Abstract
PURPOSE
Patients with cirrhosis often experience muscle cramps with varying severity. We investigated the factors associated with the prevalence and morbidity associated with muscle cramps.
METHODS
A total of 150 adult patients with cirrhosis were enrolled consecutively. Cramp questionnaire with visual analogue scale for pain, Chronic Liver Disease Questionnaire (CLDQ), and blood for measurement of 25-(OH) vitamin D levels were obtained after informed consent.
RESULTS
A total of 101 patients (67%) reported muscle cramps in the preceding 3 months. Patients with cramps had significantly lower serum albumin (3.1 ± 0.6 g/dL vs 3.3 ± 0.7 g/dL, P = .04) and CLDQ scores (107 ± 37 vs 137 ± 34, P < .0001) compared with those without cramps. The median composite symptom score, defined as product of frequency and severity of cramps, in the study cohort was 12 with a range of 0.3 to 200. There were no clinical or biochemical predictors for occurrence of any cramps or severe cramps (composite symptom score > 12). Muscle cramps (P < .001) and hepatic encephalopathy (P = .009) were associated independently with decreased CLDQ scores. Vitamin D deficiency was seen in 66% of the study cohort, but the serum 25-(OH) vitamin D levels were not significantly different between patients with and without cramps (18.0 ± 8.9 ng/mL vs 19.6 ± 9.5 ng/mL, P = .49).
CONCLUSIONS
Muscle cramps are associated with significantly diminished quality of life in patients with cirrhosis. More research is needed to better understand their mechanism to develop effective treatment.
Keywords: Chronic liver disease questionnaire, Cirrhosis, Muscle cramps
The association of muscle cramps with cirrhosis was first reported by Konikoff and Theodor1 in 1986 when they made their observation of repeated painful muscle cramps by patients with cirrhosis. They proposed that the strikingly high incidence and uniformity of the phenomenon may justify the inclusion of painful muscle cramps among the recognized symptoms of cirrhosis.1 Subsequently, several studies have confirmed that muscle cramps are a common symptom of cirrhosis.2–4 Patients experiencing muscle cramps often describe them as sudden, uncomfortable squeezing or contraction of a muscle, lasting seconds to minutes. An electromyogram of a cramping muscle reveals involuntary repetitive firing of motor unit action potentials at high rates (up to 150 per second) producing a sustained muscle contraction.5,6 These cramps are not associated with any progressive motor neuron disease or chronic neuromuscular disability and are different from muscle contracture, dystonia, and tetany.5,6 The prevalence is varied and ranges from 29% to 88% depending on the inclusion criteria used by the investigators.2,3,7–9
The exact mechanism behind the occurrence of muscle cramps remains elusive.10 Potential primary hypotheses include neurologic, muscular, endocrine, or electrolyte imbalance.2,4,9–11 Diuretic use in cirrhotic patients also has been implicated as a cause of muscle cramps through its effects on serum electrolyte balance and plasma volume. However, Abrams et al2 found that patients with congestive heart failure had a lower prevalence of cramps compared with patients with cirrhosis despite higher diuretic use, suggesting that diuretic use may not be the primary precipitating factor for muscle cramps. A subsequent study by Angeli et al3 also reported that cramps were associated with cirrhosis irrespective of its cause, diuretic consumption, serum electrolyte alterations, or differences in Child’s classification. However, additional analysis in their cohort for pathophysiologic associations revealed that the presence of ascites, lower mean arterial pressure, and higher plasma renin activity were independent predictive factors for the occurrence of cramps.3
Although muscle cramps are benign in nature, their occurrence is associated with bodily pain and decreased physical and social functioning. General health-related quality of life, measured by instruments such as the Medical Outcome Study Short Form-36 and the Nottingham Health Profile questionnaires, is diminished in cirrhotic patients with cramps.12,13 However, the Chronic Liver Disease Questionnaire (CLDQ) is a more sensitive and specific instrument to assess quality of life in patients with liver disease,14,15 but the effect of muscle cramps on the quality of life as measured by the CLDQ is not known.
Patients with certain medical conditions, such as hyper-parathyroidism and vitamin D insufficiency after bariatric surgery, also report muscle cramps.16–19 Several recent studies have established that vitamin D deficiency is common in patients with cirrhosis and is associated with muscle pain.20–22 It would thus be of interest to investigate the relationship between vitamin D levels and muscle cramps in patients with cirrhosis. The current study was designed to determine the prevalence, characteristics, and predictors of muscle cramps in patients with cirrhosis and their impact on quality of life as measured by the CLDQ. The objectives of this study were to determine the prevalence of and factors associated with muscle cramps in patients with cirrhosis; to examine the relationship between vitamin D levels and the prevalence of muscle cramps; and to examine the relationship between muscle cramps and health-related quality of life in patients with cirrhosis.
MATERIALS AND METHODS
This study was approved by the institutional review board of Indiana University Purdue University at Indianapolis (Study No. 1002-50). Patients with an established diagnosis of cirrhosis (clinical, histologic, or radiologic) as determined by the treating hepatologist, aged 18 years or more, and attending liver clinics and liver transplant clinics at Indiana University Hospital, Indianapolis, Indiana, were enrolled consecutively during a 6-month period (June 2010 to January 2011) after informed consent was obtained by a single physician investigator (HC). Patients with cramps had ongoing symptoms of “true cramps” defined by painful, involuntary contraction of skeletal muscles, occurring at rest or strong enough to wake the patient from sleep in the preceding 12 weeks. Patients with evidence of disease associated with muscle cramps, such as vascular occlusive disease, peripheral neuropathy, end-stage renal disease on hemo-dialysis, congestive heart failure, and postphlebitis syndrome, were excluded. True cramps were differentiated from muscle contracture defined as an involuntary state of sustained muscle contraction occurring during exercise and not relieved by stretching the involved muscle; dystonia, defined as simultaneous contraction of both agonist and antagonist muscle groups that occur with specific tasks; and tetany, defined as a syndrome of sensory (paresthesias) and motor (muscle spasms) neuron hyperexcitability.23
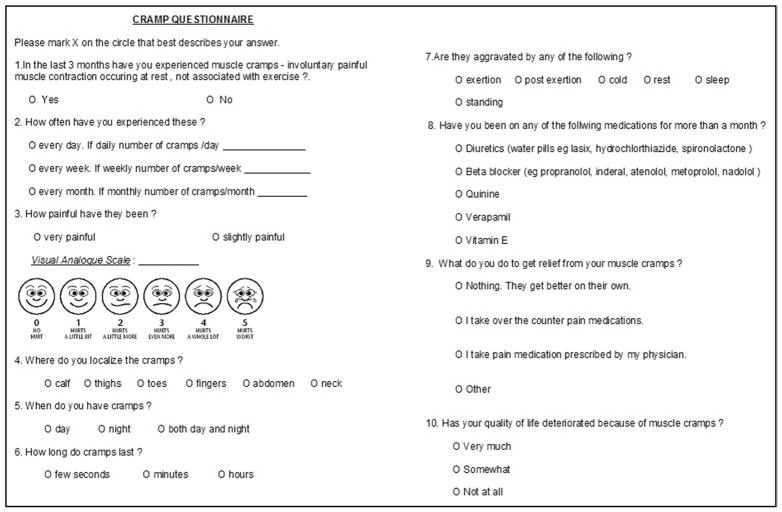
All subjects completed the cramp questionnaire (Figure 1) and the CLDQ. The cramp questionnaire was modified slightly from a prior published questionnaire and included description of muscle cramps (onset, precipitating events, frequency, duration, relief, and localization) and severity of pain by visual analogue scale (Wong-Baker FACES Pain Rating Scale).24,25 The CLDQ is a disease-specific instrument designed to assess health-related quality of life in patients with chronic liver disease.14 It includes 29 items and is designed to measure the 6 domains of quality of life; abdominal symptoms, fatigue, systemic symptoms, activity, emotional functions, and worry. Each of the variables is rated on a scale of 1 to 7. The responses are interpreted as for (1) “all the time,” (2) “most of the time,” (3) “a good proportion of time,” (4) “some of the time,” (5) “a little proportion of the time,” (6) “hardly anytime,” and (7) “never.” The cumulative CLDQ score is calculated by adding up the numeric value for each of the responses. Therefore, patients with lower scores are assumed to have greater impairment in quality of life compared with those with higher scores. Likewise, cumulative scores also can be calculated for each of the 6 categories. This instrument has been shown to have adequate internal reliability, validity, and sensitivity.
Figure 1.
Cramp questionnaire to capture description of muscle cramps (onset, precipitating events, frequency, duration, relief, and localization) and severity of pain by visual analogue scale for pain (Wong-Baker FACES Pain Rating Scale).
To assess both symptom frequency and severity simultaneously, a composite symptom score (CSS) was calculated by multiplying the frequency of cramps per week with the severity of cramps. Because cramps can occur at variable frequency and severity, we defined clinically significant cramps as those with CSS value greater than the median value for the study cohort. Other study variables, such as demographics (age, gender, race, and diuretic use), physical findings (body mass index, mean arterial pressure, ascites, hepatic encephalopathy), serum metabolic panel (sodium, potassium, creatinine, total calcium), and hepatic profile (albumin, total protein, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total protein, total bilirubin, international normalized ratio) within the preceding 12 weeks (closest to the enrollment day), were extracted from medical records. In subjects who consented for blood draws (n = 86), a sample was obtained and serum was stored at −20°C for analysis of circulating levels of 25-(OH) vitamin D3 and 25-(OH) vitamin D2 using the LC/MS-MRM (liquid chromatography/mass spectrometry-multiple reaction monitoring) assay (Intelimmune, North Webster, Ind). Total 25-(OH) vitamin D levels were calculated from the sum of 25-(OH) vitamin D2 and 25-(OH) vitamin D3.
Statistical Analysis
Descriptive statistics, such as means, standard deviation, ranges, and percentages, were used to characterize the study patients. Comparisons among groups were made by the use of Student t test for the continuous variables and chi-square test for the categoric variables. Univariable and multivariable logistic regression analyses were conducted to explore the variables that are independently associated with the occurrence of muscle cramps and clinically significant muscle cramps (CSS > median CSS) in the study cohort. Variables with univariate P values < .1 were entered into the multivariate analyses. The strength of association was reported as odds ratio (OR) with 95% confidence interval (CI) and P values. A P value of < .05 was considered to be statistically significant. When analyzing the predictors of the CLDQ, the values were log-transformed to account for the large range and asymmetric distribution of data. Univariable linear regression analysis was performed to identify potential predictors of the CLDQ, and the variables with a P value < .05 in the univariable analysis were then entered into multivariable analysis to identify independent predictors. Quality-control procedures, database management, and statistical analyses were performed using SAS software for Windows, version 9.2 (SAS Institute, Cary, NC).
RESULTS
A total of 150 patients were prospectively enrolled in this study. The mean age was 56 ± 10 years, and 39% were male (Table 1). The cause of cirrhosis was viral hepatitis in 42%, alcoholic liver disease in 22%, and nonalcoholic steatohepatitis in 19%. A total of 101 patients (67%) reported muscle cramps in the preceding 12 weeks. The lower half of the body was the most common site for muscle cramps with patients reporting in locations such as thighs (43%), calves (70%), and toes (50%). However, patients also experienced cramps in the upper half of the body in locations such as the neck (9%), abdominal wall (12%), and fingers (47%). Sixty-five percent of the patients reported cramps lasting for several minutes, whereas 21% reported cramps lasting for a few seconds.
Table 1.
Selected Characteristics of Study Cohort (n = 150)
| Mean age (± SD), y | 56 ± 10 |
| Male (%) | 41 |
| Caucasian (%) | 53 |
| BMI (kg/m2) | 29 ± 6 |
| Mean arterial pressure (mm Hg) | 92 ± 14 |
| Cause of cirrhosis | |
| Chronic viral hepatitis (%) | 42 |
| Alcoholic (%) | 22 |
| NASH or cryptogenic (%) | 19 |
| All others (%) | 17 |
| Child-Pugh class | |
| Class A (%) | 29 |
| Class B (%) | 47 |
| Class C (%) | 24 |
| Mean MELD score (± SD) | 13 ± 5 |
| 25-(OH) vitamin D (ng/mL) | 13 ± 6 |
| Proportion with muscle cramps (%) | 67 |
| Proportion with CSS > 12 (%) | 49 |
SD = standard deviation; BMI = body mass index; NASH = nonalcoholic steatohepatitis; MELD = Model for End-Stage Liver Disease; CSS = Composite Symptom Score.
Patients with or without cramps were similar in age (55 ± 10 years vs 57 ± 10 years, P = .3), gender (male 58% vs 61%, P = .6), diuretic use (66% vs 54% P = .1), presence of ascites (67% vs 63%, P = .7), presence of hepatic encephalopathy (50% vs 39%, P = .2), serum sodium (135 ± 11 mmol/L vs 137 ± 4 mmol/L, P = .07), serum potassium (4.0 ± 0.6 vs 4.0 ± 0.5, P = .3), Child-Pugh score (8 ± 2 vs 8 ± 2, P = .9), and Model for End-Stage Liver Disease score (13 ± 6 vs 12 ± 5, P = .4) (Table 2). Univariable logistic regression analysis revealed serum albumin (OR, 0.55; 95% CI, 0.32–0.95; P = .03) and international normalized ratio (OR, 2.9; 95% CI, 0.88–9.72; P = .07) as the only significant predictors for the occurrence of cramps (Table 3). However, this relationship did not persist when these 2 variables were controlled for each other in the multivariable analysis.
Table 2.
Select Demographic, Biochemical, and Chronic Liver Disease Questionnaire Scores in Cirrhotic Patients with and without Muscle Cramps
| Patients with Cramps (n = 101, 67%) | Patients without Cramps (n = 49, 33%) | P | |
|---|---|---|---|
| Mean age (± SD), y | 55 ± 10 | 57 ± 10 | .28 |
| Male (%) | 58 | 61 | .85 |
| Caucasian (%) | 79 | 78 | 1.0 |
| BMI (kg/m2) | 29 ± 7 | 29 ± 5 | .9 |
| Mean arterial pressure (mm Hg) | 91 ± 15 | 95 ± 11 | .11 |
| Proportion with hepatic encephalopathy (%) | 50 | 39 | .22 |
| Proportion with ascites (%) | 67 | 63 | .71 |
| Diuretic usage (%) | 66 | 54 | .12 |
| Serum total calcium, mg/dL (mean ± SD) | 8.9 ± 0.6 | 9.0 ± 0.6 | .16 |
| Serum albumin, g/dL (mean ± SD) | 3.1 ± 0.7 | 3.3 ± 0.7 | .04 |
| Serum sodium, mmol/L (mean ± SD) | 136 ± 5 | 137 ± 4 | .12 |
| Serum potassium, mmol/L (mean ± SD) | 4.0 ± 0.6 | 4.0 ± 0.5 | .30 |
| Serum creatinine, mg/dL (mean ± SD) | 1.0 ± 0.6 | 1.0 ± 0.5 | .88 |
| International normalized ratio | 1.4 ± 0.4 | 1.3 ± 0.3 | .05 |
| Child Pugh score (mean ± SD) | 8 ± 2 | 8 ± 2 | .9 |
| MELD score (mean ± SD) | 13.3 ± 5.7 | 12.1 ± 4.5 | .16 |
| 25-(OH) vitamin D (ng/mL) | 18.0 ± 8.9 | 19.6 ± 9.5 | .49 |
| CLDQ score (mean ± SD) | 107 ± 37 | 137 ± 34 | <.0001 |
| Abdominal symptoms | 12 ± 5 | 16 ± 5 | <.05 |
| Fatigue | 15 ± 7 | 20 ± 8 | <.0001 |
| Systemic symptoms | 20 ± 7 | 28 ± 6 | <.0001 |
| Activity | 12 ± 5 | 16 ± 5 | <.05 |
| Emotional function | 30 ± 12 | 37 ± 13 | <.01 |
| Worry | 19 ± 9 | 22 ± 9 | .06 |
SD = standard deviation; BMI = body mass index; MELD = Model for End-Stage Liver Disease; CLDQ = Chronic Liver Disease Questionnaire.
Table 3.
Variables Associated with Prevalence and Severity of Muscle Cramps and Quality of Life in Patients with Cirrhosis: Summary of Univariable and Multivariable Analysis
| Univariable Analysis
|
Multivariable Analysis
|
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | |
| Occurrence of cramps | ||||
| Albumin | 0.55 (0.32–0.95) | .03 | 0.62 (0.35–1.08) | .09 |
| INR | 2.93 (0.88–9.72) | .07 | 2.14 (0.62–7.38) | .23 |
| Severe cramps (CSS > 12) | ||||
| Age | 0.96 (0.93–1.0) | .03 | Not applicable | |
| CLDQ | Univariable Analysis
|
Multivariable Analysis
|
||
|---|---|---|---|---|
| Parameter Estimate | P | Parameter Estimate | P | |
| Serum albumin | 13.15 | .0048 | 3.69 | .51 |
| Hepatic encephalopathy | −19.30 | .0020 | −15.46 | .009 |
| Muscle cramps | −30.21 | <.0001 | −28.05 | <.001 |
| Serum total calcium | 12.88 | .0164 | 7.07 | .27 |
CSS = Composite Symptom Score; INR = international normalized ratio; CLDQ = Chronic Liver Disease Questionnaire.
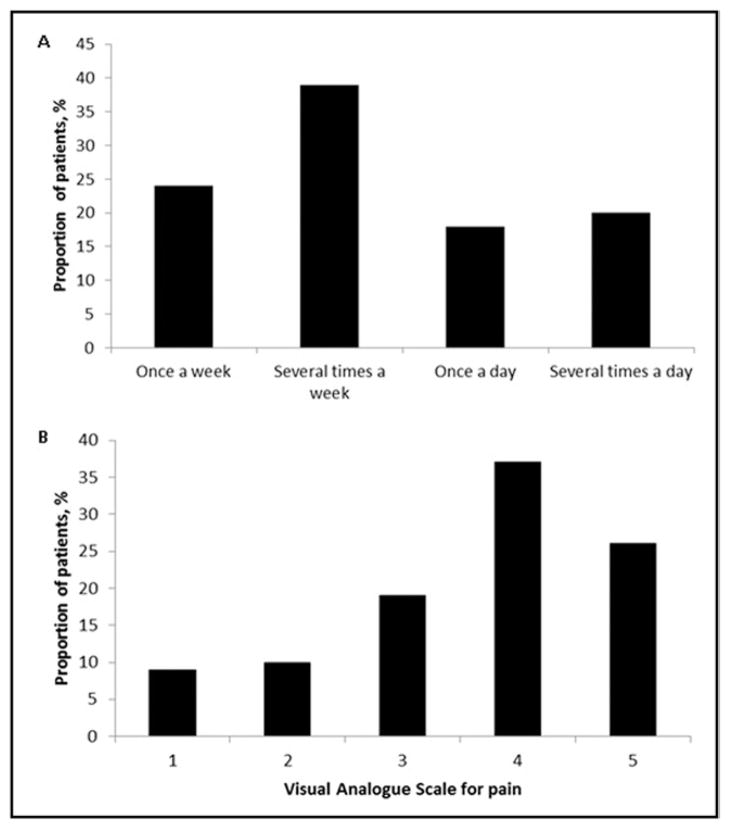
Pain associated with muscle cramps was reported with variable frequency and intensity. In the current cohort, patients reported the following frequency of cramps: once per week (24%), several times per week (39%), once per day (18%), and several times per day (20%) (Figure 2A). Cramps lasting several hours were reported in 15% of the patients in the group with muscle cramps. Although 19% reported mild pain (≤2 on a scale of 5), the majority of patients (62%) reported severe pain (>3 on a scale of 0–5) (Figure 2B). Some 66% of the patients had cramps during both day and night times, whereas 34% of patients experienced cramps exclusively during the night. There was a wide variation in the CSS ranging from 0.3 to 200 with a median value of 12 in patients who reported cramps. Clinically significant cramps, that is, patients with CSS > 12, were found in 49% of the patients. Univariable logistic regression analysis revealed age as the only variable significantly associated with CSS > 12 (OR, 0.96; 95% CI, 0.93–1.0; P = .03) (Table 3).
Figure 2.
Characteristics of muscle cramps as reported by the patients. A, Frequency of muscle cramps as reported by the patients (n = 101). B, Self-reported severity of cramps using visual analogue scale for pain by Wong-Baker FACES Pain Rating Scale in patients with cramps.
Vitamin D deficiency, as defined by 25-(OH) vitamin D level less than 20 ng/mL, was seen in 66% of the study cohort. Vitamin D insufficiency, as defined by 25-(OH) vitamin D levels between 21 and 29 ng/mL, was seen in 23% of the study cohort. Serum 25-(OH) vitamin D levels were not different between the patients with and without cramps (18.0 ± 8.9 ng/mL vs 19.6 ± 9.5 ng/mL, P = .49) (Table 2) and were not predictive of occurrence of muscle cramps, CSS > 12, or lower CLDQ score (Table 3).
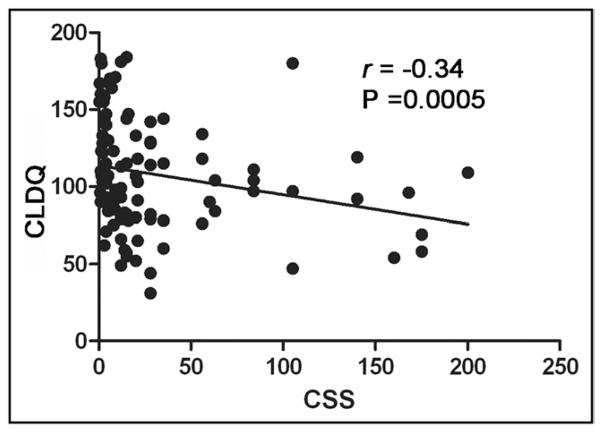
Cirrhotic patients with cramps had a significantly lower CLDQ score (107 ± 37 vs 137 ± 34, P < .0001) when compared with cirrhotic patients without cramps (Table 2). The decreased CLDQ scores in cirrhotic patients with cramps was a result of lower scores in all 6 domains (abdominal symptoms, fatigue, systemic symptoms, activity, emotional functions, and worry) of the CLDQ (Table 2). Univariable linear regression analysis revealed serum albumin (P = .005), muscle cramps (P < .0001), total calcium (P = .02), and hepatic encephalopathy (P = .002) as significant predictors of CLDQ score (Table 3). Multivariable analysis after adjusting for these variables revealed only muscle cramps (β = −28.05, P < .001) and hepatic encephalopathy (β = −15.46, P = .009) as independent predictors of lower CLDQ scores (Table 3). Although both frequency and severity of muscle cramps were significantly associated with decreased CLDQ scores, the association with decreased CLDQ score was stronger for the CSS (r = −0.34 and P = .0005) (Figure 3).
Figure 3.
Significant negative correlation between the CSS and the CLDQ. CLDQ = Chronic Liver Disease Questionnaire; CSS = composite symptom score.
DISCUSSION
Patients with cirrhosis often report muscle cramps with varied frequency and severity. Because of the intermittent nature of the symptoms, lack of objective findings, and inability to measure an individuals’ predisposition to muscle cramping by means of any diagnostic tool, these symptoms are often dismissed. The current study highlights the high prevalence (67%) of muscle cramps in cirrhotic patients and describes in detail various characteristics, such as location (lower extremities > upper extremities), duration (lasting several minutes in up to 65%), severity of associated pain (63% had > 3 on a 0–5 scale), and prevalence of clinically significant cramps (49% had CSS > 12). Muscle cramps from hypokalemia are a known side effect of furosemide.26,27 In our experience, providers often dose reduce or discontinue diuretics when cirrhotic patients have muscle cramps. Despite a thorough and systematic evaluation in the current prospective study, we failed to show any predictors for occurrence of muscle cramps, including serum potassium level, diuretic use, presence of ascites, Child-Pugh score, and Model for End-Stage Liver Disease score. The exact pathophysiology of muscle cramps thus continues to be poorly understood. An additional finding in the current study is the high prevalence of inadequate vitamin D levels, with 89% of our study cohort having vitamin D levels less than 29 ng/mL. It is possible that this relatively high prevalence of vitamin D deficiency/insufficiency did not allow us to detect any association between the 25-(OH) vitamin D levels and the occurrence of muscle cramps. Our group has previously reported a 68% prevalence of vitamin D deficiency/insufficiency in a study sample from the Third National Health and Nutrition Examination Survey (n = 1287).28 However, caution should be exercised with this perception because of seasonal variability of vitamin D levels29 and wide variability in vitamin D deficiency in the general population (30%–89%).29,30
Muscle cramps, although nonlethal, have a significant impact on health-related quality of life. The current study shows that the cirrhotic patients with muscle cramps and hepatic encephalopathy had significantly lower health-related quality of life. A unique finding in the current study is the effect of muscle cramps on health-related quality of life that is significant, impressive, and independent from the effect of hepatic encephalopathy. The lower CLDQ score in patients with hepatic encephalopathy is consistent with a recently published clinical trial.31 Another novel finding in the current study is the significant negative correlation between the CLDQ and the CSS, that is, higher CSS (product of frequency of cramps/week and severity of pain) is associated with lower CLDQ score. This finding may allow us to use the CSS as an additional outcome measure in clinical trials designed to measure the effectiveness of any therapy for muscle cramps.
The treatment of muscle cramps still remains rather empirical.32,33 Quinine sulfate was the most widely used agent for relief of muscle cramps, but it has fallen out of favor because of the Food and Drug Administration warning prohibiting the off-label use of quinine in light of its potential cardiotoxicity.34–36 Some hepatologists often, for lack of a better therapy, resort to infusion of albumin on the basis of a single pilot study in which 9 of 12 patients had improvement in muscle cramps after albumin infusions.3 Prior neurophysiologic studies have shown that cramps arise from spontaneous discharges of motor nerves rather than from within the muscle itself.10 It was speculated that decreased intravascular volume associated with hypoalbuminemia in liver disease results in increased muscle membrane hyper-excitability by lowering the “threshold frequency” required for the induction of a muscle cramp. In the current study, cirrhotic patients with cramps had significantly lower serum albumin levels, although the magnitude of difference (3.1 ± 0.6 g/dL vs 3.3 ± 0.7 g/dL) does not seem to be clinically significant. However, multivariable analysis failed to show that albumin levels were predictive of muscle cramps (Table 3). Muscle cramps can be experimentally induced by repetitive electrical stimulation of peripheral nerves, and the minimum electrical stimulation frequency (Hz) at which a muscle cramps is termed “threshold frequency.”37 Prior studies suggest that threshold frequency is generally indicative of an individual’s predisposition to muscle cramping.37,38 Because of the lack of an effective therapy and high placebo response rates, there is confusion with regard to optimal therapy. The novel use of threshold frequency as a tool to quantitatively measure the response to various therapeutic interventions for muscle cramps is thus highly desirable.
Certain aspects of our study merit further discussion. Although our study was conducted in a prospective fashion, the characteristics of the cramps were reported by the patients in a retrospective fashion. Although this may not necessarily affect the prevalence of cramps because of the dichotomous nature of the question, the frequency and severity may not have been optimally captured. Future studies may need to be performed with longitudinal follow-up possibly using a cramp diary or an electronic event marker to better capture cramp frequency. The current study did not evaluate the association of serum ionized calcium and magnesium levels with the occurrence of cramps because these measures were not available in the majority of patients.
CONCLUSIONS
Muscle cramps are highly prevalent in patients with cirrhosis, and their occurrence is associated with a significant decrease in health-related quality of life. There are no significant predictors for the occurrence of muscle cramps. Further studies need to be conducted to systematically evaluate the therapeutic efficacy of various agents possibly using the CSS and threshold frequency as additional study outcome measures.
CLINICAL SIGNIFICANCE.
Some 67% of patients with cirrhosis reported muscle cramps in the current study.
Diuretic use, presence of ascites, severity of liver disease, and serum vitamin D levels were not associated with the occurrence of muscle cramps.
Muscle cramps and hepatic encephalopathy were associated independently with a significant decrease in health-related quality of life in patients with cirrhosis.
Acknowledgments
Funding: Supported in part by K24 DK069290 to Dr Chalasani.
Footnotes
Conflict of Interest: None.
Authorship: All authors had access to the data and played a role in writing this manuscript.
References
- 1.Konikoff F, Theodor E. Painful muscle cramps. A symptom of liver cirrhosis? J Clin Gastroenterol. 1986;8:669–672. doi: 10.1097/00004836-198612000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Abrams GA, Concato J, Fallon MB. Muscle cramps in patients with cirrhosis. Am J Gastroenterol. 1996;91:1363–1366. [PubMed] [Google Scholar]
- 3.Angeli P, Albino G, Carraro P, et al. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology. 1996;23:264–273. doi: 10.1002/hep.510230211. [DOI] [PubMed] [Google Scholar]
- 4.Marotta PJ, Graziadei IW, Ghent CN. Muscle cramps: a ‘complication’ of cirrhosis. Can J Gastroenterol. 2000;14(Suppl D):21D–25D. doi: 10.1155/2000/214916. [DOI] [PubMed] [Google Scholar]
- 5.Simchak AC, Pascuzzi RM. Muscle cramps. Semin Neurol. 1991;11:281–287. doi: 10.1055/s-2008-1041233. [DOI] [PubMed] [Google Scholar]
- 6.AAEM glossary of terms in clinical electromyography. Muscle Nerve. 1987;10:G4–G60. [PubMed] [Google Scholar]
- 7.Kobayashi Y, Kawasaki T, Yoshimi T, et al. Muscle cramps in chronic liver diseases and treatment with antispastic agent (eperisone hydrochloride) Dig Dis Sci. 1992;37:1145–1146. doi: 10.1007/BF01300302. [DOI] [PubMed] [Google Scholar]
- 8.Konikoff F. The therapeutic benefit of vitamin E in patients with liver disease. J Hepatol. 1994;21:687–688. doi: 10.1016/s0168-8278(94)80124-x. [DOI] [PubMed] [Google Scholar]
- 9.Baskol M, Ozbakir O, Coskun R, et al. The role of serum zinc and other factors on the prevalence of muscle cramps in non-alcoholic cirrhotic patients. J Clin Gastroenterol. 2004;38:524–529. doi: 10.1097/01.mcg.0000129059.69524.d9. [DOI] [PubMed] [Google Scholar]
- 10.Jansen PH, Joosten EM, Vingerhoets HM. Muscle cramp: main theories as to aetiology. Eur Arch Psychiatry Neurol Sci. 1990;239:337–342. doi: 10.1007/BF01735062. [DOI] [PubMed] [Google Scholar]
- 11.Kugelmas M. Preliminary observation: oral zinc sulfate replacement is effective in treating muscle cramps in cirrhotic patients. J Am Coll Nutr. 2000;19:13–15. doi: 10.1080/07315724.2000.10718908. [DOI] [PubMed] [Google Scholar]
- 12.Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120:170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Oh EG, Lee WH, et al. Symptom experience in Korean patients with liver cirrhosis. J Pain Symptom Manage. 2006;31:326–334. doi: 10.1016/j.jpainsymman.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Guyatt G, Kiwi M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bondini S, Kallman J, Dan A, et al. Health-related quality of life in patients with chronic hepatitis B. Liver Int. 2007;27:1119–1125. doi: 10.1111/j.1478-3231.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 16.Turken SA, Cafferty M, Silverberg SJ, et al. Neuromuscular involvement in mild, asymptomatic primary hyperparathyroidism. Am J Med. 1989;87:553–557. doi: 10.1016/s0002-9343(89)80613-8. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita Y, Masuoka K, Miyakoshi S, et al. Vitamin D insufficiency underlies unexpected hypocalcemia following high dose glucocorticoid therapy. Bone. 2008;42:226–228. doi: 10.1016/j.bone.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Atreja A, Abacan C, Licata A. A 51-year-old woman with debilitating cramps 12 years after bariatric surgery. Cleve Clin J Med. 2003;70:417–418. 420, 423–426. doi: 10.3949/ccjm.70.5.417. [DOI] [PubMed] [Google Scholar]
- 19.Korpershoek HW, Witteman EM, Meinardi JR, et al. Severe vitamin D deficiency and hypocalcaemia after bariatric surgery. Ned Tijdschr Geneeskd. 2010;154:A827. [PubMed] [Google Scholar]
- 20.Malham M, Jorgensen SP, Ott P, et al. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922–925. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 22.Crawford BA, Labio ED, Strasser SI, et al. Vitamin D replacement for cirrhosis-related bone disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:689–699. doi: 10.1038/ncpgasthep0637. [DOI] [PubMed] [Google Scholar]
- 23.Jansen PH, Joosten EM, Vingerhoets HM. Muscle cramp as a feature of neuromuscular disease. Five neuromuscular disorders, accompanied by frequent muscle cramps. Acta Neurol Belg. 1992;92:138–147. [PubMed] [Google Scholar]
- 24.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- 25.Bodkin CL, Kennelly KD, Boylan KB, et al. Defining normal duration for after discharges with repetitive nerve stimulation: a pilot study. J Clin Neurophysiol. 2009;26:45–49. doi: 10.1097/WNP.0b013e3181968f00. [DOI] [PubMed] [Google Scholar]
- 26.Leemhuis MP, Struyvenberg A. Significance of hypokalaemia due to diuretics. Neth J Med. 1973;16:18–28. [PubMed] [Google Scholar]
- 27.Hutcheon D, Vincent ME, Sandhu RS. Clinical use of diuretics in congestive heart failure. J Clin Pharmacol. 1981;21:668–672. doi: 10.1002/j.1552-4604.1981.tb05681.x. [DOI] [PubMed] [Google Scholar]
- 28.Liangpunsakul S, Chalasani N. Serum vitamin D concentrations and unexplained elevation in ALT among US adults. Dig Dis Sci. 2011;56:2124–2129. doi: 10.1007/s10620-011-1707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orelind E, Feinglass J, Moran M, et al. Correlates of vitamin D insufficiency in an affluent adult population. South Med J. 2012;105:78–81. doi: 10.1097/SMJ.0b013e3182459145. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan CP, Johnson TM, 2nd, Goode PS, et al. Vitamin D and lower urinary tract symptoms among US men: results from the 2005–2006 National Health and Nutrition Examination Survey. Urology. 2011;78:1292–1297. doi: 10.1016/j.urology.2011.07.1415. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal A, Younossi ZM, Bass NM, et al. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy—a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853–861. doi: 10.1111/j.1365-2036.2011.04808.x. [DOI] [PubMed] [Google Scholar]
- 32.Guay DR. Are there alternatives to the use of quinine to treat nocturnal leg cramps? Consult Pharm. 2008;23:141–156. doi: 10.4140/tcp.n.2008.141. [DOI] [PubMed] [Google Scholar]
- 33.Young G. Muscle cramps: quinine derivatives likely to be effective but not recommended for routine use due to toxicity; vitamin B complex, naftidrofuryl and calcium channel blockers possibly effective. Evid Based Med. 2010;15:114–115. doi: 10.1136/ebm1090. [DOI] [PubMed] [Google Scholar]
- 34.Corbani A, Manousou P, Calvaruso V, et al. Muscle cramps in cirrhosis: the therapeutic value of quinine. Is it underused? Dig Liver Dis. 2008;40:794–799. doi: 10.1016/j.dld.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 35.El-Tawil S, Al Musa T, Valli H, et al. Quinine for muscle cramps. Cochrane Database Syst Rev. 2010:CD005044. doi: 10.1002/14651858.CD005044.pub2. [DOI] [PubMed] [Google Scholar]
- 36.FDA warns of risks with unapproved use of malaria drug qualaquin. [Accessed July 8, 2010];FDA News Release. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm218383.htm.
- 37.Stone MB, Edwards JE, Huxel KC, et al. Threshold frequency of an electrically induced cramp increases following a repeated, localized fatiguing exercise. J Sports Sci. 2010;28:399–405. doi: 10.1080/02640410903508854. [DOI] [PubMed] [Google Scholar]
- 38.Miller KC, Knight KL. Electrical stimulation cramp threshold frequency correlates well with the occurrence of skeletal muscle cramps. Muscle Nerve. 2009;39:364–368. doi: 10.1002/mus.21170. [DOI] [PubMed] [Google Scholar]