Abstract
Acute liver damage caused by acetaminophen overdose is a significant clinical problem and could benefit from new therapeutic strategies. Objective. This study investigated the hepatoprotective effect of Thymus vulgaris essential oil (TEO), which is used popularly for various beneficial effects, such as its antiseptic, carminative, and antimicrobial effects. The hepatoprotective activity of TEO was determined by assessing serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) in mice. Their livers were then used to determine myeloperoxidase (MPO) enzyme activity and subjected to histological analysis. In vitro antioxidant activity was evaluated by assessing the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH•)-scavenging effects of TEO and TEO-induced lipid peroxidation. TEO reduced the levels of the serum marker enzymes AST, ALT, and ALP and MPO activity. The histopathological analysis indicated that TEO prevented acetaminophen-induced necrosis. The essential oil also exhibited antioxidant activity, reflected by its DPPH radical-scavenging effects and in the lipid peroxidation assay. These results suggest that TEO has hepatoprotective effects on acetaminophen-induced hepatic damage in mice.
1. Introduction
Acetaminophen (APAP) at large doses causes serious liver injury that may develop into liver failure [1]. Hepatotoxicity induced by acetaminophen occurs through a biotransformation reaction that forms the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) through the cytochrome P-450 mixed function of oxidase system. The metabolite is normally detoxified through a conjugation reaction with reduced glutathione (GSH). However, at large doses of acetaminophen, NAPQI levels increase, ultimately depleting GSH levels. Subsequently, sulfhydryl groups of hepatic proteins may react with the reactive metabolite, resulting in hepatic necrosis [2, 3]. Hepatocellular degeneration and necrosis are also associated with elevated enzyme markers, such as serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) that indicate hepatotoxicity [4]. Liver injury induced by acetaminophen in mice is a commonly used experimental model for screening substances with potential hepatoprotective activity [5]. Growing interest has been observed in the analysis of these natural entities for their potential benefits to human health. Accelerating research of plants used in folk medicine to treat liver diseases and boost liver function has been performed. In plants, essential oils are natural mixtures of terpenes, mainly monoterpenes and sesquiterpenes, which have been increasingly used in complementary therapies because essential oils are usually rich sources of phytochemical mixtures [6].
Essential oils extracted from fresh leaves and flowers can be used as aroma additives in food, pharmaceuticals, and cosmetics [7]. The leaves of thyme (Thymus vulgaris) can be used fresh or dried as a spice. Thyme also possesses various beneficial effects, including antiseptic, carminative, antimicrobial, and antioxidative effects [8]. Recently, in our laboratory it was showed that constituents, thymol and carvacrol, of Thymus vulgaris L. essential oil present effects on the inflammatory response [9]. Besides, essential oils and phenolic compounds, such as thymol in thyme, have antioxidative properties and may have hepatoprotective properties [8, 10]. To our knowledge, scarce information is available about the effects of Thymus vulgaris essential oil (TEO) in experimental hepatotoxicity models. Therefore, the present study investigated the hepatoprotective effect of TEO on acetaminophen-induced hepatic damage in mice.
2. Methods
2.1. Extraction of Essential Oil
The fresh leaves of Thymus vulgaris L. were collected from the Proffessor Irenice Silva Medicinal Plant Garden on the campus of the State University of Maringá, Paraná, Brazil. The leaves were identified and authenticated by botanist Maria Aparecida Sert. A voucher specimen was deposited in the Herbarium of the Department of Botany, State University of Maringá (number 11329). The essential oil was obtained by hydrodistillation using a Clevenger-type apparatus. Approximately 556 g of the leaves was subjected to steam distillation for 2 h. The oil was dried over sodium sulfate and stored in an amber flask at 4°C. The TEO yield was 1.76% v/w.
2.2. Essential Oil Analysis
2.2.1. Gas Chromatography-Mass Spectrometry (GC-MS)
Gas chromatographic (GC) analysis was performed with a Thermo Electron Corporation, Focus GC model, under the following conditions: DB-5 capillary column (30 m × 0.32 mm, 0.50 mm); column temperature, 60°C (1 min) to 180°C at 3°C/min; injector temperature 220°C; detector temperature 220°C; split ratio 1 : 10; carrier gas He; flow rate: 1.0 mL/min. The volume injected 1 μL diluted in chloroform (1 : 10). The GC/MS analysis was performed in a Quadrupole mass spectrometer (Thermo Electron Corporation, DSQ II model), operating at 70 V. Identification of the individual components was based on comparison of their GC retention indices (RI) on apolar columns and comparison with mass spectra of authentic standard purchased from Sigma-Aldrich literature data.
2.2.2. Nuclear Magnetic Resonance (NMR)
1H (300.06 MHz) and 13C NMR (75.45 MHz) spectra were recorded in deuterated chloroform (CDCl3) solution in a Mercury-300BB spectrometer, with δ (ppm) and spectra referred to CDCl3 (δ 7.27 for 1H and 77.00 for 13C) as internal standard.
2.3. Animals
Male Balb/c mice, weighing 24 ± 2 g, were provided by the Central Animal House of the State University of Maringá. The animals were housed at 22 ± 2°C under a 12/12 h light/dark cycle. Prior to the experiments, the animals fasted overnight, with water provided ad libitum. The experimental protocols were approved by the Ethical Committee in Animal Experimentation of the State University of Maringá (CEAE/UEM 126/2010).
2.4. Treatment of Animals
The experimental animals were divided into six groups of five animals each. Firstly, each group received orally during seven days the following treatment: Group I, the mice did not receive any treatment. In Group II, the mice received TEO vehicle (saline that contained 0.1% Tween 80). In Groups III–V, the mice were pretreated with TEO at doses of 125, 250, and 500 mg/kg, respectively. In Group VI, the mice were pretreated with the standard drug, silymarin (200 mg/kg). After this time, the animals fasted for 8 h and then received oral acetaminophen on the seventh day at a dose of 250 mg/kg in Groups II–VI. Group I orally received saline that contained 0.1% Tween 80 (APAP vehicle). After 12 h, the mice were anesthetized with halothane, and blood was collected for the determination of serum AST, ALT, and alkaline phosphatase (ALP). The livers were then used to determine myeloperoxidase (MPO) enzyme activity and for histological analysis.
2.5. Determination of Serum ALT, AST, and ALP Levels
Blood samples were collected and centrifuged at 3000 ×g for 15 min at 4°C. Serum ALT, AST, and ALP levels were then measured using the Analyze Gold enzymatic test kit.
2.6. Determination of MPO Activity
The livers were used to determine MPO enzyme activity in the homogenate supernatant of the liver sections, which were placed in potassium phosphate buffer that contained hexadecyltrimethylammonium bromide in a Potter homogenizer. The homogenate was stirred in a vortex and centrifuged. Ten microliters of the supernatant was added to each well in triplicate in a 96-well microplate. Two hundred microliters of the buffer solution that contained 16.7 mg O-dianisidine dihydrochloride (Sigma), 90 mL double-distilled water, 10 mL potassium phosphate buffer, and 50 μL of 1% H2O2 was added. The enzymatic reaction was stopped by the addition of sodium acetate. Enzyme activity was determined by absorbance measured at 460 nm using a Spectra Max Plus microplate spectrophotometer.
2.7. Histopathological Analysis
The livers were washed in 0.9% (w/v) sodium chloride solution and placed in 10% neutral buffered formalin for fixation. Subsequently, the livers were processed to paraffin embedded and sectioned in semiserial at a 6 μm thickness on a Leica rotary microtome (Leica Microsystems, Gladesville, New South Wales, Australia). The sections were stained with hematoxylin and eosin to evaluate tissue morphology using light microscopy (Olympus BX-41, Tokyo, Japan). The graded lesions were subjectively classified as absent, mild, moderate, or severe according to lesion area.
2.8. Lipid Peroxidation Assay
A lipid peroxidation assay was performed as previously reported with a minor modification [11]. Egg yolk homogenates were prepared as lipid-rich media. Briefly, 0.1 mL of TEO (5, 50, 500, 2500, and 5000 μg/mL) in methanol was thoroughly mixed with 0.5 mL of egg yolk homogenate (10%, v/v, diluted with pure water) and made up to 1 mL with pure water. Ferrous sulfate (50 μL, 70 mM) was added to induce lipid peroxidation, and the mixture was incubated for 30 min at 37.5°C. Afterward, 1.5 mL of 20% acetic acid (v/v, pH 3.5, diluted with pure water) and 1.5 mL of 0.8% (w/v) thiobarbituric acid in 1.1% sodium dodecyl sulfate (w/v, diluted with pure water) were added, and the resulting mixture was vortexed and heated at 95°C for 60 min. After cooling, 5 mL of 1-butanol was added to each tube and centrifuged at 5000 rotations per minute for 15 min. The organic upper layer was collected and measured spectrophotometrically at 532 nm using a Beckman DU-65 spectrophotometer. The essential oil was diluted in methanol (the solvent expressed no antioxidant activity). Ascorbic acid was used as a positive control. The inhibition of lipid peroxidation was calculated as follows: Inhibition (%) = (1 − A sample/A control) × 100. A control was considered the absorbance of the control (i.e., methanol, instead of the sample). The IC50 value, representing the concentration of the essential oil that caused 50% inhibition of lipid peroxidation in the Fe2+/ascorbate system, was determined by linear regression analysis from the obtained inhibition (%) values.
2.9. DPPH Assay
Free radical-scavenging capacity (RSC) was evaluated by measuring the 2,2-diphenyl-1-picrylhydrazil (DPPH)-scavenging activity of TEO. The DPPH assay was performed as previously described [12], with minor modifications. The samples (60–2500 μg/mL) in methanol were mixed with 1 mL of a 25 mM DPPH• solution (Sigma, St. Louis, MO, USA), with the addition of 95% methanol to a final volume of 4 mL. The absorbance of the resulting solutions and blank (i.e., with the same chemicals, with the exception of the sample) were recorded against ascorbic acid (Chem Cruz; used as a positive control) after 30 min at room temperature. For each sample, four replicates were recorded. The disappearance of DPPH• was measured spectrophotometrically at 515 nm using a Beckman DU-65 spectrophotometer. The percentage of RSC was calculated using the following equation: RSC (%) = 100 × (A blank − A sample/A blank). The IC50 value, representing the concentration of the essential oil that caused 50% RSC inhibition, was determined by linear regression analysis from the obtained RSC values.
3. Results
The thyme essential oil showed a predominance of carvacrol (45.54%), α-terpineol (22.96%), and endo-borneol (14.29%) as the major components (data not shown). In the acute toxicological study, TEO tested orally showed an LD50 value of 4.000 mg/kg. All doses used in the present study were lowest of LD50 values observed. Consequently, no apparent behavioural side effects were observed in the animals during our studies. The high LD50 values also suggest that the TEO was relatively safe and nontoxic to the animals.
We evaluated the effects of TEO on serum enzyme markers. As shown in Table 1, acetaminophen-induced hepatic damage markedly elevated serum ALT, AST, and ALP enzyme levels compared with the normal animals. Pretreatment with 250 and 500 mg/kg TEO but not 125 mg/kg TEO for 7 days prior to acetaminophen administration markedly reduced serum ALT, AST, and ALP levels compared with vehicle-treated controls. The effect of TEO was also comparable to silymarin, a standard hepatoprotective agent.
Table 1.
The effect of Thymus essential oil on biomarkers of hepatic damage.
| Groups and designe of treatment | ALT | AST | ALP | MPO |
|---|---|---|---|---|
| (IU L−1) | ||||
| Group I, control, Tween 80 | 48.88 ± 2.05 | 113.3 ± 15.39 | 138.40 ± 10.67 | 0.058 ± 0.009 |
| Group II, APAP control (250 mg kg−1) | 11130 ± 973.40a | 6860 ± 140.00a | 181.90 ± 24.75a | 0.251 ± 0.149ª |
| Group III, 125 mg kg−1 of TEO + APAP | 3847 ± 3673 | 3201 ± 2731 | 110.40 ± 35.74 | 0.101 ± 0.008 |
| Group IV, 250 mg kg−1 of TEO + APAP | 261.10 ± 84.41b | 176.0 ± 76.50b | 71.37 ± 11.49b | 0.073 ± 0.009b |
| Group V, 500 mg kg−1 of TEO + APAP | 110.70 ± 35.79b | 110.8 ± 22.53b | 79.20 ± 1.591b | 0.069 ± 0.009b |
| Group VI, 200 mg kg−1 of SLM + APAP | 388.80 ± 148.10b | 286.7 ± 150.40b | 101.40 ± 40.01b | 0.091 ± 0.003b |
Values are mean ± SEM. 5 mice in each group (n = 5), P < 0.05 values are considered statistically significant. a P < 0.05 acetaminophen (APAP) treated group compared with animals in control groups. b P < 0.05 mice treated with the Thyme essential oil (TEO) or Silymarin (SLM) compared with acetaminophen group.
The activity of MPO in TEO-pretreated mice that received doses of 250 and 500 mg/kg was significantly decreased (0.073 ± 0.008 and 0.069 ± 0.008 IU/L, resp.) compared with the group that received acetaminophen only (0.251 ± 0.149 IU/L; Table 1).
The histopathological analysis of control group (APAP vehicle) did not show hepatocellular damage (Figure 1(a)). However, the acetaminophen-treated group showed severe injury characterized by hemorrhagic and necrotic areas, presence of inflammatory infiltrate and piknotic nucleus, (Figure 1(b)). Considering the sylimarin group (standard drug), although the hepatic parenchymal did not present homogenous, necrotic areas were not observed. Also, cellular nucleus was more basophilic than of that control mice and many cells showed cytoplasm vacuolization, showing minor injuries (Figure 1(c)). The group of animals treated with TEO 125 mg/Kg showed interspersed necrotic areas with non-necrotic areas, with cytoplasm vacuolization and hemorrhagic points, characteristics of moderate injuries (Figure 1(d)), differently to that observed after TEO 250 mg/kg treatment, where mild injuries were observed, characterized by basophilic nucleus almost piknotic with shrinkage level (Figure 1(e)). Besides, the group treated with 500 mg/kg of TEO showed the hepatic parenchyma with similar morphology to the control group (Figure 1(f)). Therefore, TEO appeared to provide significant protection against hepatocyte injury.
Figure 1.
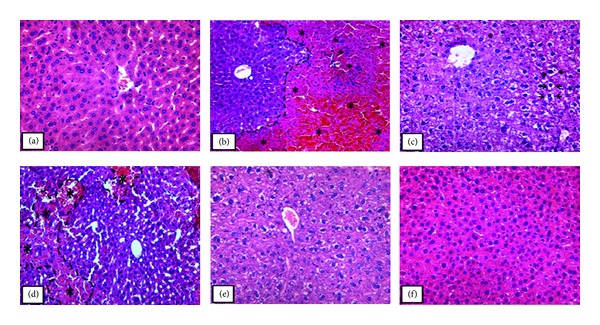
Photomicrograph of the liver in mice that received orally (a) saline, (b) acetaminophen on last day of treatment (250 mg/kg), (c) silymarin (200 mg/kg), and ((d)–(f)) acetaminophen, after being treated for 7 days with the essential oil of Thymus vulgaris (TEO), 125, 250, and 500 mg/kg, respectively. In (a) the liver showed normal morphology; (b) presence of necrosis and hemorrhagic points (*) in the defined area; ((c) and (e)) parenchyma stands out for having vacuolated hepatocytes (arrows); (d) observed necrotic areas (*); (f) hepatic parenchyma morphology similar to that observed in the control. Original magnification 40x in (a), (c), (e), and (f); original magnification 20x in (b) and (d). The sections stained with hematoxylin and eosin.
In the DPPH test, the ability of TEO to act as a donor for hydrogen atoms or electrons in the transformation of DPPH• in its reduced form (DPPH-H) was measured spectrophotometrically. The RSC of TEO at concentrations of 60–2500 μg/mL showed significant antioxidant activity in vitro (IC50 = 1377 ± 1.6970 μg/mL; Figure 2(a), Table 2). The IC50 value of ascorbic acid (i.e., the positive control) was 4.40 ± 0.07928 μg/mL in the DPPH assay (Figure 2(b) and Table 2).
Figure 2.
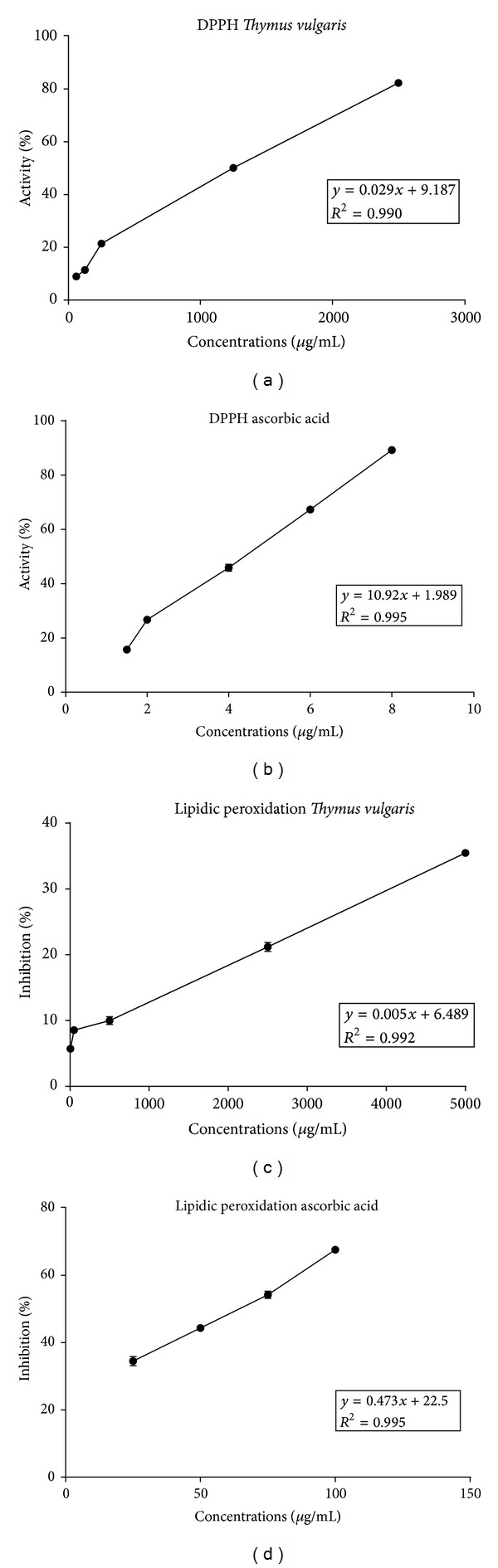
Antioxidant activity of the essential oil from Thymus vulgaris. The figure shows the percentage of neutralization of DPPH by (a) the essential oil of T. vulgaris and (b) ascorbic acid in the DPPH assay (µg/mL). The inhibition of lipid peroxidation (LP) in the Fe2+/ascorbate system induced by (c) the essential oil of T. vulgaris and (d) ascorbic acid in the TBA assay is also shown.
Table 2.
Summary of IC50 values of thyme essential oil (TEO) and ascorbic acid.
| DPPH IC50 (μg/mL) ± SD |
Lipid peroxidation IC50 (μg/mL) ± SD |
|
|---|---|---|
| TEO | 1377 ± 1.6970 | 8461 ± 7.7781 |
| Ascorbic acid | 4.40 ± 0.07928 | 63.00 ± 3.3870 |
Egg yolk lipids undergo rapid lipid peroxidation when incubated in the presence of ferrous sulfate. The effect of TEO on nonenzymatic peroxidation is shown in Figure 2(c) and Table 2. At concentrations of 5–5000 μg/mL, TEO significantly inhibited lipid peroxidation (IC50 = 8461 ± 7.778 μg/mL). The IC50 value of ascorbic acid (i.e., the positive control) was 63.00 ± 3.3870 μg/mL in the lipid peroxidation assay (Figure 2(d) and Table 2). Therefore, TEO was significantly correlated with total antioxidant activity (R > 0.99), demonstrating that TEO had antioxidant activity.
4. Discussion
In the present study, it was evaluated the hepatoprotective effect of TEO using the hepatotoxicity model induced by acetaminophen. This drug in an overdose (i.e., at doses that are different from analgesic doses that are safely and effectively used therapeutically) can induce severe hepatotoxicity in experimental animals and humans [13–15]. In our work, hepatotoxicity was reflected by a marked elevation of the levels of serum marker enzymes (AST, ALT, and ALP), increased MPO activity, and histopathologic alterations. These enzymes in serum are useful quantitative markers of the extent and type of hepatocellular damage. High levels of AST indicate a loss of the functional integrity of the liver, similar to the effects seen in viral hepatitis, cardiac infraction, and muscle injury. The ALT enzyme catalyzes the conversion of alanine to pyruvate and glutamate and is released in a similar manner. Therefore, ALT is more specific to the liver and thus is a better parameter for detecting liver injury [16–18].
Acetaminophen is converted to a toxic reactive intermediate called N-acetyl-p-benzoquinone imine (NAPQI) following metabolism by number of isozymes of cytochrome P-450 (CYPs), that is, CYP 2E1 [19], CYP 1A2 [20], CYP 2A6 [21], CYP 3A4, and CYP 2D6 [22]. NAPQI could be bound covalently to cellular proteins, including mitochondrial proteins [23] and that in turn leads to mitochondrial dysfunction. Mitochondrial respiration is inhibited, which results in the formation of reactive oxygen species (ROS) and peroxynitrite in the mitochondria [24, 25]. The massive production of reactive species (ROS) may lead to depletion of protective physiological moieties (glutathione and α-tocopherol), ensuing widespread propagation of the alkylation as well as peroxidation, causing damage to the macromolecules in vital biomembranes [26]. However, reduced glutathione is one of the main defense mechanisms against oxidative stress reducing peroxides and hydroperoxides [27]. In addition, the oxidative stress can induce a mitochondrial membrane permeability transition and adenosine-5′-triphosphate (ATP) depletion which results in membrane permeabilization, membrane rupture, and cell apoptosis [28–31].
We assessed the hepatoprotective effect of TEO in acetaminophen-induced hepatic damage in mice, and the results suggested that pretreatment with TEO could be protecting the functional integrity of hepatocytes and the cellular membrane from damage by toxic reactive metabolites produced by acetaminophen biotransformation [32, 33].
Inflammation also plays a central role during drug-induced acute hepatitis and products of arachidonic acid metabolism have been extensively involved in inflammatory processes [34]. The histopathological analysis of the livers obtained from the TEO-pretreated group showed mild sinusoidal congestion, less inflammatory cell infiltration, and well-preserved hepatocytes with less of an area of necrosis compared with the severe centrilobular necrosis observed in acetaminophen-treated mice. These results suggest that the anti-inflammatory properties of TEO are partially involved in the hepatoprotective effect of this essential oil. Similarly, previous studies have shown that extracts of plants protect the liver from acetaminophen overdose, suggesting that the hepatoprotective effect can be considered an expression of the functional improvement of hepatocytes that results from accelerated cellular regeneration [35, 36]. Thus, the cytoprotective effects of silymarin, a natural product, are also mainly attributable to its antioxidant and free radical-scavenging properties. Silymarin can interact directly with cell membrane components to prevent abnormalities in the content of the lipid fraction that is responsible for maintaining normal fluidity [37].
Furthermore, the free radical-initiated oxidation of cellular membrane lipids can lead to cellular necrosis and is now accepted to be important in various pathological conditions. High acetaminophen doses significantly elevated reactive oxygen species levels, ultimately depleting the levels of superoxide dismutase (SOD) and GSH in liver tissue. This oxidative stress contributes to the initiation and progression of liver damage [33]. Apoptosis is a form of cell death and has deleterious consequences as observed in many diseases, including acquired immunodeficiency syndrome, cancer, and neurodegenerative disorders [38–40]. Apoptosis is induced by different stimuli such as oxidants, xenobiotics, glucocorticoids, and irradiation [41] which converge to trigger a common pathway of cell death, activating proteases as caspase-3 found only in cells undergoing apoptosis. Protease caspase-3 can cleave and inactivate a nuclear protein poly (ADP-ribose) polymerase (PARP), an enzyme used for DNA repair. Many bioactive substances exert their effect on apoptosis acting in cell cycle progression and/or triggering apoptotic cell death. However, the effects of essential oils inducing or inhibiting apoptosis via mitochondrial stress and caspase activation are controversial [42–45]. Thus, various essential oils have been shown to have antioxidant activity and have been used as antioxidant drugs in many diseases [46–48]. The ability of natural products to reduce acetaminophen-induced lipid peroxidation could be as a result of their antioxidant constituents [49]. The mechanisms of TEO in reduction of free radical species and their effects on cells and tissues damage should be elucidated.
5. Conclusion
TEO pretreatment improves the hepatotoxicity induced by acetaminophen in mice. The effects of TEO partially involve the antioxidative effect of this essential oil. However, further detailed studies are required to investigate the mechanism by which TEO exerts its effects and determine the specific constituents that are responsible for this action.
Acknowledgments
The authors thank Jailson Araujo Dantas and Celia Regina Miranda for technical assistance. This study was supported by grants from the Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Conflict of Interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicological Sciences. 2002;67(2):322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 2.Laskin DL, Pilaro AM. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicology and Applied Pharmacology. 1986;86(2):204–215. doi: 10.1016/0041-008x(86)90051-7. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Mitchell JR. Neutrophil accumulation exacerbates acetaminophen induced liver injury. The FASEB Journal. 1989;3, article A920 [Google Scholar]
- 4.Cigremis Y, Turel H, Adiguzel K, et al. The effects of acute acetaminophen toxicity on hepatic mRNA expression of SOD, CAT, GSH-Px, and levels of peroxynitrite, nitric oxide, reduced glutathione, and malondialdehyde in rabbit. Molecular and Cellular Biochemistry. 2009;323(1-2):31–38. doi: 10.1007/s11010-008-9961-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee WM, Ostapowicz G. Acetaminophen: pathology and clinical presentation of hepatotoxicity. In: Kaplowitz N, DeLeve LD, editors. Drug-Induced Liver Disease. New York, NY, USA: Marcel Dekker; 2003. pp. 327–344. [Google Scholar]
- 6.Daferera DJ, Tarantilis PA, Polissiou MG. Characterization of essential oils from Lamiaceae species by Fourier transform Raman spectroscopy. Journal of Agricultural and Food Chemistry. 2002;50(20):5503–5507. doi: 10.1021/jf0203489. [DOI] [PubMed] [Google Scholar]
- 7.Javanmardi J, Khalighi A, Kashi A, Bais HP, Vivanco JM. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. Journal of Agricultural and Food Chemistry. 2002;50(21):5878–5883. doi: 10.1021/jf020487q. [DOI] [PubMed] [Google Scholar]
- 8.Baranauskiene R, Venskutonis PR, Viškelis P, Dambrauskiene E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris) Journal of Agricultural and Food Chemistry. 2003;51(26):7751–7758. doi: 10.1021/jf0303316. [DOI] [PubMed] [Google Scholar]
- 9.Fachini-Queiroz FC, Kummer R, Estevão-Silva CF, et al. Constituents of Thymus vulgaris L. Essential Oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/657026.657026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson DA, Frankel EN, Aeschbach R, German JB. Inhibition of endothelial cell-mediated oxidation of low-density lipoprotein by rosemary and plant phenolics. Journal of Agricultural and Food Chemistry. 1997;45(3):578–582. [Google Scholar]
- 11.Dasgupta N, De B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chemistry. 2004;88(2):219–224. [Google Scholar]
- 12.Espín JC, Soler-Rivas C, Wichers HJ. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. Journal of Agricultural and Food Chemistry. 2000;48(3):648–656. doi: 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Abril ER, Bethea NW, McCuskey RS. Ethanol binging enhances hepatic microvascular responses to acetaminophen in mice. Microcirculation. 2004;11(7):625–632. doi: 10.1080/10739680490503456. [DOI] [PubMed] [Google Scholar]
- 14.Hwang H-J, Kwon M-J, Kim I-H, Nam T-J. Chemoprotective effects of a protein from the red algae Porphyra yezoensis on acetaminophen-induced liver injury in rats. Phytotherapy Research. 2008;22(9):1149–1153. doi: 10.1002/ptr.2368. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y-L, Piao D-M, Han X-H, Nan J-X. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biological and Pharmaceutical Bulletin. 2008;31(8):1523–1529. doi: 10.1248/bpb.31.1523. [DOI] [PubMed] [Google Scholar]
- 16.Mitra SK, Venkataranganna MV, Sundaram R, Gopumadhavan S. Protective effect of HD-03, a herbal formulation, against various hepatotoxic agents in rats. Journal of Ethnopharmacology. 1998;63(3):181–186. doi: 10.1016/s0378-8741(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 17.Sallie R, Tredger JM, Williams R. Drugs and the liver. Part 1: testing liver function. Biopharmaceutics and Drug Disposition. 1991;12(4):251–259. doi: 10.1002/bdd.2510120403. [DOI] [PubMed] [Google Scholar]
- 18.Williamson EM, Okpako DT, Evans FJ. Selection, Preparation and Pharmacological Evaluation of Plant Material. Chichester, UK: John Wiley & Sons; 1996. [Google Scholar]
- 19.Tanaka E, Terada M, Misawa S. Cytochrome P450 2E1: its clinical and toxicological role. Journal of Clinical Pharmacy and Therapeutics. 2000;25(3):165–175. doi: 10.1046/j.1365-2710.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- 20.Venkatakrishnan K. Human cytochromes p450 mediating phenacetin 0-deethylation in vitro: validation of the high affinity component as an index of CYP1A2 activity. Journal of Pharmaceutical Sciences. 1998;87(12):1502–1507. doi: 10.1021/js980255z. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Koenigs LL, Thompson SJ, et al. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chemical Research in Toxicology. 1998;11(4):295–301. doi: 10.1021/tx9701687. [DOI] [PubMed] [Google Scholar]
- 22.Dong H, Haining RL, Thummel KE, Rettie AE, Nelson SD. Involvement of human cytochrome P450 2D6 in the bioactivation of Acetaminophen. Drug Metabolism and Disposition. 2000;28(12):1397–1400. [PubMed] [Google Scholar]
- 23.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Seminars in Liver Disease. 1990;10(4):267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicological Sciences. 2001;62(2):212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- 26.Aldridge WN. Mechanisms of toxicity. New concepts are required in toxicology. Trends in Pharmacological Sciences. 1981;2:228–231. [Google Scholar]
- 27.Gutierrez MB, Miguel BS, Villares C, Tunon MJ, Gonzales-Callego J. Oxidative Stress Induced by Cremphor EL is not accompanied by changes in NF-KB activation or iNOS expression. Toxicol. 2006;222:125–131. doi: 10.1016/j.tox.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Qian T, Herman B, Lemasters JJ. The mitochondrial permeability transition mediates both necrotic and apoptotic death of hepatocytes exposed to Br-A23187. Toxicology and Applied Pharmacology. 1999;154(2):117–125. doi: 10.1006/taap.1998.8580. [DOI] [PubMed] [Google Scholar]
- 29.Kim J-S, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochemical and Biophysical Research Communications. 2003;304(3):463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim J-S, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124(2):494–503. doi: 10.1053/gast.2003.50059. [DOI] [PubMed] [Google Scholar]
- 31.Kon K, Kim J-S, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40(5):1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 32.Pryor WA. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annual Review of Physiology. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- 33.Van de Straat R, De Vries J, Vermeulen NPE. Role of hepatic microsomal and purified cytochrome P-450 in one-electron reduction of two quinone imines and concomitant reduction of molecular oxygen. Biochemical Pharmacology. 1987;36(5):613–619. doi: 10.1016/0006-2952(87)90710-6. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Alvarez V, Bobadilla-Lugo RA, Muriel P, Favari L, Villanueva-Lopez C. Effects of leukotriene synthesis inhibition on acute liver damage induced by carbon tetrachloride. Pharmacology. 1993;47(5):330–336. doi: 10.1159/000139114. [DOI] [PubMed] [Google Scholar]
- 35.Sadasivan S, Latha PG, Sasikumar JM, Rajashekaran S, Shyamal S, Shine VJ. Hepatoprotective studies on Hedyotis corymbosa (L.) Lam. Journal of Ethnopharmacology. 2006;106(2):245–249. doi: 10.1016/j.jep.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Tabassum N, Chattervedi S, Aggrawal SS, Ahmed N. Hepatoprotective studies on Phyllanthus niruri on paracetamol induced liver cell damage in albino mice. JK Practitioner. 2005;12(4):211–212. [Google Scholar]
- 37.Muriel P, Mourelle M. Prevention by silymarin of membrane alterations in acute CCl4 liver damage. Journal of Applied Toxicology. 1990;10(4):275–279. doi: 10.1002/jat.2550100408. [DOI] [PubMed] [Google Scholar]
- 38.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 39.Ingawale DK, Mandlik SK, Naik SR. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): a critical discussion. Environmental Toxicology and Pharmacology. 2013;37(1):118–133. doi: 10.1016/j.etap.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 40.-Tan DX, Hardeland R, Manchester LC, Galano A, Reiter RJ. Cyclic-hidroxymelatonin (C3HOM), a potent antioxidant, scavanges free radical and suppresses oxidative reactions. Current Medicinal Chemistry. 2013 doi: 10.2174/0929867321666131129113146. [DOI] [PubMed] [Google Scholar]
- 41.Payne CM, Bernstein C, Bernstein H. Apoptosis overview emphasizing the role of oxidative stress, DNA damage and signal-transduction pathways. Leukemia and Lymphoma. 1995;19(1-2):43–93. doi: 10.3109/10428199509059662. [DOI] [PubMed] [Google Scholar]
- 42.Cha J-D, Moon S-E, Kim H-Y, Cha I-H, Lee K-Y. Essential oil of artemisia capillaris induces apoptosis in KB cells via mitochondrial stress and caspase activation mediated by MAPK-stimulated signaling pathway. Journal of Food Science. 2009;74(9):T75–T81. doi: 10.1111/j.1750-3841.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 43.Usta J, Kreydiyyeh S, Knio K, Barnabe P, Bou-Moughlabay Y, Dagher S. Linalool decreases HepG2 viability by inhibiting mitochondrial complexes I and II, increasing reactive oxygen species and decreasing ATP and GSH levels. Chemico-Biological Interactions. 2009;180(1):39–46. doi: 10.1016/j.cbi.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Koo H-N, Hong S-H, Kim C-Y, et al. Inhibitory effect of apoptosis in human astrocytes CCF-STTG1 cells by lemon oil. Pharmacological Research. 2002;45(6):469–473. doi: 10.1006/phrs.2002.0986. [DOI] [PubMed] [Google Scholar]
- 45.Cha J-D, Kim Y-H, Kim J-Y. Essential oil and 1,8-cineole from Artemisia lavandulaefolia induces apoptosis in KB Cells via mitochondrial stress and caspase activation. Food Science and Biotechnology. 2010;19(1):185–191. [Google Scholar]
- 46.Grover AK, Samson SE. Antioxidants and vision health: facts and fiction. Molecular and Cellular Biochemistry. 2013 doi: 10.1007/s11010-013-1908-z. [DOI] [PubMed] [Google Scholar]
- 47.Sendra E, Viuda MM, Nasser ABD, et al. Chemical composition and antioxidant and anti-Listeria activities of essential oils obtained from some Egyptian plants. Journal of Agricultural and Food Chemistry. 2010;58(16):9063–9070. doi: 10.1021/jf101620c. [DOI] [PubMed] [Google Scholar]
- 48.Zeng W-C, Zhu R-X, Jia L-R, Gao H, Zheng Y, Sun Q. Chemical composition, antimicrobial and antioxidant activities of essential oil from Gnaphlium affine . Food and Chemical Toxicology. 2011;49(6):1322–1328. doi: 10.1016/j.fct.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Anoush M, Eghbal MA, Fathiazad F, Hamzeiy H, Kouzehkonani NS. The protective effects of garlic extract against acetaminophen-induced oxidative stress and Glutathione depletion. Pakistan Journal of Biological Sciences. 2009;12(10):765–771. doi: 10.3923/pjbs.2009.765.771. [DOI] [PubMed] [Google Scholar]


