Abstract
Background and Objectives
The presence of sleep apnea (SA) among surgical patients has been associated with significantly increased risk of perioperative complications. Although regional anesthesia has been suggested as a means to reduce complication rates among SA patients undergoing surgery, no data are available to support this association. We studied the association of the type of anesthesia and perioperative outcomes in patients with SA undergoing joint arthroplasty.
Methods
Drawing on a large administrative database (Premier Inc), we analyzed data from approximately 400 hospitals in the United States. Patients with a diagnosis of SA who underwent primary hip or knee arthroplasty between 2006 and 2010 were identified. Perioperative outcomes were compared between patients receiving general, neuraxial, or combined neuraxial-general anesthesia.
Results
We identified 40,316 entries for unique patients with a diagnosis for SA undergoing primary hip or knee arthroplasty. Of those, 30,024 (74%) had anesthesia-type information available. Approximately 11% of cases were performed under neuraxial, 15% under combined neuraxial and general, and 74% under general anesthesia. Patients undergoing their procedure under neuraxial anesthesia had significantly lower rates of major complications than did patients who received combined neuraxial and general or general anesthesia (16.0%, 17.2%, and 18.1%, respectively; P = 0.0177). Adjusted risk of major complications for those undergoing surgery under neuraxial or combined neuraxial-general anesthesia compared with general anesthesia was also lower (odds ratio, 0.83 [95% confidence interval, 0.74–0.93; P = 0.001] vs odds ratio, 0.90 [95% confidence interval, 0.82–0.99; P = 0.03]).
Conclusions
Barring contraindications, neuraxial anesthesia may convey benefits in the perioperative outcome of SA patients undergoing joint arthroplasty. Further research is needed to enhance an understanding of the mechanisms by which neuraxial anesthesia may exert comparatively beneficial effects.
The presence of sleep apnea (SA) among patients undergoing surgery has been associated with significantly increased risk of perioperative complications.1–3 The impact of this problem is compounded by the increasing prevalence of the disease, which is estimated to affect as many as one-fourth of patients presenting for surgery.3,4 Despite this challenge, no proven interventions to reduce complication rates among SA patients are available, and even the impact of perioperative use of positive airway pressure remains equivocal.1,2 In an attempt to reduce the risk for adverse outcomes among surgical patients with SA, the current American Society of Anesthesiology practice advisory recommends the use of regional anesthesia.5 However, no data are available to support this approach. Although we have previously studied outcomes in patients with SA3 using a different database (National Inpatient Sample), this data source did not allow for the study of the impact of the type of anesthesia on outcomes. In addition, this patient group is of particular interest as (1) the type of surgery allows for the utilization of a number of different anesthetic approaches, and (2) the prevalence of SA among orthopedic patients is high compared with other surgical populations.3 Therefore, we sought to study the impact of the type of anesthesia on perioperative outcomes in SA patients undergoing joint arthroplasty using large-scale, population-based data. We hypothesized that the use of neuraxial or a combination of neuraxial and general anesthesia would be associated with more beneficial perioperative outcomes in SA patients undergoing primary hip or knee arthroplasty compared with general anesthesia.
METHODS
Data Source, Ethics Approval
Data collected between 2006 and 2010 from an administrative database (Premier Inc, Charlotte, North Carolina) containing discharge information from approximately 400 acute care hospitals located throughout the United States were used for this study.6 The Premier database provides specific and granular information about present diagnoses and procedures carried out during inpatient visits. Data integration is strictly regulated and controlled via rigorous data validation assurance procedures. This study was exempt from consent requirements by the Hospital for Special Surgery Institutional Review Board as data are deidentified and in accordance with the Health Insurance Portability and Accountability Act. 7 The same data set was used previously by our group for various other projects, including the evaluation of rates of critical care utilization in patients receiving total joint arthroplasty8 and differential outcomes in patients undergoing unilateral hip or knee9 or bilateral total knee arthroplasty.10 However, it had not been used to evaluate the impact of anesthesia-specific information on outcomes in SA patients undergoing hip and knee arthroplasty.
Study Sample
Data were included in the study cohort if patients had (1) International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes for primary total hip or knee arthroplasty (81.51; 81.54) and (2) an ICD-9-CM code for SA (786.03, 780.51, 780.53, 780.57, 327.20–327.27, 327.29). Patients included were admitted between December 2005 and September 2010 to a participating institution. Of those, 2617 patients (8%) had multiple procedures and were excluded from analysis. Utilizing information provided in billing data, the cohort was subdivided into 3 groups according to the type of anesthesia received: (1) general, (2) neuraxial, and (3) combined neuraxial and general anesthesia. Patients who could not be allocated to these groups were excluded from the analysis. The influence of this exclusion on outcomes was assessed by performing a sensitivity analysis by including patients without a designation for anesthesia type (ie, “others”) in the regression analysis. Patients receiving a peripheral nerve block in addition to their primary anesthetic, as identified by billing codes, were included in the study sample and were allocated to the respective primary anesthesia-type groups. The proportion of patients who had only peripheral nerve blocks listed as an anesthetic was less than 1%, and entries were added to the “others” category.
Demographic Variables
Patient characteristics were compared among anesthesia groups. Demographic variables included age, sex, ethnicity (white, African American, Hispanic, other), and procedure type (primary hip or knee arthroplasty). The prevalence of individual comorbidities and overall comorbidity burden was assessed using the method described by Deyo et al.11 The prevalence of comorbid conditions was determined using ICD-9 codes. Age and comorbidity burden were also evaluated as both categorical and continuous variables.
Complication Variables
Cases with major complications were identified by the presence of respective ICD-9-CM diagnoses and billing codes (as listed in Appendix 1). Thirty-day mortality rate was defined as any cases of death within 30 days during any admission. This information was provided by Premier Inc’s discharge status data. Major individual complications included deep venous thrombosis, pulmonary embolism, cerebrovascular event, pulmonary complications, cardiac complications (except myocardial infarction), pneumonia, all infections, acute renal failure, gastrointestinal complications, acute myocardial infarction, and 30-day mortality. A combined complications variable was defined as incurring 1 or more of the major complications listed above. Moreover, a cardiac complications variable (consisting of acute myocardial infarction and cardiac complications other than myocardial infarction) and a pulmonary complications variable (consisting of pulmonary complications, pneumonia, and pulmonary embolism) were established as separate outcomes.
In addition, the incidence of blood product transfusion, mechanical ventilation, and critical care service utilization was computed by identifying appropriate ICD-9-CM or billing codes (Table 3). Differences in length of hospital stay and cost of hospitalization were analyzed as continuous variables and as binary variables dichotomized based on the 75th percentile. Entries above the 75th percentile were categorized as prolonged hospitalizations or increased cost of hospitalization, respectively.
TABLE 3.
Results From the Multivariate Regression for Various Outcomes
| Multivariate Regression
| |||||||
|---|---|---|---|---|---|---|---|
| Outcome | Neuraxial vs General
|
Combined Neuraxial/General vs General
|
Combined Neuraxial/General vs Neuraxial
|
C–Statistic | |||
| OR (95% CI) | P* | OR (95% CI) | P* | OR (95% CI) | P* | ||
| Combined complications | 0.825 (0.74–0.93) | 0.0012 | 0.898 (0.82–0.99) | 0.03 | 1.088 (0.95–1.25) | 0.2399 | 0.64 |
| Pulmonary complications | 0.825 (0.65–1.05) | 0.1154 | 0.772 (0.63–0.95) | 0.0137 | 0.935 (0.70–1.26) | 0.659 | 0.63 |
| Cardiac complications | 0.904 (0.78–1.05) | 0.1859 | 0.936 (0.83–1.06) | 0.3121 | 1.035 (0.86–1.24) | 0.7069 | 0.70 |
| Blood transfusion | 0.919 (0.81–1.04) | 0.1776 | 0.87 (0.78–0.97) | 0.0116 | 0.947 (0.81–1.10) | 0.4821 | 0.64 |
| Mechanical ventilation | 0.636 (0.50–0.80) | 0.0001 | 0.644 (0.53–0.79) | <0.0001 | 1.012 (0.76–1.35) | 0.9334 | 0.59 |
| Critical care service admission | 0.433 (0.34–0.55) | <0.0001 | 0.666 (0.56–0.79) | <0.0001 | 1.538 (1.16–2.03) | 0.0025 | 0.61 |
| Prolonged length of stay | 0.752 (0.69–0.82) | <0.0001 | 0.70 (0.65–0.76) | <0.0001 | 0.93 (0.83–1.04) | 0.202 | 0.60 |
| Increased cost of hospitalization | 0.883 (0.80–0.98) | 0.0139 | 0.704 (0.64–0.77) | <0.0001 | 0.797 (0.70–0.90) | 0.0004 | 0.57 |
General anesthesia is the reference group. Displayed are ORs, 95% CIs, and P values. Prolonged length of stay and increased cost of hospitalization are defined as values exceeding the 75th percentile.
Readers may wish to evaluate statistical significance at a modified α level of 0.05/3 comparisons = 0.0167 (Bonferroni correction). Under this α level, statistical significance is achieved when P < 0.0167 for a given group comparison.
Statistical Analysis
The study goal was to analyze if the type of anesthesia is associated with differences in perioperative outcomes. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, North Carolina). To facilitate analysis of weighted data, SAS procedures SURVEYMEANS, SURVEYFREQ, SURVEYREG, and SURVEYLOGISTIC were utilized for descriptive analyses and modeling efforts.
Univariate Analysis
Weighted means and 95% confidence intervals (CIs) were described for continuous variables with nonskewed distributions, and medians and interquartile ranges (IQRs) were described for variables with skewed distributions. One-way analysis of variance F test or nonparametric Kruskal-Wallis test, whenever applicable, was used to compare means or medians for continuous variables among more than 2 groups.
Unweighted frequency and weighted percentages were reported for categorical variables. A χ2 test was performed to evaluate the association of 2 categorical variables.
Multivariate Regression Analysis
Binary outcomes of incidence of combined major complications, pulmonary complications, cardiac complications, use of blood product transfusion, need for mechanical ventilation, utilization of critical care services, prolonged length of hospital stay, and increased cost of hospitalization as defined above were considered. For each outcome, a weighted logistic regression was used to evaluate its association with the type of anesthesia while controlling for age group, sex, ethnicity, Deyo Index group, and type of surgery. These adjustments were included in the final model because they were considered clinically relevant or had P < 0.2 in the univariate analyses. As a secondary analysis, we added peripheral nerve block as a covariate to evaluate its independent influence on outcomes using the same regression model. Odds ratios, 95% CI, and P values were reported from the multivariate analysis. The conventional threshold of statistical significance (ie, 2-sided P < 0.05) was used to determine significance of variables. However, because pairwise comparisons were performed, the issue of multiplicity adjustment may be a concern. Readers may wish to evaluate statistical significance at a modified α level of 0.05/3 comparisons = 0.0167 based on the Bonferroni correction. Under this α level, statistical significance was achieved when P < 0.0167 for a given group comparison.
The influence of “other” type of anesthesia was assessed by performing a sensitivity analysis in the multivariate regression analysis: once by including and once by excluding “others” entries,12 where “others” entries were treated as a separate group.
Model discrimination and calibration were performed by using the C-statistic and the Hosmer-Lemeshow (H-L) test,13 respectively. The C-statistic, representing the area under the receiver operating characteristic curve, measures the level of model discrimination between observed data at different levels of the outcome. Whereas a C-statistic of 0.7 or above was considered acceptable, it has been suggested that in the setting of large database research a low C-statistics was not a result of a weak model, but rather a consequence of patient entries in cohorts simply becoming more alike.14 The H-L test was performed so that the probability predictions from the model reflected the true occurrence of events in the data. Nonsignificant P values from the H-L test were considered a well-calibrated model; however, significant P values could claim adequate calibration when applied to large numbers of patients.15
RESULTS
We identified a total of 40,316 entries for unique patients with a diagnosis for SA who underwent hip or knee arthroplasty. Of those, 30,024 (74%) had a listing for the type of anesthesia received and were included into the analysis. Approximately 11% of cases were performed under neuraxial, 15% under combined neuraxial and general, and 74% under general anesthesia. The frequencies for the use of peripheral nerve blocks were 8%, 1%, and 1.5% in the general, neuraxial, and general/neuraxial anesthesia group, respectively.
Patient-related demographics are presented in Table 1. Patients who underwent surgery under neuraxial anesthesia were on average older than those in the neuraxial/general or general groups. No difference was found in the prevalence of various comorbidities between groups (Fig. 1) or in the distribution of Deyo comorbidity categories (Table 1). Patients undergoing their procedure under neuraxial anesthesia had significantly lower rates of major complications compared with those undergoing surgery under neuraxial/general or general anesthesia (16.0%, 17.2%, and 18.1%; P = 0.0177). Rates of pulmonary, gastrointestinal, and infectious complications as well as acute renal failure in particular were higher in those undergoing surgery involving a general anesthetic compared with a neuraxial approach (Table 2). Utilization of transfusions, mechanical ventilation, and critical care services was significantly lower in the neuraxial and neuraxial/general groups than in the general anesthesia group. Patients in the neuraxial and general/neuraxial groups had shorter medians of lengths of hospitalizations compared with the general group (2.6 [IQR, 2.2–3.3], 2.6 [IQR, 2.2–3.6], 2.8 [IQR, 2.3–3.6]; P < 0.001). Median costs of care were US $15,827 (IQR, US $13,292–$18,743) for the neuraxial, US $15,357 (IQR, US $13,132–$18,192) for the neuraxial/general, and US $15,345 (IQR, US $12,551–$19,335) for the general anesthesia group, respectively (P < 0.001).
TABLE 1.
Patient- and Procedure-Related Characteristics, Subgrouped by Type of Anesthesia
| Patient-Related Demographics
| |||||||
|---|---|---|---|---|---|---|---|
| Neuraxial | Neuraxial/General | General | P | ||||
| n | 3066 | 4259 | 22,699 | ||||
| % | 11.10% | 15.30% | 73.60% | ||||
| Comorbidity burden | |||||||
| Average Deyo Index (SE) | 0.97 (0.92–1.01) | 0.99 (0.96–1.03) | 1.00 (0.98–1.01) | 0.3896 | |||
| n | % | n | % | n | % | P | |
| Deyo Index category | |||||||
| 0 | 1445 | 47.5 | 1964 | 47.4 | 10,586 | 46.5 | 0.2447 |
| 1 | 661 | 21.2 | 858 | 20.1 | 4552 | 20.1 | |
| 2 | 620 | 20.5 | 915 | 20.7 | 4935 | 22.0 | |
| ≥3 | 340 | 10.8 | 522 | 11.9 | 2626 | 11.4 | |
| Age | |||||||
| Average age (CI), y | 64.2 (63.9–4.6) | 63.9 (63.6–64.2) | 63.0 (62.9–63.2) | <0.0001 | |||
| n | % | n | % | n | % | P | |
| Age category, y | |||||||
| <45 | 54 | 1.9 | 92 | 2.1 | 570 | 2.5 | <0.0001 |
| 45–54 | 423 | 14.3 | 585 | 14.2 | 3723 | 16.5 | |
| 55–64 | 1085 | 34.5 | 1525 | 35.5 | 8335 | 36.7 | |
| 65–74 | 1050 | 34.9 | 1443 | 33.4 | 7249 | 31.6 | |
| >75 | 454 | 14.3 | 614 | 14.8 | 2822 | 12.6 | |
| Sex | |||||||
| Female | 1404 | 46.2 | 1994 | 46.6 | 10475 | 45.8 | 0.6683 |
| Male | 1662 | 53.8 | 2265 | 53.4 | 12224 | 54.2 | |
| Ethnicity | |||||||
| White | 2435 | 82.4 | 3602 | 84.2 | 17893 | 76.8 | <0.0001 |
| African American | 144 | 4.8 | 289 | 6.0 | 1966 | 7.9 | |
| Hispanic | 38 | 1.0 | 51 | 1.3 | 409 | 2.1 | |
| Other | 449 | 11.7 | 317 | 8.5 | 2431 | 13.2 | |
| Procedure type | |||||||
| Total hip arthroplasty | 672 | 21.3 | 1043 | 24.0 | 5788 | 24.8 | 0.0005 |
| Total knee arthroplasty | 2394 | 78.7 | 3216 | 76.0 | 16911 | 75.2 | |
| Admission type | |||||||
| Emergent | 13 | 0.5 | 135 | 4.2 | 680 | 3.8 | <0.0001 |
| Urgent | 112 | 3.8 | 238 | 3.9 | 929 | 3.9 | |
| Elective | 2937 | 95.6 | 3882 | 91.8 | 21,037 | 92.0 | |
| Other | 4 | 0.1 | 4 | 0.1 | 53 | 0.3 | |
The P value indicates testing of the null hypothesis (= no difference across all 3 groups: neuraxial, neuraxial/general, and general): 1-way analysis of variance, Kruskal-Wallis test (for continuous outcomes), χ2 test (for categorical outcomes).
FIGURE 1.
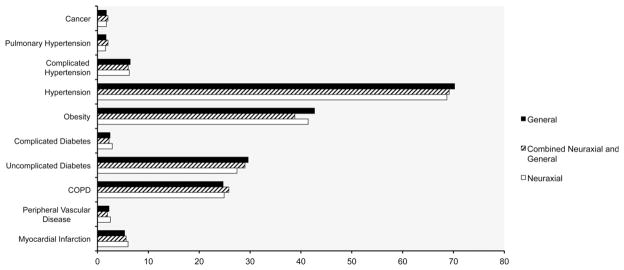
Prevalence of comorbidities. The figure depicts the prevalence (%) of comorbidities among patients by different types of anesthesia. There were no significant differences between groups.
TABLE 2.
Incidence of Major In-hospital Complications, Mortality, and Resource Utilization by Group of Anesthesia
| Complications
| |||||||
|---|---|---|---|---|---|---|---|
| Neuraxial
|
Neuraxial/General
|
General
|
P | ||||
| n | % | n | % | n | % | ||
| Deep venous thrombosis | 9 | 0.4 | 17 | 0.4 | 143 | 0.6 | 0.1327 |
| Pulmonary embolism | 15 | 0.5 | 20 | 0.4 | 144 | 0.6 | 0.1018 |
| Cerebrovascular event | 4 | 0.1 | 4 | 0.1 | 23 | 0.1 | 0.9383 |
| Pulmonary complications | 49 | 1.8 | 79 | 1.9 | 475 | 2.2 | 0.2622 |
| Cardiac (non–myocardial infarction) | 277 | 9.0 | 401 | 9.3 | 2103 | 9.2 | 0.9 |
| Pneumonia | 44 | 1.3 | 54 | 1.1 | 343 | 1.6 | 0.0605 |
| All infections | 125 | 3.8 | 192 | 4.2 | 1022 | 4.6 | 0.1166 |
| Acute renal failure | 82 | 2.8 | 125 | 2.7 | 701 | 3.2 | 0.1172 |
| Gastrointestinal complication | 29 | 0.9 | 43 | 0.9 | 298 | 1.3 | 0.0657 |
| Acute myocardial infarction | 11 | 0.4 | 10 | 0.2 | 54 | 0.2 | 0.258 |
| Mortality (30-d) | 3 | 0.1 | 9 | 0.2 | 48 | 0.2 | 0.2879 |
| Blood transfusion | 438 | 12.7 | 592 | 12.4 | 3230 | 13.8 | 0.0273* |
| Mechanical ventilation | 119 | 2.8 | 139 | 2.8 | 945 | 4.4 | <0.0001* |
| Critical care services admission | 97 | 3.1 | 190 | 4.8 | 1647 | 6.9 | <0.0001* |
(MI = myocardial infarction).
The P value indicates testing of the null hypothesis (= no difference across all three groups: neuraxial [N], neuraxial/general [NG], and general [G]): χ2 test (for categorical outcomes).
Significant P values from post hoc comparisons: for outcome of blood transfusion: P = 0.0173(G vs NG); for outcome of mechanical ventilation: P < 0.0001 (G vs NG) and P < 0.001 (G vs N); for critical care service admission: P < 0.0001(G vs NG), P = 0.0015 (N vs NG), and P < 0.001 (G vs N).
There were no significant odds ratio (OR) estimate differences in the sensitivity analysis with or without missing data of type of anesthesia. When controlling for covariates, the odds for combined major complications were lower among patients receiving neuraxial anesthesia compared with general anesthesia (Table 3). The same was true when specifically looking at the outcome of pulmonary complications, but the type of anesthesia had no effect on the risk for cardiac complications, respectively (Table 3).
Furthermore, the odds for the outcomes of utilization of mechanical ventilation, requirement for critical care services, prolonged length of stay, and increased cost were also beneficially associated with neuraxial versus general anesthesia (Table 3). No difference was found for the odds of requiring blood transfusions between neuraxial and general anesthesia.
When entering the presence or absence of a peripheral nerve block into the regression models, the use of a peripheral nerve block was not associated with any significant impact on the outcomes of combined, pulmonary, and cardiac complications (OR, 0.95 [CI, 0.85–1.06; P = 0.3661]; OR, 0.93 [CI, 0.74–1.16; P = 0.4989]; OR, 1.11 [CI, 0.96–1.28; P = 0.1596]) or need for transfusion (OR, 1.03 [CI, 0.92–1.17; P = 0.1596]), respectively.
However, decreased odds for the need of mechanical ventilation, critical care services, and prolonged length of stay were seen, respectively (OR 0.66 [CI, 0.51–0.86; P = 0.0018]; OR, 0.46 [CI, 0.36–0.57; P < 0.0001]; OR, 0.84 [CI, 0.77–0.92; P < 0.0001]). The odds for increased cost above the 75th percentile for those with a peripheral nerve block were increased (OR, 1.4 [CI, 1.28–1.53; P < 0.0001]).
The C-statistic ranged from 0.6 to 0.7. The H-L tests showed nonsignificant P values for all models except for those with the outcomes mechanical ventilation and utilization of critical care services.
DISCUSSION
In this population-based study, we were able to show differential perioperative outcomes in patients with a diagnosis of SA who undergo hip or knee arthroplasty procedures under neuraxial versus general anesthesia. Patients with SA who received a neuraxial versus a general anesthetic for their surgery had reduced odds for major complications, requirement for mechanical ventilation, and intensive care services, as well as decreased odds for prolonged hospitalization and increased cost. Therefore, without proving a causal relationship, our results suggest potential beneficial associations of the use of regional anesthesia in SA patients with regard to perioperative complication incidence and risk. To the best of our knowledge, this is the first large-scale, population-based study to describe these associations.
We found significant discrepancies in the utilization of the various anesthesia types among patients with SA. Only one-fourth of patients received a neuraxial anesthetic with or without a concomitant general technique. The lower utilization of regional anesthesia among the orthopedic patient populations has previously been identified in our study of unilateral hip and knee and bilateral knee arthroplasty recipients.9,10 Although the choice of anesthetic technique may vary by, among other reasons, regional custom, anesthesiologist’s and surgeon’s preference, and patient wishes, the low rate of utilization found in this study is surprising. Given the fact that hip and knee arthroplasties are amenable to regional anesthesia and that the American Society of Anesthesiologists advisory on the perioperative and postoperative management of patients with SA has identified regional anesthesia as a preferred anesthetic option for these patients,5 a higher rate of utilization might have been expected. Moreover, patients in whom epidural analgesia or peripheral nerve blocks have been applied intraoperatively were recently shown to exhibit lower postoperative opioid-related distress scores.16
We identified favorable odds in regard to complications, resource utilization, and cost among patients receiving neuraxial versus general anesthesia in this study. A potentially beneficial influence of regional anesthesia on perioperative outcomes after orthopedic surgery has previously been reported in a number of meta-analyses.17–19 However, outcomes studied were limited, and benefits were shown mostly related to reduced blood loss and effects on thromboembolic events. Unfortunately, these reports are limited by a number of factors including analyses of studies with relatively small sample sizes, representation of experiences of specialized centers, and inclusion of investigations that do not reflect recent medical practice.
A further limitation of existing studies is the lack of data on outcomes among subpopulations of patients, which may arguably be affected by different levels of risk for adverse events. In this context, patients with SA have been identified as a group of individuals at especially high risk for perioperative complications, especially those affecting the pulmonary system.1,3 Reasons for these findings may include the high prevalence of comorbid disease such as obesity and diabetes,3 higher baseline levels of systemic and pulmonary inflammation that are compounded by perioperative insults,20 decreased pharyngeal sphincter function thus promoting risk of aspiration,3,21 and increased sensitivity to the respiratory depressant effects of opioids potentially leading to respiratory compromise.22 Unlike other studies in orthopedic patients,18,23 our findings indicate that neuraxial anesthesia confers only insignificantly reduced odds for requirement of blood transfusion in patients with SA when compared with general anesthesia. Evidence regarding reduced need of blood product substitution evoked by neuraxial anesthesia has, however, not remained undisputed. A meta-analysis by Mauermann et al18 concluded that patients undergoing total hip arthroplasty under neuraxial anesthesia had lower overall odds of receiving blood transfusions (OR, 0.26); however, the very wide CI (0.06–1.05) indicated a high degree of variability within the included studies. Moreover, there are currently no data available on comparative risk for transfusion in patients with SA, a patient population with potentially distinct risk factors for blood loss and transfusion.
In a case-control study including 1016 cases of SA patients and controls, Cashman et al24 reported no difference in perioperative cardiovascular and respiratory complications following total hip arthroplasty, but a higher incidence of acute renal failure in SA patients. These findings could indicate that awareness for the condition along with a perioperative monitoring and treatment protocols could be capable of, at least to a certain degree, reducing the associated risk.
Although only a secondary outcome, we attempted to identify the independent impact of the addition of a nerve block on outcomes. Although the regression models including this variable were less robust and have to be interpreted accordingly, we found that the addition of a peripheral nerve block did not affect the risk of complications. However, some benefit was seen in regard to the need of mechanical ventilation and use of critical care services. Peripheral nerve blocks may confer better pain control postoperatively and thus may reduce the risk of opioid-related respiratory depression, which may explain our findings to some degree.16,25,26
A number of factors must be mentioned as limitations, most of which are related to the nature of data source analysis. First, the Premier Inc database comprises only events that occurred during the index admission in which the surgery was performed; thus, postdischarge events (except 30-day mortality) could not be analyzed. Also, we did not obtain detailed information about a number of clinical events from the database: blood loss, administration of medication (including cardioprotective drugs and anticoagulation agents), and other therapeutic interventions were not taken into account. However, as all of this information is recorded from a variety of clinical settings, our results are based on “real world” practice, rather then presenting conclusions from randomized or controlled circumstances in single academic institutions, as is frequently the case with prospective studies. Furthermore, ICD-9 coding is used to identify complications in this database (Table 3). In addition, the timing of complication recording may be contingent with institutional practices; that is, the exact occurrence time of the complication within the time of hospital stay cannot be determined. It must also be mentioned that coding errors can occur despite rigorous quality checks by the database vendor. As this confounding factor is equally distributed across the whole data set, the resulting bias is likely of little relevance to comparative results. To ensure accuracy and quality, extensive data validation procedures are performed before distribution. Multiple analyses have been performed and published across a number of specialties, confirming the rigor of the database construct.27–29
Moreover, it is not possible to correlate the diagnosis of SA as defined by ICD-9 code with the severity of the condition. As only patients with a present diagnosis code for SA were identified, the actual incidence of SA is likely considerably higher than reported in this study. It is possible that patients with problematic airways were preferentially managed using general anesthesia. Weingarten et al30 recently reported that the severity of SA as determined by preoperative polysomnography was not associated with the rate of perioperative complications. However, these results were based on bariatric surgery in fully diagnosed and treated patients, and the authors conclude that this does not necessarily apply to unrecognized and/or untreated SA.30 In addition, we do not have information on the continuous or perioperative use of positive airway pressure therapy as a therapeutic strategy for SA. The individual risk of either anesthetic technique (neuraxial or general) was not analyzed, including complications such as nerve damage, blood vessel puncture, intubation-related oropharyngeal injury, or failed intubation. However, all of these complications are known to occur with relatively low incidence. Individual contraindications have to be taken into consideration as certain comorbidities can potentially prohibit the use of one or the other technique (for instance, anticoagulation for neuraxial or pulmonary comorbidity for general anesthesia). Although interesting, the combined neuraxial/general anesthesia group poses additional difficulty in interpretation, as patients with failed neuraxial techniques (for instance, those with inadequate intraoperative analgesia) were likely included into this category as well as those with a planned combined approach. Finally, the group of patients who also received a peripheral nerve block in this cohort was relatively small, and no information for the reason for time of placement can be derived from the database. In addition, the subset of patients who received a peripheral nerve block as the only anesthetic for joint arthroplasty was less than 1% and thus not further analyzed in this study.
In conclusion, in this population-based cohort of orthopedic patients with SA, we were able to show that neuraxial anesthesia and combined neuraxial-general anesthesia are associated with improved odds in regard to major perioperative morbidity, resource utilization, and cost, compared with general anesthesia. Therefore, barring any contraindications, neuraxial anesthesia could potentially convey benefits in perioperative outcome of patient with SA undergoing joint arthroplasty. Further research is needed, however, to examine cause and effect of type of anesthesia and outcome and elucidate potential mechanisms by which neuraxial anesthesia may confer beneficial effects compared with the general approach.
Acknowledgments
This work should be attributed to the Department of Anesthesiology, Hospital for Special Surgery, Weill Medical College of Cornell University.
This study received funding from the Anna-Maria and Stephen Kellen Physician-Scientist Career Development Award and the Department of Anesthesiology, Hospital for Special Surgery, New York, NY (to Dr. Memtsoudis). Contributions of Dr. Mazumdar, Ms. Chiu, and Ms. Sun on this project were supported in part by funds from the Clinical Translational Science Center, New York, NY, and National Center for Advancing Translational Sciences, Rockville, MD (UL1-RR024996).
APPENDIX 1. ICD-9-CM Diagnosis Codes for Major Complications
| Complications | ICD-9-CM Diagnosis Codes |
|---|---|
| Deep venous thrombosis | 451.1, 451.2, 451.8, 451.9, 453.2, 453.4, 453.8, 453.9 |
| Pulmonary embolism | 415.1 |
| Cerebrovascular event | 433.01, 433.11, 433.21,433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 997.02 |
| Pulmonary complications | 514, 518.4, 518.5, 518.81, 518.82 |
| Cardiac (non–myocardial infarction) | 426.0, 427.41, 427.42, 429.4, 997.1, 427.4, 427.3, 427.31, 427.32 |
| Pneumonia | 481, 482.00- 482.99, 483,485, 486, 507.0, 997.31, 997.39 |
| All infections | 590.1, 590.10, 590.11, 590.8, 590.81, 590.2, 590.9, 595.0, 595.9, 599.0, 567.0 480, 480.0, 480.1, 480.2, 480.8, 480.9, 481, 482.0, 482.1, 482.2, 482.3, 482.30, 482.31, 482.32, 482.39, 482.4, 482.40, 482.41, 482.42, 482.49, 482.5, 482.8, 482.81, 482.82, 482.83, 482.84, 482.89, 482.9, 483, 483.0, 483.1, 483.8, 485, 486, 487, 997.31, 038, 038.0, 038.1, 038.10, 038.11, 038.12, 038.19, 038.2, 038.3, 038.4, 038.40, 038.41, 038.42, 038.43, 038.44, 038.49, 038.8, 038.9, 790.7, 998.0, 958.4, 998.5, 998.59, 998.89, 785, 785.50, 785.52, 785.59, 999.39, 999.31, 999.3 |
| Acute renal failure | 584, 584.5, 584.9 |
| Gastrointestinal complication | 997.4, 560.1, 560.81, 560.9, 536.2, 537.3 |
| Acute myocardial infarction | 410.XX |
| Blood transfusion | 99.0, 99.01, 99.02, 99.03, 99.04, 99.05, 99.06, 99.07, 99.08, 99.09, (HCPCS codes) P9010, P9011, P9012, P9016, P9017, P9019, P9020, P9021, P9022, P9023, P9031, P9032, P9033, P9034, P9035, P9036, P9037, P9038, P9039, P9040 |
| Mechanical ventilation | 93.90, 96.7, 96.70, 96.71, 96.72, (CPT code) 94002, 94656, 94003, 94657 |
Footnotes
The authors declare no conflict of interest.
References
- 1.Kaw R, Pasupuleti V, Walker E, Ramaswamy A, Foldvary-Schafer N. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141:436–441. doi: 10.1378/chest.11-0283. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 3.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–121. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 4.Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 2009;10:753–758. doi: 10.1016/j.sleep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093. doi: 10.1097/00000542-200605000-00026. quiz 1117–1118. [DOI] [PubMed] [Google Scholar]
- 6.Premier Inc. Charlotte, NC: Premier Perspective Database; [Accessed December 30, 2012]. Available at: https://www.premierinc.com/quality-safety/tools-services/prs/data/perspective.jsp. [Google Scholar]
- 7.US Department of Health and Human Services. OCR Privacy Brief: Summary of the HIPAA Privacy Rule. Washington, DC: Office for Civil Rights, HIPAA Compliance Assistance; 2003. [Google Scholar]
- 8.Memtsoudis SG, Sun X, Chiu YL, et al. Utilization of critical care services among patients undergoing total hip and knee arthroplasty: epidemiology and risk factors. Anesthesiology. 2012;117:107–116. doi: 10.1097/ALN.0b013e31825afd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memtsoudis SG, Sun X, Chiu YL, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118:1046–1058. doi: 10.1097/ALN.0b013e318286061d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stundner O, Chiu YL, Sun X, et al. Comparative perioperative outcomes associated with neuraxial versus general anesthesia for simultaneous bilateral total knee arthroplasty. Reg Anesth Pain Med. 2012;37:638–644. doi: 10.1097/AAP.0b013e31826e1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Groenwold RH, Donders AR, Roes KC, Harrell FE, Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175:210–217. doi: 10.1093/aje/kwr302. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer DW, Lemeshow SA. Goodness-of-fit test for the multiple logistic regression model. Commun Stat. 1980;A10:1043–1069. [Google Scholar]
- 14.Merkow RP, Hall BL, Cohen ME, et al. Relevance of the C-statistic when evaluating risk-adjustment models in surgery. J Am Coll Surg. 2012;214:822–830. doi: 10.1016/j.jamcollsurg.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med. 2007;35:2052–2056. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- 16.Yadeau JT, Liu SS, Rade MC, Marcello D, Liguori GA. Performance characteristics and validation of the Opioid-Related Symptom Distress Scale for evaluation of analgesic side effects after orthopedic surgery. Anesth Analg. 2011;113:369–377. doi: 10.1213/ANE.0b013e31821ae3f7. [DOI] [PubMed] [Google Scholar]
- 17.Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91:935–942. doi: 10.1302/0301-620X.91B7.21538. [DOI] [PubMed] [Google Scholar]
- 18.Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103:1018–1025. doi: 10.1213/01.ane.0000237267.75543.59. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpagnano GE, Lacedonia D, Foschino-Barbaro MP. Non-invasive study of airways inflammation in sleep apnea patients. Sleep Med Rev. 2011;15:317–326. doi: 10.1016/j.smrv.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Sabate JM, Jouet P, Merrouche M, et al. Gastroesophageal reflux in patients with morbid obesity: a role of obstructive sleep apnea syndrome? Obes Surg. 2008;18:1479–1484. doi: 10.1007/s11695-008-9508-9. [DOI] [PubMed] [Google Scholar]
- 22.Blake DW, Chia PH, Donnan G, Williams DL. Preoperative assessment for obstructive sleep apnoea and the prediction of postoperative respiratory obstruction and hypoxaemia. Anaesth Intensive Care. 2008;36:379–384. doi: 10.1177/0310057X0803600309. [DOI] [PubMed] [Google Scholar]
- 23.Guay J. The effect of neuraxial blocks on surgical blood loss and blood transfusion requirements: a meta-analysis. J Clin Anesth. 2006;18:124–128. doi: 10.1016/j.jclinane.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Cashman J, Bican O, Patel R, Jacovides C, Dalsey C, Parvizi J. Total joint arthroplasty in patients with obstructive sleep apnea: strategies for reduction of perioperative complications. Surg Technol Int. 2011;XXI:261–266. [PubMed] [Google Scholar]
- 25.Meftah M, Wong AC, Nawabi DH, Yun RJ, Ranawat AS, Ranawat CS. Pain management after total knee arthroplasty using a multimodal approach. Orthopedics. 2012;35:e660–e664. doi: 10.3928/01477447-20120426-19. [DOI] [PubMed] [Google Scholar]
- 26.Hogan MV, Grant RE, Lee L., Jr Analgesia for total hip and knee arthroplasty: a review of lumbar plexus, femoral, and sciatic nerve blocks. Am J Orthop. 2009;38:E129–E133. [PubMed] [Google Scholar]
- 27.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 28.Blanchette CM, Wang PF, Joshi AV, Kruse P, Asmussen M, Saunders W. Resource utilization and costs of blood management services associated with knee and hip surgeries in US hospitals. Adv Ther. 2006;23:54–67. doi: 10.1007/BF02850347. [DOI] [PubMed] [Google Scholar]
- 29.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 30.Weingarten TN, Flores AS, McKenzie JA, et al. Obstructive sleep apnoea and perioperative complications in bariatric patients. Br J Anaesth. 2011;106:131–139. doi: 10.1093/bja/aeq290. [DOI] [PubMed] [Google Scholar]


