Abstract
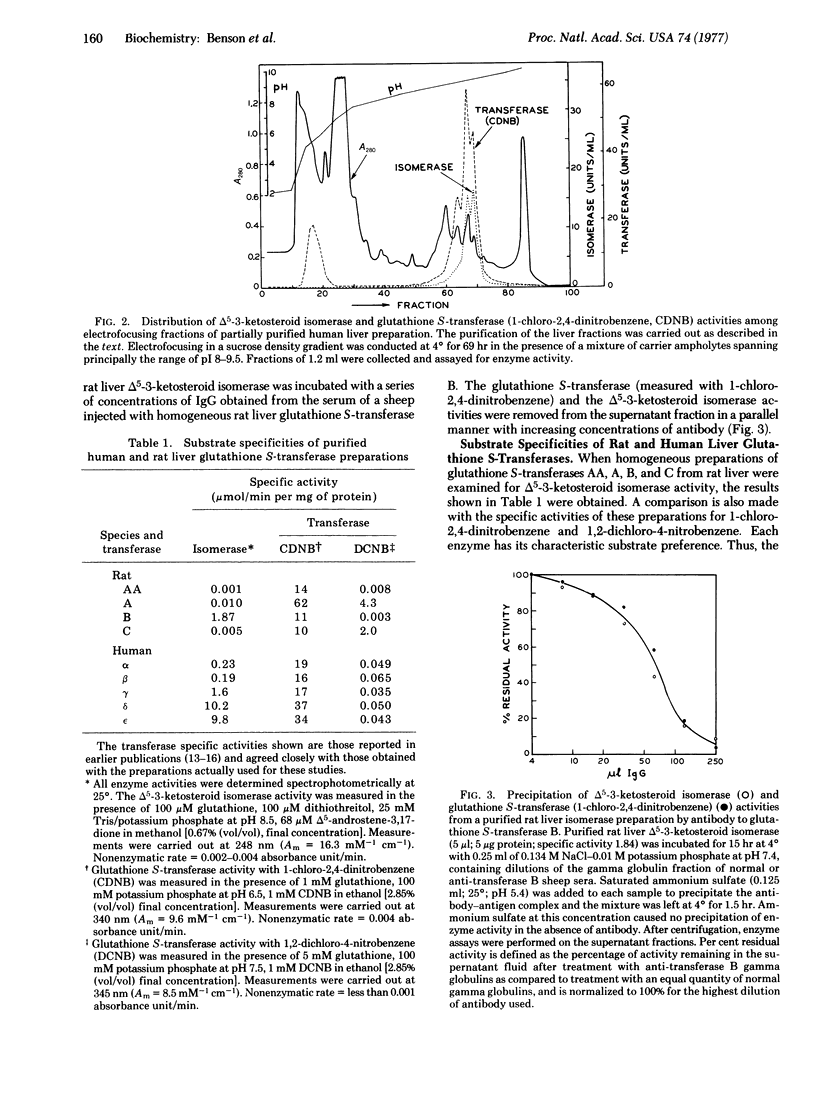
Soluble, glutathione-stimulated delta 5-3-ketosteroid isomerase (EC 5.3.3.A) activity of human and rat liver resides in very basic proteins with molecular weights of about 45,000 which are present in high concentrations in these tissues. Physiochemical and immunological evidence is presented for the identity of the proteins responsible for this enzymatic activity with the glutathione S-transferases (RX:glutathione R-transferase, EC 2.5.1.18) that conjugate glutathione with a variety of electrophilic compounds. In the rat, the steroid isomerase is associated principally with the major transferase (B), which is also known as ligandin, and has the versatility to bind various hydrophobic compounds such as bilirubin, corticosteroids, and metabolites of a number of carcinogens. Other rat liver-glutathione S-transferase species are far less active in the steroid isomerization reaction. The delta 5-3-ketosteroid isomerase activity of human liver is more uniformly distributed among the five glutathione S-transferases that have been described. Steroid isomerization differs fundamentally from other reactions promoted by glutathione S-transferases in that glutathione is not consumed in the reaction. However, because the transferase enzymes promote nucleophilic attack by glutathione on a variety of largely foreign organic substrates, a similar mechanism may be involved in the isomerase reaction. Delta 5-3-ketosteroids are among the few known naturally occurring substrates for these enzymes.
Full text
PDF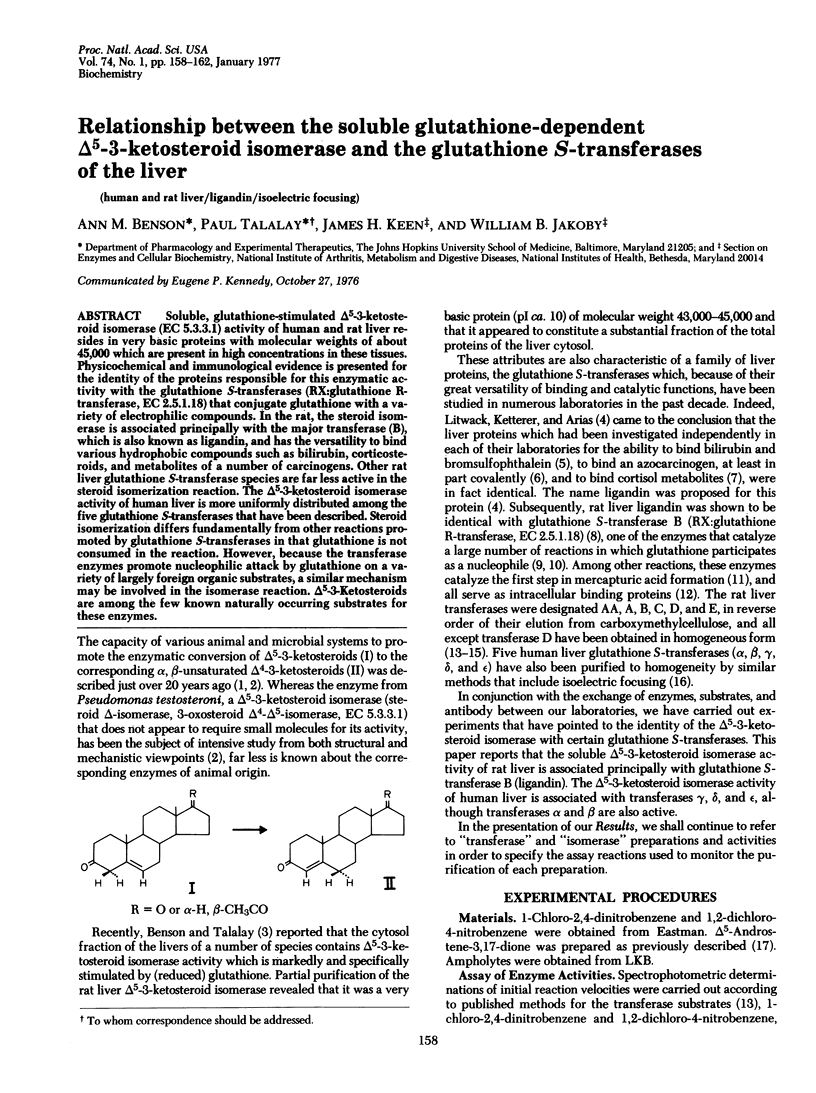



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzold F. H., Benson A. M., Covey D. F., Robinson C. H., Talalay P. The delta 5-3-ketosteroid isomerase reaction: catalytic mechanism, specificity and inhibition. Adv Enzyme Regul. 1976;14:243–267. doi: 10.1016/0065-2571(76)90016-9. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Talalay P. Role of reduced glutathione in the delta(5)-3-kitosteroid isomerase reaction of liver. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1073–1079. doi: 10.1016/0006-291x(76)90482-4. [DOI] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:173–219. doi: 10.1002/9780470122778.ch5. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Keen J. H., Jakoby W. B. Glutathione S-transferase in the formation of cyanide from organic thiocyantes and as an organic nitrate reductase. Biochem Biophys Res Commun. 1975 May 19;64(2):501–506. doi: 10.1016/0006-291x(75)90349-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Fleischner G., Gatmaitan Z., Arias I. M., Jakoby W. B. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferase AA from rat liver. Arch Biochem Biophys. 1976 Aug;175(2):710–716. doi: 10.1016/0003-9861(76)90563-4. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Habig W. H., Jakoby W. B. Mechanism for the several activities of the glutathione S-transferases. J Biol Chem. 1976 Oct 25;251(20):6183–6188. [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Ketterer B., Ross-Mansell P., Whitehead J. K. The isolation of carcinogen-binding protein from livers of rats given 4-dimethylaminoazobenzene. Biochem J. 1967 May;103(2):316–324. doi: 10.1042/bj1030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACK L. Enzymic cis-trans isomerization of maleylpyruvic acid. J Biol Chem. 1961 Nov;236:2835–2840. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- Morey K. S., Litwack G. Isolation and properties of cortisol metabolite binding proteins of rat liver cytosol. Biochemistry. 1969 Dec;8(12):4813–4821. doi: 10.1021/bi00840a024. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Habig W. H., Jakoby W. B. Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem. 1974 Nov 25;249(22):7140–7147. [PubMed] [Google Scholar]
- Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Organic anion-binding protein in rat liver: drug induction and its physiologic consequence. Proc Natl Acad Sci U S A. 1969 Sep;64(1):168–170. doi: 10.1073/pnas.64.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Studies of Y and Z, two hepatic cytoplasmic organic anion-binding proteins: effect of drugs, chemicals, hormones, and cholestasis. J Clin Invest. 1971 Nov;50(11):2242–2252. doi: 10.1172/JCI106721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALALAY P., WANG V. S. Enzymic isomerization of delta5-3-ketosteroids. Biochim Biophys Acta. 1955 Oct;18(2):300–301. doi: 10.1016/0006-3002(55)90079-2. [DOI] [PubMed] [Google Scholar]


