Abstract
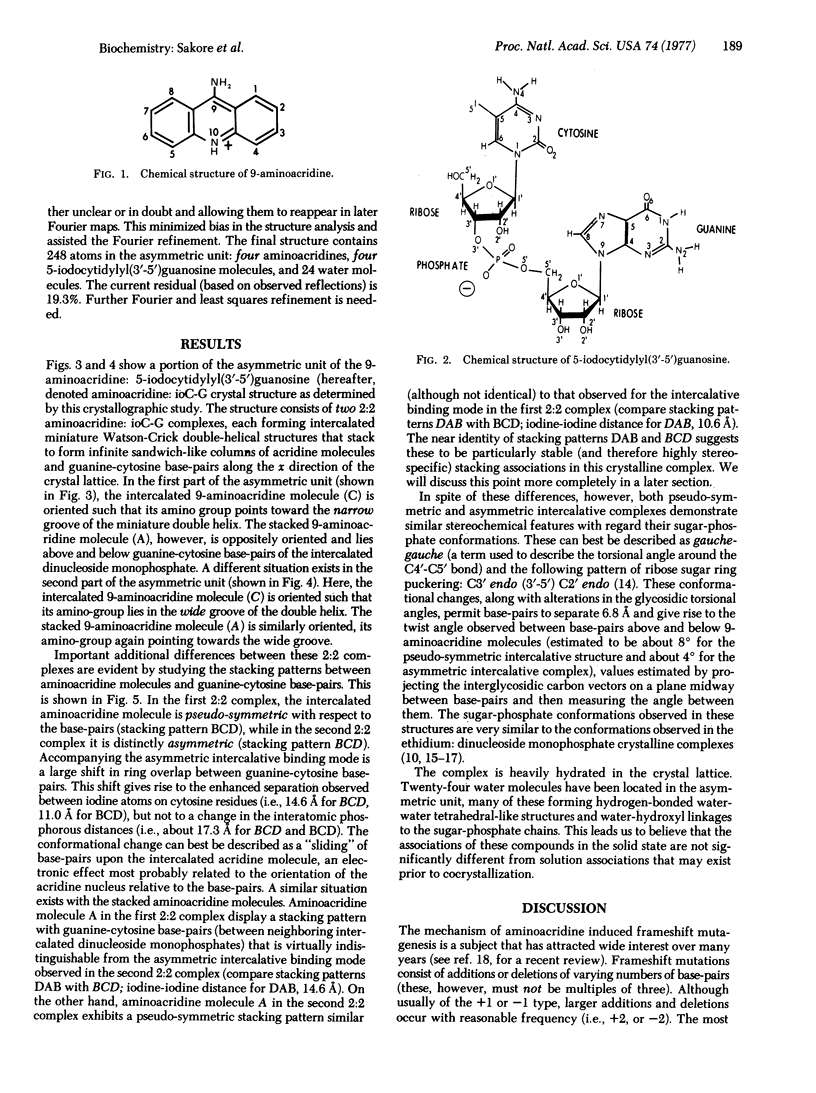
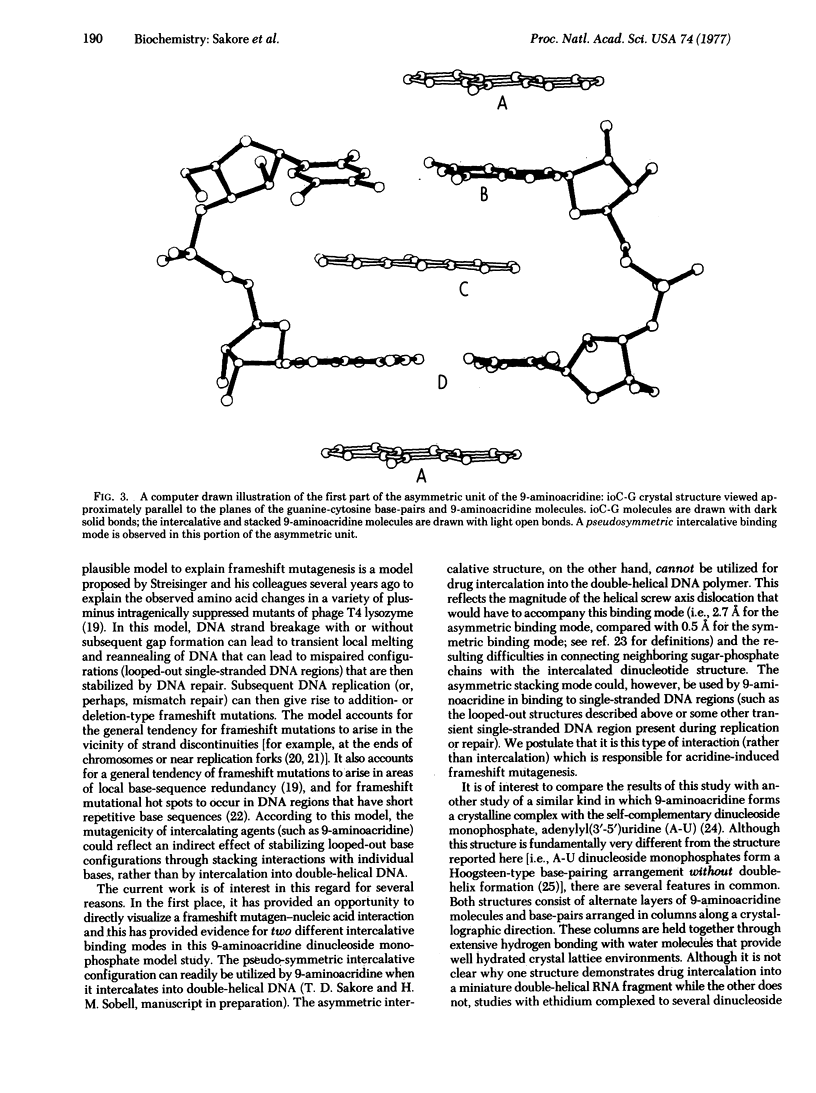
9-Aminoacridine forms a crystalline complex with the dinucleoside monophosphate, 5-iodocytidylyl(3'-5')guanosine. We have solved the three-dimensional structure of this complex by x-ray crystallography and have observed two distinct intercalative binding modes by this drug to miniature Watson-Crick double helical structures. The first of these involves a pseudosymmetric stacking interaction between 9-aminoacridine molecules and guanine-cytosine base-pairs. This configuration may be used by 9-aminoacridine when intercalating into DNA. The second configuration is an asymmetric interaction, largely governed by stacking forces between acridine and guanine rings. This type of association may play an important role in the mechanism of frameshift mutagenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Reinhardt C. G. Evidence for sequence preferences in the intercalative binding of ethidium bromide to dinucleoside monophosphates. J Mol Biol. 1975 Sep 15;97(2):133–162. doi: 10.1016/s0022-2836(75)80031-3. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom D. M., Drake J. W. Mechanics of frameshift mutagenesis in bacteriophage T4: role of chromosome tips. Proc Natl Acad Sci U S A. 1970 Mar;65(3):617–624. doi: 10.1073/pnas.65.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Nastasi M., Morris J. M., Rayner D. M., Seligy V. L., Szabo A. G., Williams D. F., Williams R. E., Yip R. W. Structural implications of the electronic spectra of quinacrine-deoxyribonucleic acid complexes in the ultraviolet region (250-300 nm). J Am Chem Soc. 1976 Jun 23;98(13):3979–3986. doi: 10.1021/ja00429a039. [DOI] [PubMed] [Google Scholar]
- Newton A., Masys D., Leonardi E., Wygal D. Association of induced frameshift mutagenesis and DNA replication in Escherichia coli. Nat New Biol. 1972 Mar 8;236(62):19–22. doi: 10.1038/newbio236019a0. [DOI] [PubMed] [Google Scholar]
- Okada Y., Streisinger G., Owen J. E., Newton J., Tsugita A., Inouye M. Molecular basis of a mutational hot spot in the lysozyme gene of bacteriophage T4. Nature. 1972 Apr 14;236(5346):338–341. doi: 10.1038/236338a0. [DOI] [PubMed] [Google Scholar]
- Pigram W. J., Fuller W., Hamilton L. D. Stereochemistry of intercalation: interaction of daunomycin with DNA. Nat New Biol. 1972 Jan 5;235(53):17–19. doi: 10.1038/newbio235017a0. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Day R. O., Rich A. Nucleic acid-mutagen interactions: crystal structure of adenylyl-3',5'-uridine plus 9-aminoacridine. Nature. 1975 Jan 31;253(5490):324–327. doi: 10.1038/253324a0. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Jain S. C. Stereochemistry of actinomycin binding to DNA. II. Detailed molecular model of actinomycin-DNA complex and its implications. J Mol Biol. 1972 Jul 14;68(1):21–34. doi: 10.1016/0022-2836(72)90259-8. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Gilbert S. G., Jain S. C., Sakore T. D. Organization of DNA in chromatin. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3068–3072. doi: 10.1073/pnas.73.9.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. Drug-nucleic acid interaction: X-ray crystallographic determination of an ethidium-dinucleoside monophosphate crystalline complex, ethidium: 5-iodouridylyl(3'-5')adenosine. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):137–146. doi: 10.1098/rstb.1975.0076. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Jain S. C., Sobell H. M. X-ray crystallographic visualization of drug-nucleic acid intercalative binding: structure of an ethidium-dinucleoside monophosphate crystalline complex, Ethidium: 5-iodouridylyl (3'-5') adenosine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):628–632. doi: 10.1073/pnas.72.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]



