Abstract
The fidelity of protein synthesis is substantially greater than the specificity of codon-anticodon recognition that would be expected from the known energetics of base-pairing in solution. To test the suggestion that the specificity of recognition may be increased by "kinetic proofreading" associated with GTP hydrolysis [J. J. Hopfield (1974) Proc. Natl. Acad. Sci. USA 71, 4135-4139], we have studied the interaction of ternary complexes of polypeptide elongation factor Tu, aminoacyl-tRNA, and GTP with poly(U)-programed ribosomes. With most noncognate ternary complexes, including two that pair correctly with the 5' and 3' bases of UUU, rejection occurred without GTP hydrolysis, presumably by the reverse of the initial binding reaction. However, with complexes containing Leu- or Ile-tRNAs, which may pair correctly with the 3' and middle bases, GTP hydrolysis was stimulated though the aa-tRNA was not retained on the ribosome. These results demonstrate the existence of a GTP-dependent proofreading step in aminoacyl-tRNA recognition on ribosomes. They also suggest that the 5' base of the codon is more prone than the middle base to errors that can be corrected by proofreading.
Full text
PDF
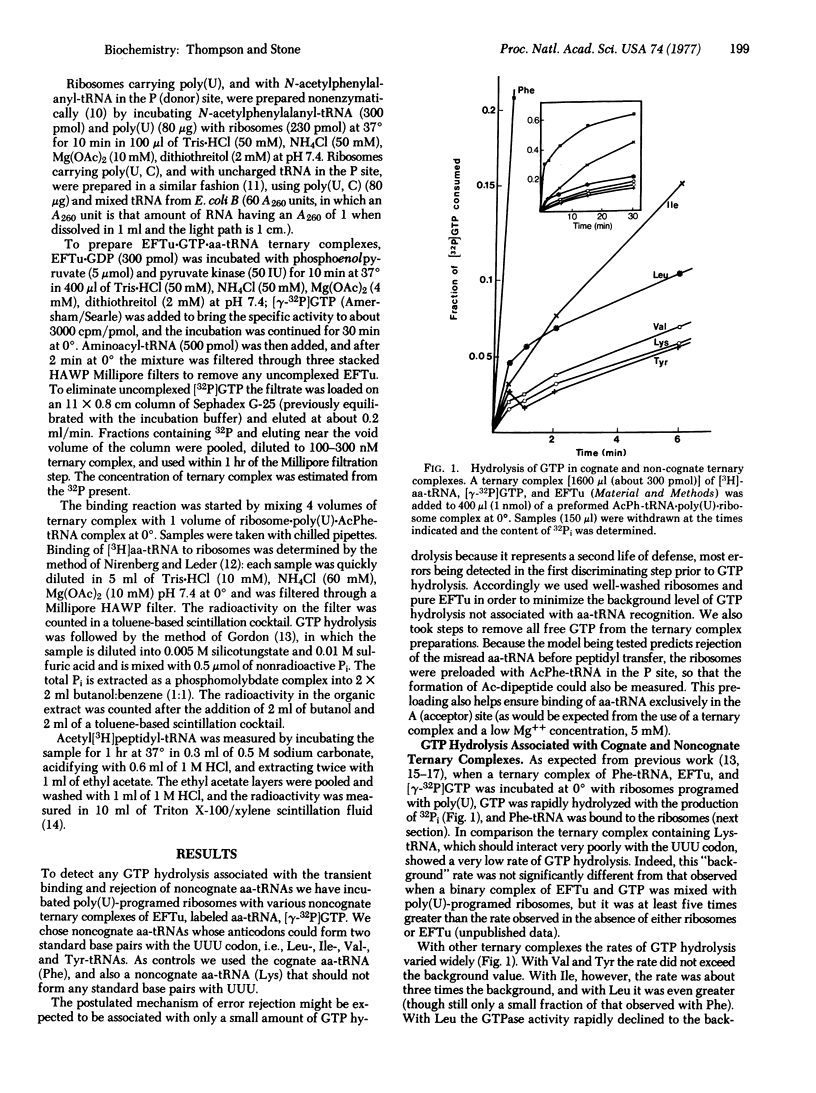

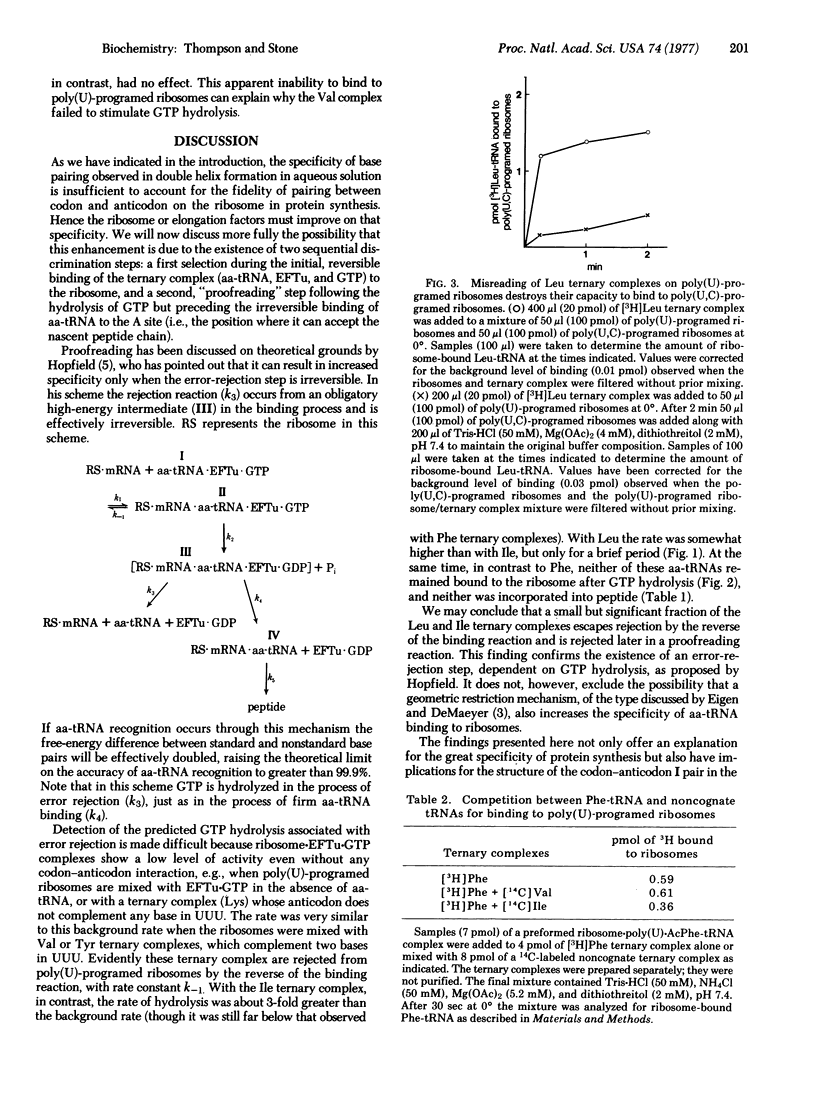

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank H. U., Söll D. Purification of five leucine transfer ribonucleic acid species from Escherichia coli and their acylation by heterologous leucyl-transfer ribonucleic acid synthetase. J Biol Chem. 1971 Aug 25;246(16):4947–4950. [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Gorini L., Davis B. D. Misreading of RNA codewords induced by aminoglycoside antibiotics. Mol Pharmacol. 1965 Jul;1(1):93–106. [PubMed] [Google Scholar]
- Eigen M., de Maeyer L. Chemical means of information storage and readout in biological systems. Naturwissenschaften. 1966 Feb;53(3):50–57. doi: 10.1007/BF00594747. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN S. M., WEINSTEIN I. B. LACK OF FIDELITY IN THE TRANSLATION OF SYNTHETIC POLYRIBONUCLEOTIDES. Proc Natl Acad Sci U S A. 1964 Oct;52:988–996. doi: 10.1073/pnas.52.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J., Landy A., Brenner S., Smith J. D. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature. 1968 Mar 16;217(5133):1019–1024. doi: 10.1038/2171019a0. [DOI] [PubMed] [Google Scholar]
- Gordon J. Hydrolysis of guanosine 5'-triphosphate associated wh binding of aminoacyl transfer ribonucleic acid to ribosomes. J Biol Chem. 1969 Oct 25;244(20):5680–5686. [PubMed] [Google Scholar]
- Hopfield J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot Y., de Groot N., Rappoport S., Hamburger A. D. Modified aminoacyl-tRNA. 3. A general procedure for the synthesis of dipeptidyl transfer RNA. Biochim Biophys Acta. 1967 Dec 19;149(2):532–539. [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Requirement of granosine 5'-triphosphate for ribosomal binding of aminoacyl-SRNA. Proc Natl Acad Sci U S A. 1968 Feb;59(2):554–560. doi: 10.1073/pnas.59.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Ono Y., Skoultchi A., Waterson J., Lengyel P. Stoichiometry of aminoacyl-transfer RNA binding and GTP cleavage during chain elongation and translocation. Nature. 1969 Aug 16;223(5207):697–701. doi: 10.1038/223697a0. [DOI] [PubMed] [Google Scholar]
- SO A. G., DAVIE E. W. THE EFFECTS OF ORGANIC SOLVENTS ON PROTEIN BIOSYNTHESIS AND THEIR INFLUENCE ON THE AMINO ACID CODE. Biochemistry. 1964 Aug;3:1165–1169. doi: 10.1021/bi00896a027. [DOI] [PubMed] [Google Scholar]
- SZER W., OCHOA S. COMPLEXING ABILITY AND CODING PROPERTIES OF SYNTHETIC POLYNUCLEOTIDES. J Mol Biol. 1964 Jun;8:823–834. doi: 10.1016/s0022-2836(64)80163-7. [DOI] [PubMed] [Google Scholar]
- Seeds N. W., Retsema J. A., Conway T. W. Properties of ribosomal binding sites for phenylalanyl-transfer ribonucleic acid. J Mol Biol. 1967 Aug 14;27(3):421–430. doi: 10.1016/0022-2836(67)90048-4. [DOI] [PubMed] [Google Scholar]
- Shorey R. L., Ravel J. M., Shive W. The effect of guanylyl-5'-methylene diphosphonate on binding of aminoacyl-transfer ribonucleic acid to ribosomes. Arch Biochem Biophys. 1971 Sep;146(1):110–117. doi: 10.1016/s0003-9861(71)80047-4. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Martin F. H., Doty P. Self-complementary oligoribonucleotides: effects of helix defects and guanylic acid-cytidylic acid base pairs. J Mol Biol. 1971 Apr 28;57(2):217–229. doi: 10.1016/0022-2836(71)90342-1. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Nucleotide sequence of E. coli B tRNA1-Val. Nature. 1969 Apr 19;222(5190):278–279. doi: 10.1038/222278a0. [DOI] [PubMed] [Google Scholar]
- Yarus M., Barrell B. G. The sequence of nucleotides in tRNA Ile from E. coli B. Biochem Biophys Res Commun. 1971 May 21;43(4):729–734. doi: 10.1016/0006-291x(71)90676-0. [DOI] [PubMed] [Google Scholar]


