-sensitive while SJL/J mice are salt-resistant 41. We now report that hGRK4γ 142V mice on mixed C57BL/6J and SJL/J background have increased blood pressure.
We have reported that hGRK4γ 142V transgenic mice have increased blood pressure and impaired ability to excrete a sodium load 11. The impaired sodium excretion is mainly due to a dysfunction of the D1 dopamine receptor 4, 6-8, 11, 14, 42. Dopamine, produced by the renal proximal tubule is important in the regulation of sodium excretion and blood pressure 4, 6-8, 11, 14, 42. While the renal dopaminergic system keeps the blood pressure from increasing following a moderate sodium load 4, 6-8, 11, 14, 42, the renin-angiotensin system, including the AT1R, is crucial in sodium retention and maintenance of blood pressure, especially under conditions of sodium deficit 5, 8, 15. Both GRK4 and AT1R exist in VSMCs, but whether or not GRK4-mediated regulation of blood pressure involves the AT1R in VSMCs is not known. Our present study found that compared with hGRK4γ WT transgenic mice, hGRK4γ 142V transgenic mice have higher arterial AT1R expression and Ang II-mediated aortic vasoconstriction. Ang II-mediated increase in intracellular calcium is also increased to a greater extent in hGRK4γ 142V- than hGRK4γ WT-transduced A10 aortic cells The stimulatory effect of hGRK4γ 142V on AT1R receptor expression and function is physiologically relevant because the intravenous infusion of Ang II increased while the intravenous of infusion of an AT1R antagonist, candesartan, decreased blood pressure to a greater degree and longer extent in hGRK4γ 142V than hGRK4γ WT transgenic mice. In the current study, the transgenic mice are on 50% C57BL/6 Jackson and 50% SJL Jackson mouse background. GRK4 and AT1R protein expression are greater in C57BL/6 Jackson than SJL Jackson mice 41. hGRK4γ 142V transgenic mice on C57BL/6 background are also hypertensive that is caused in part by decreased renal D1 receptor function 11, 13, 34 and increased renal AT1R expression 34. The increase in blood pressure in hGRK4γ 142V in C57BL/6 and SJL Jackson mice is not mitigated by the 50% SJL Jackson genetic background, and thus, independent of the presence of the salt-resistant phenotype.
As a kinase, GRK4 phosphorylates ligand-unoccupied and -occupied GPCRs as their primary substrates, such as the D1 dopamine receptor 6-11. Increased GRK4 activity augments D1 receptor phosphorylation in kidney 6, 7, 8, 10, 11, 14. However, our present study found that increased GRK4 activity decreases AT1R phosphorylation, which seems counterintuitive, at first glance. Our experiments uncover a possible mechanism; there is a linkage between GRK4 and AT1R in VSMCs, and it is interesting to find that the GRK4/AT1R linkage is decreased in A10 cells transduced with hGRK4γ 142V, which may therefore cause decrease in AT1R phosphorylation in the hGRK4γ 142V A10-transduced cells. The decreased phosphorylation of AT1R in hGRK4γ 142V A10-transduced cells may be involved in the GRK4γ 142V-mediated up-regulation of AT1R expression, because in present study, we found that a decreased AT1R degradation accompanies the decreased AT1R phosphorylation in hGRK4γ 142V-transduced A10 cells. The pathway leading to the lower binding of hGRK4γ 142V with AT1R receptor is not known, which needs to be elucidated in the future.
The regulation hGRK4γ-mediated regulation of AT1R expression is complicated, as in our present study, we found that in addition to hGRK4γ 142V-mediated decrease in AT1R degradation, AT1R transcription is also increased, as evidenced by increased AT1R mRNA in hGRK4γ 142V-transduced A10 cells. The activity of NF-κB, a regulator of AT1R promoter activity, is increased, accompanied by an increase in its binding to the AT1R promoter in hGRK4γ 142V-transduced A10 cells. In the presence of an NF-κB inhibitor, the increase in AT1R expression in hGRK4γ 142V-transduced A10 cells is abolished, confirming the important role of NF-κB in this process.
Conclusion and Perspectives
Our previous study found that increased renal GRK4 expression causes the attenuated renal D1 dopamine receptor-mediated natriuresis and diuresis and increased renal AT1R-mediated sodium excretion that play a role in the pathogenesis of the hypertension in SHRs14, 16. The present study reinforces the role of GRK4 in hypertension and shows that a constitutively increased activity of GRK4 increases arterial AT1R receptor expression and function, which may be involved in the abnormalities of conduit vessels in essential hypertension. The results imply that the inhibition of GRK4 expression or activity, based on the chemical or biological medicine, may be an effective therapeutic approach for essential hypertension.
Supplementary Material
Novelty and Significance.
What Is New ?
The gene variant of G protein coupled receptor kinase 4 (GRK4), GRK4γ 142V, is associated with hypertension. Our previous study found that increased renal GRK4 activity attenuated renal D1 dopamine receptor and increased renal AT1R functions. In these studies, we report for the first time that GRK4 is expressed in VSMCs of the aorta and GRK4γ 142V decreased AT1R degradation, via decreased phosphorylation and increased AT1R expression, via NF-κΒ. In A10 cells, expression of GRK4γ 142V augmented the Ang II-mediated increase in intracellular Ca2+ levels. In transgenic mice on novel C57Bl/6J and SJL/J background, Ang II-induced vasoconstriction was increased in the aorta from GRK4γ 142V transgenic mice, compared with GRK4γ WT transgenic mice. Finally the hypertension in GRK4γ 142V transgenic mice was related to an increase in Ang II-mediated vasoconstriction.
What Is Relevant ?
The present study reinforces the role of GRK4 in hypertension and shows that a constitutively increased activity of GRK4 increases arterial AT1R receptor expression and function, which may be involved in the abnormalities of conduit vessels in essential hypertension. The results imply that the inhibition of GRK4 expression or activity, based on the chemical or biological medicine, may be an effective therapeutic approach for essential hypertension.
Summary
The present study reinforces the role of GRK4 in hypertension and shows that a constitutively increased activity of GRK4 increases arterial AT1R receptor expression and function, which may be involved in the abnormalities of conduit vessels in essential hypertension.
Acknowledgments
Sources of Funding
These studies were supported in part by grants from National Natural Science Foundation of China (30925018, 31130029, 81070559, 81270337), National Basic Research Program of China (2013CB531104, 2012CB517801), and National Institutes of Health, USA (R37HL023081, and P01HL074940).
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors except for Dr. Pedro A. Jose who is the Scientific Director of Hypogen, Inc., which owns, US Patent Number 6,660,474 for G protein-related kinase mutants in essential hypertension.
REFERENCES
- 1.Ezzati M, Riboli E. Can noncommunicable diseases be prevented? Lessons from studies of populations and individuals. Science. 2012;337:1482–1487. doi: 10.1126/science.1227001. [DOI] [PubMed] [Google Scholar]
- 2.Eurosurveillance editorial team. WHO launches the World Health Statistics 2012. Euro Surveill. 2012;17(20):20175. [PubMed] [Google Scholar]
- 3.Mitchell GF, Dunlap ME, Warnica W, Ducharme A, Arnold JM, Tardif JC, Solomon SD, Domanski MJ, Jablonski KA, Rice MM, Pfeffer MA. Long-term trandolapril treatment is associated with reduced aortic stiffness: The prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49:1271–1277. doi: 10.1161/HYPERTENSIONAHA.106.085738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris RC, Zhang MZ. Dopamine, the kidney, and hypertension. Curr Hypertens Rep. 2012;14:138–143. doi: 10.1007/s11906-012-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrera M, Coffman TM. The kidney and hypertension: Novel insights from transgenic models. Curr Opin Nephrol Hypertens. 2012;21:171–178. doi: 10.1097/MNH.0b013e3283503068. [DOI] [PubMed] [Google Scholar]
- 6.Zeng C, Villar VA, Eisner GM, Williams SM, Felder RA, Jose PA. G protein-coupled receptor kinase 4: Role in blood pressure regulation. Hypertension. 2008;51:1449–1455. doi: 10.1161/HYPERTENSIONAHA.107.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris RC. Abnormalities in renal dopamine signaling and hypertension: The role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61–65. doi: 10.1097/MNH.0b013e32834de2cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and g protein-coupled receptor kinase 4 in the kidney: Role in blood pressure regulation. Biochim Biophys Acta. 2010;1802:1259–1267. doi: 10.1016/j.bbadis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belmonte SL, Blaxall BC. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ Res. 2011;109:309–319. doi: 10.1161/CIRCRESAHA.110.231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rankin ML, Marinec PS, Cabrera DM, Wang Z, Jose PA, Sibley DR. The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol. 2006;69:759–769. doi: 10.1124/mol.105.019901. [DOI] [PubMed] [Google Scholar]
- 11.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speirs HJ, Katyk K, Kumar NN, Benjafield AV, Wang WY, Morris BJ. Association of G-protein-coupled receptor kinase 4 haplotypes, but not HSD3B1 or PTP1B polymorphisms, with essential hypertension. J Hypertens. 2004;22:931–936. doi: 10.1097/00004872-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, Felder RA, Schnackenberg CG, Sibley DR, Eisner GM, Jose PA. The elevated blood pressure of human GRK4γ A142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol. 2007;292:H2083–2092. doi: 10.1152/ajpheart.00944.2006. [DOI] [PubMed] [Google Scholar]
- 14.Sanada H, Yatabe J, Midorikawa S, Katoh T, Hashimoto S, Watanabe T, Xu J, Luo Y, Wang X, Zeng C, Armando I, Felder RA, Jose PA. Amelioration of genetic hypertension by suppression of renal G protein-coupled receptor kinase type 4 expression. Hypertension. 2006;47:1131–1139. doi: 10.1161/01.HYP.0000222004.74872.17. [DOI] [PubMed] [Google Scholar]
- 15.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatabe J, Sanada H, Midorikawa S, Hashimoto S, Watanabe T, Andrews PM, Armando I, Wang X, Felder RA, Jose PA. Effects of decreased renal cortical expression of G protein-coupled receptor kinase 4 and angiotensin type 1 receptors in rats. Hypertens Res. 2008;31:1455–1464. doi: 10.1291/hypres.31.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feihl F, Liaudet L, Waeber B. The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep. 2009;11:182–189. doi: 10.1007/s11906-009-0033-6. [DOI] [PubMed] [Google Scholar]
- 18.Ruilope LM, Schaefer A. Efficacy of Sevikar® compared to the combination of perindopril plus amlodipine on central arterial blood pressure in patients with moderate-to-severe hypertension: Rationale and design of the sevitension study. Contemp Clin Trials. 2011;32:710–716. doi: 10.1016/j.cct.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Hasenkamp S, Telgmann R, Staessen JA, Hagedorn C, Dordelmann C, Bek M, Brand-Herrmann SM, Brand E. Characterization and functional analyses of the human G protein-coupled receptor kinase 4 gene promoter. Hypertension. 2008;52:737–746. doi: 10.1161/HYPERTENSIONAHA.108.114512. [DOI] [PubMed] [Google Scholar]
- 20.Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA. Dopamine D1 receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–679. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]
- 21.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–1078. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
- 22.Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, Felder RA, Jose PA. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int. 2004;66:2167–2180. doi: 10.1111/j.1523-1755.2004.66007.x. [DOI] [PubMed] [Google Scholar]
- 23.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45:804–810. doi: 10.1161/01.HYP.0000155212.33212.99. [DOI] [PubMed] [Google Scholar]
- 24.Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA. Gα12- and Gα13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–610. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- 25.Zeng C, Wang Z, Hopfer U, Asico LD, Eisner GM, Felder RA, Jose PA. Rat strain effects of AT1 receptor activation on D1 dopamine receptors in immortalized renal proximal tubule cells. Hypertension. 2005;46:799–805. doi: 10.1161/01.HYP.0000184251.01159.72. [DOI] [PubMed] [Google Scholar]
- 26.Zeng C, Wang Z, Li H, Yu P, Zheng S, Wu L, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. D3 dopamine receptor directly interacts with D1 dopamine receptor in immortalized renal proximal tubule cells. Hypertension. 2006;47:573–579. doi: 10.1161/01.HYP.0000199983.24674.83. [DOI] [PubMed] [Google Scholar]
- 27.Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husain M, Jones-Carson J, Song M, McCollister BD, Bourret TJ, Vazquez-Torres A. Redox sensor Ssrb Cys203 enhances Salmonella fitness against nitric oxide generated in the host immune response to oral infection. Proc Natl Acad Sci U S A. 2010;107:14396–14401. doi: 10.1073/pnas.1005299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Q, Wen W, Qu S, Li G, Egan KM, Chen K, Deming SL, Shen H, Shen CY, Gammon MD, Blot WJ, Matsuo K, Haiman CA, Khoo US, Iwasaki M, Santella RM, Zhang L, Fair AM, Hu Z, Wu PE, Signorello LB, Titus-Ernstoff L, Tajima K, Henderson BE, Chan KY, Kasuga Y, Newcomb PA, Zheng H, Cui Y, Wang F, Shieh YL, Iwata H, Le Marchand L, Chan SY, Shrubsole MJ, Trentham-Dietz A, Tsugane S, Garcia-Closas M, Long J, Li C, Shi J, Huang B, Xiang YB, Gao YT, Lu W, Shu XO, Zheng W. Replication and functional genomic analyses of the breast cancer susceptibility locus at 6q25.1 generalize its importance in women of Chinese, Japanese, and European ancestry. Cancer Res. 2011;71:1344–1355. doi: 10.1158/0008-5472.CAN-10-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedrosa R, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Giα3 protein-coupled dopamine D3 receptor-mediated inhibition of renal NHE3 activity in SHR proximal tubular cells is a PLC-PKC-mediated event. Am J Physiol Renal Physiol. 2004;287:F1059–1066. doi: 10.1152/ajprenal.00139.2004. [DOI] [PubMed] [Google Scholar]
- 31.Monck JR, Reynolds EE, Thomas AP, Williamson JR. Novel kinetics of single cell Ca2+ transients in stimulated hepatocytes and A10 cells measured using fura-2 and fluorescent videomicroscopy. J Biol Chem. 1988;263:4569–4575. [PubMed] [Google Scholar]
- 32.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 33.Ming J, Li T, Zhang Y, Xu J, Yang G, Liu L. Regulatory effects of myoendothelial gap junction on vascular reactivity after hemorrhagic shock in rats. Shock. 2009;31:80–86. doi: 10.1097/SHK.0b013e31817d3eF2-11. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z AL, Nazarov V, Zeng C, Eisner GM, Felder RA, Wong LJ, Jose PA. AT1 receptors and hypertension in human γ A142V transgenic mice. Am J Hypertens. 2005;18:137A. [Google Scholar]
- 35.Palczewski K. GTP-binding-protein-coupled receptor kinases--two mechanistic models. Eur J Biochem. 1997;248:261–269. doi: 10.1111/j.1432-1033.1997.00261.x. [DOI] [PubMed] [Google Scholar]
- 36.Bengra C, Mifflin TE, Khripin Y, Manunta P, Williams SM, Jose PA, Felder RA. Genotyping of essential hypertension single-nucleotide polymorphisms by a homogeneous pcr method with universal energy transfer primers. Clin Chem. 2002;48:2131–2140. [PubMed] [Google Scholar]
- 37.Williams SM, Ritchie MD, Phillips JA, 3rd, Dawson E, Prince M, Dzhura E, Willis A, Semenya A, Summar M, White BC, Addy JH, Kpodonu J, Wong LJ, Felder RA, Jose PA, Moore JH. Multilocus analysis of hypertension: A hierarchical approach. Hum Hered. 2004;57:28–38. doi: 10.1159/000077387. [DOI] [PubMed] [Google Scholar]
- 38.Lohmueller KE, Wong LJ, Mauney MM, Jiang L, Felder RA, Jose PA, Williams SM. Patterns of genetic variation in the hypertension candidate gene GRK4: Ethnic variation and haplotype structure. Ann Hum Genet. 2006;70:27–41. doi: 10.1111/j.1529-8817.2005.00197.x. [DOI] [PubMed] [Google Scholar]
- 39.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu D, Su S, Ge D, Chen S, Huang J, Li B, Chen R, Qiang B. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 41.Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1660–1669. doi: 10.1152/ajpregu.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng CY, Wang Z, Yang ZW, He DF, Yang CM, Asico LD, Felder RA, Jose PA. [Gprotein kinase 4γ A142V overexpression induced hypertension by downregulating D1 receptors in transgenic mice]. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:411–414. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.